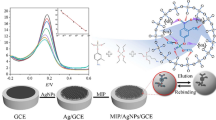
Abstract
A sensitive molecularly imprinted electrochemical sensor was successfully constructed for the detection of acrylamide (AM). It is based on a glassy carbon electrode modified with a composites prepared from gold nanoparticles, multiwalled carbon nanotubes, and chitosan along with a sol-gel-based molecularly imprinted polymer (MIP) film. The latter was prepared using AM as the template molecule, 3-aminopropyltrimethoxysilane as the functional monomer, and tetraethoxysilane as the cross-linker. The MIP sensor showed a linear current response to the target AM concentration in the range from 0.05 to 5 μg mL−1 at a working voltage of 0–0.4 V with a lower detection limit of 0.028 μg mL−1 (S/N = 3). It was successfully applied to the detection of AM in potato chips. HPLC analysis was also conducted to detect AM in the same samples to demonstrate the applicability of the electrochemical MIP sensor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acrylamide (AM), a well-known neurotoxic compound (World Health Organization 1993), has been found with especially high levels in potato chips, french fries, and other food products produced with high-temperature cooking. It is well known that AM is formed as the result of the Maillard reaction between amino acids (asparagine) and reducing sugars (mainly glucose and fructose) (Mottram et al. 2002; Stadler et al. 2002). In 2005, World Health Organization (WHO) and Food and Agriculture Organization (FAO) together announced that certain foods containing high levels of AM have the potential to cause cancer in humans (International Food Safety Authorities Network 2005). At present, a great number of methods have been developed to quantitatively analyze the AM monomer, including different extraction and cleanup procedures for different food matrixes. Most detection methods reported in the literature are mainly based on gas chromatography (GC) (Blanch et al. 2013), liquid chromatography (LC) (Backe et al. 2014), LC–MS (Bagdonaite et al. 2008), and GC–MS (Soares et al. 2006). Although these methods have reliable sensitivity, they often require qualified personnel, complex pretreatment steps, and high cost. Hence, a sensitive and selective analytical methodology, which requires an inexpensive apparatus and direct analytical determination without complicated derivatization procedures, is highly desirable for AM detection.
Electrochemical sensor has received increasing attention in many fields due to their low cost, small size, fast response time, possibility of achieving low detection limits, and strong operability (Zhu et al. 2014; Wang et al. 2014a, b). A critical aspect of the sensor development is integration of the recognition element with the transducer (Wang et al. 2013). As a recognition element of sensor, molecularly imprinted polymer (MIP) has attracted considerable attention due to characteristics of high chemical stability, high selectivity, low cost, and easy preparation (Yang et al. 2013; Hu et al. 2011). Molecular imprinting is a well-established and simple technique for the generation of recognition sites (cavities) complementary to the shape, size, and functionality of the template molecule onto synthetic materials. Moreover, the sol-gel imprinting method could improve the performance of MIP film on sensor surface since control for the thickness, porosity, and surface area of film is easier (Xu et al. 2014; Marx et al. 2004). In sol-gel imprinting process, a sol-gel inorganic framework is formed around a template molecule by noncovalently/covalently interaction between functional monomer and the template molecule (Zhang et al. 2014; Prasad et al. 2010). The method of sol-gel imprinting is becoming more important due to simple and convenient detection of compounds for different shape and materials with a good selectivity and specificity (Zhang et al. 2010; Rezaei et al. 2014). However, this simple method often makes MIP electrochemical sensor show relatively low sensitivity, because the imprinted film can result in both the slow diffusion of the analytes to the recognition sites and the inefficient communication between the binding sites and transducers (Riskin et al. 2008). Consequently, it is still a challenge to enhance the sensitivity of the signal transducer, through effective conversion of the binding signals from the molecular recognition to detectable electrical signals.
