Abstract
We describe a molecularly imprinted electrochemical sensor for selective and sensitive determination of β2-agonists. It is making use of a combination of single-wall carbon nanotubes (SWNTs) with a molecularly imprinted sol–gel. The SWNTs were introduced in order to enhance electron transport and sensitivity. The imprinted sol–gel film with its specific binding sites acts as a selective recognition element and as a preconcentrator for β2-agonists. The morphology of the imprinted film was characterized by scanning electron microscopy. The optimized sensor displays high sensitivity and excellent selectivity for the β2-agonists as shown for their determination in human serum samples.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
β2-agonists are phenylethanolamines with different substituent groups on the aromatic ring and the terminal amino group (Table 1). They are used in the symptomatic treatment of asthma and chronic bronchitis. They are also used in the prevention of exercise-induced asthma [1]. However, the drug residues accumulated in animal tissues may pose acute poisoning when consumed by humans, with symptoms of muscular tremor, cardiac palpitation, nervousness, headache, muscular pain, dizziness, nausea, vomiting, fever, and chills [2, 3]. For this reason, the use of β2-agonists in animal feeds has been banned in most countries. So it is necessary to set up accurate and quick methods to detect β2-agonists.
Several technologies including capillary electrophoresis [4, 5], chromatography [6–10], spectrophotometry [11, 12], fluorescence and chemiluminescence [13], immunoassays [14, 15] have been adopted for the determination of β2-agonists in the biological sample. The general drawbacks of capillary electrophoresis and chromatography include expensive instrumentation and time-consuming. Spectrophotometry tends to lack suitable antibodies, stability and repeatability for the determination of β2-agonists in biological samples. All of these limitations have stimulated the research and development of new devices for quick and accurate detection of drug residues. A new trend in analytical chemistry is to develop novel sensors based on molecularly imprinted polymers (MIPs) or molecularly imprinted membranes [16, 17]. Molecular imprinting is an excellent technique for the preparation of MIP with a tailor-made affinity and specificity for the template molecule [18–20], and it has been widely used in several applications involving chromatography [21], solid phase extraction [22] and sensor [23]. Specifically, MIP-based sensors attract increasing attention on account of the advantages including low cost, high specificity, stability and robustness [24–27]. Zhou et al. reported a chemiluminescence sensor using molecularly imprinted polymer as recognition elements for detection of salbutamol [28]. Zhao et al. prepared a molecular imprinted film based on chitosan/nafion/nano-silver/polyquercetin for clenbuterol sensing [29]. Andrea et al. developed a solid binding matrix/molecularly imprinted polymer-based sensor system for the determination of clenbuterol in bovine liver using differential-pulse voltammetry [30]. Liang et al. reported a potentiometric sensor based on molecularly imprinted polymers for rapid determination of clenbuterol in pig urine [31]. Among all of the developed imprinted materials, imprinted sol–gel, which is a polymeric material formed by the acid-or base-catalyzed hydrolysis and condensation of metal alkoxides, has been widely used in the development of imprinted sol–gel sensors.
The aim of this work is to construct electrochemical sensors for the detection of β2-agonists by using molecularly imprinted technique and sol–gel rigidity network. SWNTs were introduced during the preparation of imprinted sensor owing to the unique properties involving huge surface area, subtle electronic properties and catalytic ability for enhancing the sensitivity of the sensor. The MIP electrochemical sensors have the advantage of convenience, high sensitivity and low detection limit. In addition, the sensors were successfully applied to the determination of β2-agonists in human serum samples.
Experimental
Reagents and materials
SWNTs were obtained from Chengdu Organic Chemicals Co. (Sichuan, China. www.cioc.ac.cn). All β2-agonists (Salbutamol, clenbuterol, terbutaline, Metaproterenol, Fenoterol, Cimbuterol, Mabuterol, Isoxsuprine, Brombuterol, Ractopamine and Ritodrine hydrochloride) were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (China. www.nicpbp.org.cn). Tetraethoxysilane (TEOS) was purchased from Sigma (USA. www.sigma-aldrich.com). Bis-triethoxysilane endcapped polyurethane/urea (SPU) was obtained from College of Material Science and Engineering, Hubei University. All chemicals were of reagent grade and used as received. All solution was prepared with ultra-pure water (18.25 MΩ cm) by Aquapro Ultra-pure water system (Chongqi, China. www.aquapro-china.com).