Due to their unique structure, high chemical stability, and high surface-to-volume ratio, multi-walled carbon nanotubes (MWCNTs) have been employed as the medium of the electron transport and the electrocatalyst for enhancing the sensitivity of the electrochemical sensor (Xu et al. 2013; Zhu et al. 2013). Gold nanoparticles (Au NPs) are used increasingly in many electrochemical applications since they have high electrical conductivity and an abundant effective surface area (Saha et al. 2012; David et al. 2010). It was reported that Au NPs could be covalently combined with functionalized MWCNTs, which exhibited good biocompatibility with the determination of choline (Qin et al. 2010). For fabrication of nanoparticle patterns on the silicon or electrodes surface, many researchers have paid special attention to the development of the function of polyelectrolyte multilayer (Gong 2013; Gong 2014). Recently, chitosan (CS), an abundant natural cationic biopolymer, has become a hot research topic in electrochemical sensor, owing to the numerous outstanding properties of CS, such as excellent film-forming ability, high mechanical strength, adhesion, biodegradability, and nontoxic (Suginta et al. 2013; Xu et al. 2010).
In this article, we combined the advantages of Au NPs-MWCNTs composites and CS to construct an electrochemical sensor for the detection of AM by a sol-gel MIP film as recognition element on glassy carbon electrode (GCE) surface. The effect of Au NPs-MWCNTs-CS composite on the GCE and the performance of the developed MIP sensor were evaluated by cyclic voltammetry (CV) and differential pulse voltammetry (DPV) experiments. The electrochemical MIP sensor cannot only improve the sensitivity and selectivity of AM analysis but also simplify the pretreatment process of the real sample. To demonstrate the usefulness of the MIP sensor, AM in potato chips samples were detected using the MIP sensor, and the result was further compared with that of HPLC detection.
Experimental
Instruments and Reagents
CV and DPV experiments were carried out using a CHI-660 electrochemical workstation (Shanghai CH Instrument Company, China). All electrochemical experiments were performed with a conventional three-electrode system, where the modified GCE, the saturated calomel electrode, and the platinum wire act as the working electrode, the reference electrode, and the auxiliary electrode, respectively. HPLC analyses were run on an HPLC system (Waters e2695) equipped with a 2998 photo-diode array detector (PDA). A symmetry-C18 column (4.6 mm × 250 mm, 5.0 μm) was used for the separation. Transmission electron microscope (TEM) was carried out on JSM-6380 (JEOL).
Anhydrous ethanol, hydrochloric acid, potassium ferricyanide (K3[Fe(CN)6]), potassium ferrocyanide (K4[Fe(CN)6]), CS, acetone, L-asparagine, and acetic acid were obtained from Beijing Dingguo Biotechnology (Beijing, China). AM, 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC), cysteamine, chloroauric acid (HAuCl4 · 4H2O), n-hydroxysulfosuccinimide (NHS), 3-aminopropyltrimethoxysilane (APTMS), and tetraethoxysilane (TEOS) were purchased from Sigma (St. Louis, MO, USA). MWCNTs were obtained from Shenzhen Nanotech Port Co., Ltd. (Shenzhen, China). All chemicals and solvents were of commercially available analytical reagent grade, and double distilled water (DDW) was used throughout this work.
Preparation of Au NPs and Carboxyl Group modified MWCNTs
Au NPs were prepared by following a previously reported procedure (Grabar et al. 1996). The diameter of the Au NPs was about 17 nm. Briefly, 99-mL DDW and 1-mL 1 % HAuCl4 were mixed and boiled. Then, 2.4-mL 1 % trisodium citrate solution was quickly added to the refluxed HAuCl4 solution. Throughout the process, the solution color changed from yellow to gray in 2 min, then from gray to wine red, and the mixture was stirred for 15 min until the color turned to bright red. The Au NPs suspension was stored at 4 °C for further use.
The carboxyl group-modified MWCNTs (COOH–MWCNTs) were prepared according to the literature (Qin et al. 2010). Briefly, MWCNTs were chemically shortened by ultrasonic agitation in a 3:1 (v/v) mixture of concentrated sulfuric acid and nitric acid for 4 h. Then, separated MWCNTs were concentrated by centrifugation (10,000 rpm, 5 min). The resulting MWCNTs were repeatedly washed with DDW to neutrality. Thus, the obtained MWCNTs were water-soluble, since they were disconnected and functionalized with carboxylic groups on their tips and any defect on the side walls during the oxidation process.
Preparation of Au NPs-MWCNTs Composites
Because the COOH group of MWCNTs can react with NHS using EDC as catalyst to form n-hydroxysuccinimide esters, which can then react spontaneously with amine group of the cysteamine. Thus, SH-MWCNTs were obtained and the -SH of the MWCNTs may free bind to Au NPs through Au-S bond. Au NPs-MWCNTs composites were prepared with the method described in reference (Qin et al. 2010). First, the COOH–MWCNTs were successively immersed in a 1:1 (v/v) EDC/NHS mixture (50 mg mL−1 EDC and 50 mg mL−1 NHS) and cysteamine, each for 1 h, and washed with DDW by centrifugation after each incubation. Then, the MWCNTs modified with –SH were transferred into the Au NPs solution, and the mixture was kept for 1 h to assemble Au NPs onto the MWCNTs surface. Ultimately, the Au NPs-MWCNTs composites were synthesized and washed thoroughly with DDW by centrifugation and dried at room temperature (RT).
Pretreatment of GCE and MIP/Au NPs-MWCNTs-CS/GCE Preparation
Prior to use, the surface of the GCE was polished with 1.0, 0.3, and 0.05 μm alumina powder. After each polishing step, GCE was ultrasonicated in DDW and ethanol for 5 min, respectively.
The Au NPs-MWCNTs-CS/GCE was obtained as follows: Au NPs-MWCNTs (5 mL) and CS-acetic acid solution (2.0 wt%, 10 mL) were mixed by ultrasonic to form a uniform suspension. Then, 10.0 μL of the resulting suspension was dripped onto the surface of GCE and dried at RT.
MIP film was modified on the surface of the Au NPs-MWCNTs-CS/GCE by sol-gel technology. In order to prepare the AM-imprinted sol-gel, 0.055 g AM as template and 0.2 mL APTMS as functional monomer were dissolved in round-bottomed flask with tetrahydrofuran (5 mL) under magnetically stirring for 15 min. Then, 0.8 mL TEOS was added as cross-linker. After adding 0.3-mL 0.1 mol L−1 ammonia water as catalyst, the mixture solution was stirred for 2 h at RT. Finally, the MIP-Au NPs-MWCNTs-CS/GCE was fabricated by driving force of electrochemical using CV between −0.5 and +0.5 V in above sol-gel solution for 30 cycles, and dried overnight at RT (Huang et al. 2011). The resulting MIP/Au NPs-MWCNTs-CS/GCE was suspended in anhydrous ethanol with stirring for 5 min to remove the AM template. The same elution process was repeated three times. Then, the imprinted GCE was washed with DDW and dried at RT for further experiments. The process of preparation for MIP/Au NPs-MWCNTs-CS/GCE is shown in Fig. 1. The nonimprinted polymer (NIP)/Au NPs-MWCNTs-CS/GCE was prepared under the same conditions except that no template AM was used as control electrode. Moreover, the MIP/MWCNTs-CS/GCE and the NIP/MWCNTs-CS/GCE were also prepared, which is applied to demonstrate sensitivity of the MIPs sensor for AM detection.