Apparatus and procedures
A computer-controlled electrochemical workstation (CHI660C, CH Instruments, Chenhua Co., Shanghai, China. www.chinstr.com) was carried out for voltammetry measurement. A conventional three-electrode system was used in the measurements, with a bare GCE (3 mm diameter) or a modified GCE as the working electrode, a saturated calomel electrode as the reference electrode and a platinum electrode as the auxiliary electrode. The pH value of electrolyte was determined by using a 320-S acidity meter (Mettler-Toledo, Switzerland. www.mt.com). A JEOL JSM-5510LV scanning electron microscopy (SEM, Japan. www.jeol.com) was applied for characterizing the sensors. All measurements were carried out at a room temperature.
Preparation of imprinted sensor
1.5 mg purified SWNTs were dispersed into 3.0 mL 5.0 mM sodium dodecyl sulfate by ultrasonic agitation for 40 min to give a homogeneous SWNTs suspension. Then, 5.0 μL of SWNTs suspension was coated on the surface of a cleaned GCE and allowed to evaporate solvent at room temperature.
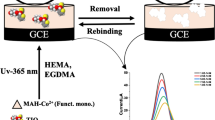
The molecularly imprinted sol–gel was prepared according to the following produce. Firstly, 1.0 mL of SPU was mixed with 10.0 mL HCl (0.01 M). The mixed solution was stirred 5 h at 30 °C to obtain a homogeneous sol. Then, a total of 1.0 mL of this sol mixed with 100.0 μL of TEOS, 300.0 μL of β2-agonists (0.01 M) was stirred for 2 h at 25 °C to obtain an imprinted sol–gel solution, while non-imprinted sol–gel film was prepared under the same experimental conditions without the addition of β2-agonists. Finally, the MIP/sol–gel/SWNTs sensor was fabricated by placing 5.0 μL of sol–gel imprinted polymer on the surface of SWNTs/GCE (Scheme. 1). The decorated sensor was left to deposit overnight at room temperature. Removal of the template molecular was achieved by immersing the dried MIP/sol–gel/SWNTs/GCE into the mixture of ethanol-pure water (1:1, v/v), and the completely removal of β2-agonists was verified by electrochemical measurement.
Results and discussion
Characterization of molecularly imprinting film
In order to characterize the morphology of SWNTs/GCE and MIP/sol–gel/SWNTs/GCE, SEM was performed. As shown in Fig. 1a, SWNTs were formed as boundless tubes because of Van der Waals forces, and distributed uniformly in sodium dodecyl sulfate. From the image of MIP/sol–gel/SWNTs film, it was clear to see that the film was uniformly cast onto the electrode surface (Fig. 1b). And the surface of the film was more complanate and compact, revealing that the fabrication sensor was achieved perfectly.
To further confirm the successful synthesis of MIP/sol–gel, FT-IR spectra measurements were conducted. Figure 2 shows the spectra of pure SAL (a), imprinting film without SAL (b) and imprinting film (c), respectively. It can be seen pure SAL shows the characteristic bands of benzene at 1,500 cm−1 and the absorption band of its –NH at 3,300 cm−1. Besides, the absorption appeared at 3,000 cm−1 is ascribed to the –OH group. For the imprinting film without SAL and imprinting films, the common absorption peak of amide (−NH) and urethane group can be observed at 3,300 cm−1 and 1,639 cm−1. The band around 1,069 cm−1 indicates Si–O–Si stretching vibrations, conforming the existence of an extensive ethoxysilane groups in the film. The distinct difference between the spectra of imprinting film without SAL and imprinting films is the characteristic bands of SAL at 1,500 cm−1, which are discernable for the imprinting film, indicating that MIP/sol–gel was achieved perfectly.
Electrochemical behaviors of imprinted sensor
To characterize the properties of the imprinted sensor, cyclic voltammetry of β2-agonists imprinted film before and after the removal of β2-agonists were investigated in phosphate buffer solution. All of the 11 β2-agonists showed similar electrochemical response in the potential range of 0.6 ~ 0.8 V. SAL was taken as an example: an apparent response current peak at 0.62 V was observed in Fig. 3A-a. However, when MIP/sol–gel/SWNTs/GCE was eluted repeatedly with the mixture of ethanol-pure water, tiny current was observed (Fig. 3A-b). This suggested that SAL was successfully removed.