Electrochemical Measurements
CV was performed to characterize the imprinted electrode and removal of the AM template by immersing the imprinted electrode in 3 mL PBS of 0.1 M pH 7.4 which contains 5 mM [Fe(CN)6]3−/4− and 0.1 M KCl. The potential of CV was set from −0.2 to +0.8 V with a scan rate of 50 mV s−1. DPV was recorded at a potential from 0 to +0.4 V, pulse amplitude 0.05 V, pulse width 0.2 s, pulse period 0.5 s. The rebinding of AM on MIP/Au NPs-MWCNTs-CS/GCE, NIP/Au NPs-MWCNTs-CS/GCE, MIP/MWCNTs-CS/GCE, and NIP/MWCNTs-CS/GCE was performed by incubating in different concentration AM solution for 30 min and evaluated using DPV, respectively. To confirm the reproducibility of the detection result, the AM with different concentrations are repeatedly detected at least three times.
Preparation of Real Sample
Potato chips were purchased from a local supermarket. Five-gram sample was put into mortar and grinded to powder. Then, the sample was transferred into 5-mL centrifuge tube containing 10 mL n-hexane to remove the fat by centrifugation (10,000 rpm, 5 min), following drying by nitrogen. After extraction with 20 mL DDW by ultrasonic for 15 min, the sample solution was filtered through 0.2-μm membrane filter. The obtained sample solution could be used for AM detection. The sample was also determined by HPLC according to the reference (Yuan et al. 2008).
Results and Discussion
Characterization of the MIP/Au NPs-MWCNTs-CS/GCE
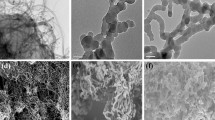
In the AM sol-gel MI process, APTMS acts as functional monomer due to its amino groups, which can interact with the oxygen atoms of AM through hydrogen bond (Wang et al. 2014a, b). Simultaneously, TEOS acts as cross-linker to form a polymeric network through Si–O bond via hydrolysis. The sol-gel MIP film was coated on the surface of the Au NPs-MWCNTs-CS/GCE by CV. Also, CV is an effective and convenient method to investigate the electron transport process of the modified electrode. During this procedure, [Fe(CN)6]3−/4− was used as the mediator between imprinted electrode and substrate solution. The CV results of the modified electrode and representative TEM images of the Au NPs/MWNCTs composite are shown in Fig. 2. Curve a in Fig. 2 is the CV of the bare electrode in [Fe(CN)6]3−/4− and KCl mixture solution, showing a pair of redox peaks. The peak current was increased significantly when the electrode surface was modified using Au NPs-MWCNTs-CS composites (Fig. 2b), which indicates that Au NPs-MWCNTs-CS composites could enhance the electrochemical signal of sensor. The TEM image (the inset of Fig. 2) depicts relatively well-dispersed Au NPs (denoted by dark spheres) in the MWNCTs matrix. This also demonstrated the successful immobilization of Au NPs on MWNCTs. Curve c in Fig. 2 shows the CV of the MIP-Au NPs-MWNTs-CS/GCE with 0.053 g AM. Compared with CV on the Au NPs-MWNTs-CS/GCE, peak current nearly disappeared. This is because the [Fe(CN)6]3−/4− could not penetrate the layer of MIP and arrive at the surface of electrode. After AM was removed from the MIP film by anhydrous ethanol, an obvious peak current was obtained again (Fig. 2d). This could be explained that by removal of the template molecule, the formation of vacant recognition sites or binding cavity made electronic transfer possible. That is, [Fe(CN)6]3−/4− could pass through the cavity and reached the surface of the electrode more easily. After incubating 0.027 g AM on the above electrode surface, the peak current (Fig. 2e) decreased comparing to that measured at the MIP electrode without the template. This demonstrates that the proposed sensor has good rebinding capacity to AM.
The CV of a bare GCE; b Au NPs/MWNCTs-CS/GCE; c MIP/Au NPs-MWNCTs-CS/GCE with 0.053-g template, d MIP/Au NPs-MWNCTs-CS/GCE after template removal, and e MIP/Au NPs-MWNCTs-CS/GCE after 0.027-g template rebinding in 3 mL PBS (0.1 M pH 7.4) containing 5 mM [Fe(CN)6]3−/4− and 0.1 M KCl at a scan rate of 50 mV s−1. Inset showed the representative TEM images of the Au NPs and MWNCTs composite
Optimization of Imprinting Conditions
The ratio of functional monomer/cross-linker and pH for sol-gel solution play a key role in the sensor performance because they influence the structure and stability of sol-gel film of sensor surface. Addition of template molecule of appropriate amount will also make the sensor obtain the optimal sensing properties and ensure high binding capacity for template molecule. In order to find the optimal imprinting conditions, the ratio of functional monomer/cross-linker, the pH for sol-gel, and the amount of added template were, respectively, investigated by DPV measurement in 0.1 M pH 7.4 PBS containing 5 mM [Fe(CN)6]3−/4− and 0.1 M KCl. A series of NIP films (monomer/cross-linker ratio: 1:3, 1:4, 1:5, 1:6) were modified on the surface of Au NPs-MWCNTs-CS/GCE, respectively. To investigate the stability of every NIP film, DPV measurements were carried out for each fabricated electrode at least three times. The change of current response on each electrode was calculated by subtracting the current response for the electrode without being washed from that for electrode with being washed by anhydrous ethanol. As shown in Fig. 3a, the change of current response was the minimum when the ratio is 1:4. It indicates that the optimal monomer/cross-linker ratio was 1:4, at which a stable sol-gel film can be formed on the surface of the Au NPs-MWCNTs-CS/GCE.
The effect of the sol-gel pH on the stability of the NIP films (at the monomer/cross-linker ratio of 1:4) was examined in the range of pH 6–9 by recording the change of current response with the same procedure, which are shown in Fig. 3b. Relative standard deviation (RSD) range is from 1.5 to 2.4 %. It was found that the change of current response was the minimum at pH 8 for the sol-gel solution, which corresponds to the optimum pH of the sol-gel solution.
The amount of template molecules in the molecule polymerization process influences the amount of available recognition sites for selective rebinding of AM on MIP film. To optimize the amount of template molecules in sol-gel MIP, we prepared three different MIP sensors with a template of 0.023, 0.055, and 0.085 g, respectively. After template molecules were removed by anhydrous ethanol, the current response of fabricated MIP sensors for AM rebinding was tested. The result implies that current response has almost not changed for AM rebinding using MIP sensor with 0.023-g template. The change of the current response for 0.055-g template is better than that for 0.085-g template. Therefore, addition amount of template molecules was 0.055 g for preparing imprinted sol-gel solution.
Performance of the MIP Sensor
AM Determination
In order to evaluate the performance of the MIP sensor, AM solution of different concentrations (from 0.05 to 15 μg mL−1) was measured by DPV using the prepared MIP/Au NPs-MWCNTs-CS/GCE. To further elucidate the molecular recognition properties and the sensitivity of the MIP sensor, NIP/Au NPs-MWCNTs-CS/GCE, MIP/MWCNTs-CS/GCE, and NIP/MWCNTs-CS/GCE were also used to detect the AM solutions, respectively. The results in Fig. 4a showed that the change of current response was proportional to the concentration of AM. As seen from Fig. 4a, when the same concentration AM was detected, the change of current response for both NIP/Au NPs-MWNCTs-CS/GCE and NIP/MWNCTs-CS/GCE was very small. This is because there are no any recognition sites for AM on the surface of NIP electrode. It is also observed that the change of current response of MIP/Au NPs-MWCNTs-CS/GCE was larger than that of MIP/MWCNTs-CS/GCE when the same concentration AM was detected, which is ascribed to the high electrical conductivity of Au NPs. These results imply that MIP/Au NPs-MWCNTs-CS/GCE has the best sensitivity. The results in Fig. 4b showed that the change of the peak current was directly proportional to the concentration of AM ranging from 0.05 to 5 μg mL−1. The linear regression equation was expressed as I (ΔμA) = 16.5 c (μg mL−1) + 2.17 (R 2 = 0.9880). The detection limit was calculated to be 0.028 μg mL−1 (S/N = 3) according to IUPAC recommendation. The error bars (RSD ranging from 2.3 to 6.9 %) were relatively small, indicating that the method is quite reproducible.
Measurement of Selectivity
Selective recognition toward the template molecule is an important capability for an MIP sensor. The acetone, L-asparagine, and acetic acid were chosen as references for investigating the special selectivity of the MIP sensor. The every compound (1 μg mL−1) and AM (1 μg mL−1) solution containing each compounds (each compound concentration, 1 μg mL−1) were determined based on the MIP sensor and NIP sensor by DPV, respectively. All results are shown in Fig. 5 (RSD 5.9–7.8 %). Compared with comparative compound, the change of current response on MIP sensor is the highest for the presence of AM, which indicates that the amounts of AM rebinding on MIP film are much more than those of comparative compound. It may be attributed to the complementarity of the size, the shape, and the position of recognition functional groups between AM and the binding sites in MIP sensor. In addition, the current shift of the NIP sensor is small due to nonspecific adsorption, which is greatly different from the recognition behavior exhibited by the proposed MIP sensor for the rebinding of AM. The MIP sensor showed much better recognition ability than that of NIPs sensor. Accordingly, it can be concluded that the MIP sensor achieved a good selectivity of recognition to AM.
Measurement of Repeatability and Stability
To investigate the repeatability of the MIP sensor, 1 μg mL−1 of AM solution was successively detected by the same electrode for 5 times under the same above mentioned procedures and conditions. The RSD of the measurements was 3.007 %. The good repeatability revealed that AM could be reversibly detected with the binding sites. That is, the MIP sensor could be used repeatedly.
To evaluate the stability of the MIP sensor, MIP/Au NPs-MWCNTs-CS/GCE was stored at 4 °C refrigerator, and AM detection was performed. Nine days later, the current response retained 84.57 % of initial current response, which suggests that the MIPs sensor has the acceptability stability.
Analysis of Food Samples and Accuracy
The feasibility of the MIP sensor for practical application was investigated by analyzing potato chips sample and comparing with AM results from HPLC analysis. The content of AM detected based on MIP sensor and HPLC was 148 μg kg−1 (RSD 5.51 %) and 134.3 μg kg−1 (RSD 2.79 %) in the same potato chips sample, respectively. This indicates that the detection result of the proposed MIP sensor agrees well with that of HPLC. Further, the recovery experiments were performed by determination of 0.2, 1.0, 2.0, 4.0 μg mL−1 AM in potato chips sample by standard addition method. The experimental results are listed in Table 1. As seen from Table 1, the recovery was in the range of 84.7 to 94.8 %, which demonstrates that the MIP sensor is feasible for determination AM in real samples.
Conclusion
An MIP sensor has been presented based on the combination of Au NPs-MWCNTs-CS composite and sol-gel molecular imprinting technique for convenient and sensitive detection of AM. The Au NPs-MWCNTs composites were introduced to enhance the electrical conductivity and expand the electrode surface area. The MIP sensor offers advantages for detection of AM, with (i) good selectivity, (ii) good sensitivity, and (iii) low cost, and was used for AM determination in potato chips samples successfully. In addition, repeatability and storage stability of the MIP sensor are also reliable.
References
Backe WJ, Yingling V, Johnson T (2014) The determination of acrylamide in environmental and drinking waters by large-volume injection-hydrophilic-interaction liquid chromatography and tandem mass spectrometry. J Chromatogr A 1334:72–78
Bagdonaite K, Derler K, Murkovic M, Agric J (2008) Determination of Acrylamide during Roasting of Coffee. Food Chem 56:6081–6086
Blanch GP, Morales FJ, Moreno FP, Castillo MLR (2013) A new approach based on off-line coupling of high-performance liquid chromatography with gas chromatography-mass spectrometry to determine acrylamide in coffee brew. J Sep Sci 36:320–324
David AG, Dwight SS, Weston LD, Matthew DM, Pinal CP, Chad AM (2010) Gold nanoparticles for biology and medicine. Angew Chem Int Ed 49:3280–3294
Gong X (2013) Controlling surface properties of polyelectrolyte multilayers by assembly pH. Phys Chem Chem Phys 15:10459
Gong X (2014) Facile formation of nanoparticle patterns by water induced flow of a polymer thin film. Res Adv 4:54494
Grabar KC, Smith PC, Musick MD, Davis JA, Walter DG, Jackson MA, Guthrie AP, Natan MJ (1996) Kinetic Control of Interparticle Spacing in Au Colloid-Based Surfaces: Rational Nanometer-Scale Architecture. J Am Chem Soc 118:1148–1153
Hu YF, Zhang ZH, Zhang HB, Luo LJ, Yao SZ (2011) Electrochemical determination of L-phenylalanine at polyniline modified carbon electrode based on β-cyclodextrin incorporated carbon nanotube composite material and imprinted sol-gel film. Talanta 84:305–313
Huang JD, Zhang XM, Lin Q, He XR, Xing XR, Huai HX, Lian WJ, Zhu H (2011) Electrochemical sensor based on imprinted sol-gel and nanomaterials for sensitive determination of bisphenol A. Food Control 22:786–791
International Food Safety Authorities Network (INFOSAN) Information Note No. 2. Acrylamide in food is a potential health hazard. Geneva, Switzerland, 1st March, 2005, http://www.fsai.ie/industry/hottopics/EN.note2.2005.acrylamide.Pdf
Marx S, Zaltsman A, Turyan I, Mandler D (2004) Preparation sensor based on molecularly imprinted sol-gel films. Anal Chem 76:120–126
Mottram DS, Wedzicha BL, Dodson AT (2002) Food chemistry: acrylamide is formed in the Maillard reaction. Nature 419:448–449
Prasad BB, Madhuri R, Tiwari MP, Sharma PS (2010) Electrochemical sensor for folic acid based on a hyperbranched molecularly imprinted polymer-immobilized sol-gel-modified pencil graphite electrode. Sens Actuators B 146:321–330
Qin X, Wang HC, Wang XS, Miao ZY, Chen LL, Zhao W (2010) Amperometric biosensors based on gold nanoparticles-decorated multiwalled carbon nanotubes-poly biocomposite for the determination of choline. Sens Actuators B 147:593–598
Rezaei B, Boroujeni MK, Ensafi AA (2014) Caffeine electrochemical sensor using imprinted film as recognition element based on polypyrrole sol-gel and gold nanoparticles hybrod nanocomposite modified pencil graphite electrode. Biosens Bioelectron 60:77–83
Riskin M, Tel-Vered R, Bourenko T, Granot E, Willner I (2008) Imprinting of molecular recognition sites through electropolymerization of functionalized Au nanoparticles: development of an electrochemical TNT Sensor Based on π-Donor − Acceptor Interactions. J Am Chem Soc 130:9726–9733
Saha K, Agasti S, Kim C, Li XN, Rotello VM (2012) Gold nanoparticles in chemical and biological sensing. Chem Rev 112:2739–2779
Soares C, Cunha C, Fernandes J (2006) Determination of acrylamide in coffee and coffee products by GC-MS using an improved SPE clean-up. Food Addit Contam 23:1276–1282
Stadler RH, Blank I, Varga N, Robert F (2002) Food chemistry: acrylamide from Maillard reaction products. Nature 419:449–450
Suginta W, Khunkaewla P, Schulte A (2013) Electrochemical biosensor applications of polysaccharides chitin and chitosan. Chem Rev 113:5458–5479
Wang ZH, Li JS, Liu XL, Yang JM, Lu XQ (2013) Preparation of an amperometric sensor for norfloxacin based on molecularly imprinted grafting photopolymerization. Anal Bioanal Chem 405:2525–2533
Wang X, Piro B, Reisberg S, Anquetin G, Rocquigny HD, Jiang P, Wang Q, Wu W, Pham MC, Dong CZ (2014a) Direct, reagentless electrochemical detection of the BIR3 domain of X-linked inhibitor of apoptosis protein using a peptide-based conducting polymer sensor. Biosens Bioelectron 61:57–62
Wang QY, Ji J, Jiang DL, Wang Y, Zhang YZ, Sun XL (2014b) An electrochemical sensor based on molecularly imprinted membranes on a P-ATP–AuNP modified electrode for the determination of acrylamide. Anal Methods 6:6452
WTO (1993) Guidelines for drinking water quality [R]. WHO: World Health Organization
Xu HF, Dai H, Chen GN (2010) Direct electrochemistry and electrocatalysis of hemoglobin protein entrapped in grahene and chirosan composite film. Talanta 81:334–338
Xu W, Liu P, Guo GH, Dong C, Zhang XH, Wang SF (2013) Electrochemical sensor based on a carbon nanotube-modified imprinted sol-gel for selective and sensitive determination of β2-agonists. Microchim Acta 180:1005–1011
Xu GL, Zhang HL, Zhong M, Zhang TT, Lu XJ, Kan XW (2014) Imprinted sol-gel electrochemical sensor for melamine direct recognition and detection. J Electroanal Chem 713:112–118
Yang YK, Fang GZ, Liu GY, Pan MF, Wang XM, Kong LJ, He XL, Wang S (2013) Electrochemical sensor based on molecularly imprinted polymer film via sol-gel technology and multi-walled carbon nanotubes-chitosan functional layer for sensitive determination of quinoxaline-2-carboxylic acid. Biosens Bioelectron 47:475–481
Yuan Y, Zhao GH, Hu XX, Wu JH, Liu J, Chen F (2008) High correlation of methylglyoxal with acrylamide formation in glucose/asparagine Maillard reaction model. Eur Food Res Technol 226:1301–1307
Zhang ZH, Hu YF, Zhang HB, Luo LJ, Yao SZ (2010) Layer-by-layer assembly sensitive electrochemical sensor for selectively probing L-histidine based on molecular imprinting sol-gel at functionalized indium tin oxide electrode. Biosens Bioelectron 26:696–702
Zhang J, Niu YH, Li SJ, Luo RQ, Wang CY (2014) A molecularly imprinted electrochemical sensor based on sol-gel techenogology and multiwalled carbon nanotubes-Nafion functional layer for determination of 2-nonylphenol in environmental samples. Sens Actuators B 193:844–850
Zhu AH, Xu GL, Li L, Yang LL, Zhou H, Kan XW (2013) Sol-gel imprinted polymers based electrochemical sensor for paracetamol recognition and detection. Anal Lett 46:7
Zhu Y, Zeng GM, Zhang Y, Tang L, Chen J, Cheng M (2014) Highly sensitive electrochemical sensor using a MWCNTs/Au NPs-modified electrode for lead(II) detection based on Pb2+-induced G-rich DNA conformation. Analyst 139:5014–5020
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 31201375), Hunan Provincial Natural Science Foundation of China (No. 2015JJ3077), and Special Fund for Agro-scientific Research in the Public Interest (No. 201303084).
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Conflict of Interest
Xia Liu declares that she has no conflict of interest. Lu-Gang Mao declares that he has no conflict of interest. Yuan-Liang Wang declares that he has no conflict of interest. Xing-Bo Shi declares that he has no conflict of interest. Yan Liu declares that she has no conflict of interest. Yang Yang declares that she has no conflict of interest. Zao He declares that she has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, X., Mao, LG., Wang, YL. et al. Electrochemical Sensor based on Imprinted Sol-Gel Polymer on Au NPs-MWCNTs-CS Modified Electrode for the Determination of Acrylamide. Food Anal. Methods 9, 114–121 (2016). https://doi.org/10.1007/s12161-015-0172-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-015-0172-0