(A) Cyclic voltammograms of the MIP/sol–gel/SWNTs/GCE: before (a) and after (b) extraction of SAL. (B) Cyclic voltammograms of different electrodes incubated in 5.0 × 10−6 M SAL solution for 10 min: MIP/sol–gel/SWNTs/GCE (a), MIP/sol–gel/GCE (b) and NIP/sol–gel/SWNTs/GCE (c). Scan rate: 0.1 V s−1. Buffer solution: 0.1 M PBS, pH 7.0
The rebinding ability of MIP/sol–gel/SWNTs/GCE with SAL was investigated by cyclic voltammetry. After being incubated in 5.0 μM SAL solution for 10 min, the MIP/sol–gel/SWNTs/GCE (Fig. 3B-a) exhibited a much higher current response than that of the GCE just modified with the imprinted sol–gel film (Fig. 3B-b) or the non-imprinted film (Fig. 3B-c). The excellent sensitivity of the MIP/sol–gel/SWNTs/GCE towards SAL was ascribed to the presence of the SWNTs functional monolayer and the imprinted sol–gel film. When SWNTs were introduced into the MIP/sol–gel/SWNTs/GCE, the current response was amplified further than that of the MIP/sol–gel/GCE. It is inferred that SWNTs can expand the current response signal by enhancing electron transfer and increasing specific surface area on the electrode interface. Owing to the inherent specificity of the imprinted film, SAL could interact with it selectivity in the cavities with rebinding groups. Thus, the MIP/sol–gel/SWNTs/GCE showed much higher responses than the NIP/sol–gel/SWNTs/GCE.
Optimization of the molecularly imprinted sol–gel polymer
Influence of the dosage of tetraethoxysilane
The role of functional monomers in sol–gel films is to assist the creation of the specific binding cavity by leaving interacting chemical functions after the polymerization, which is situated within the cavity in an optimal position for rebinding similar to active site in an enzyme. SPU was acted as functionalized monomer for molecularly imprinted, which can interact with the template β2-agonists via hydrogen-bond. TEOS was allowed for acting as cross-linker to form a polymeric network around the template, so the ratio of SPU and TEOS is very important for the sol–gel films. SAL was taken as an example: Different ratios of SPU and TEOS in the imprinting polymerization, including 20:1, 10:1, 5:1, 2:1, 1:1, 1:5, 1:10 were studied by cyclic voltammetry. It was found that the current of the imprinted SAL increased with the increasing of the dosage of TEOS in the imprinting polymerization, indicating that more SAL had been imprinted. But the sol–gel films became unstable with the increasing of the dosage of TEOS in polymerization process. Considering the above two contradiction aspects comprehensively, sol–gel film formed by mixture of SPU and TEOS (10:1, v/v) was taken as a compromise for the polymerization.
Influence of incubation time
During electrochemical determination, accumulation is an effective and simple way to improve the sensitivity. In this study, MIP/sol–gel/SWNTs/GCE was firstly immersed in the standard solution of β2-agonists at 5.0 × 10−7 M for different time. Then the peak current was investigated quantitatively by cyclic voltammetry. SAL was taken as an example: as shown in Fig. 4, when the incubation time gradually increased from 1 min to 15 min, the response currents increased gradually. The results suggested that more and more SAL molecules were re-embedded on the MIP/sol–gel/SWNTs/GCE with increment of the incubation time. However, the peak current remained constant with the incubation time up to 15 min. As a result, a time of 15 min was chosen for the MIP/sol–gel/SWNTs/GCE. In addition, 15 min was also chosen for the NIP/sol–gel/SWNTs/GCE in order to make the comparison under the same condition.
Calibration curve
The current response is directly correlated with the concentration of the β2-agonists added. SAL was taken as an example: The linear sweep voltammetry responses of SAL with stepwise increases of SAL on the MIP/sol–gel/SWNTs/GCE are shown in Fig. 5. The inset of Fig. 5 shows the calibration curves of the MIP/sol–gel/SWNTs/GCE and the NIP/sol–gel/SWNTs/GCE between the corresponding current and SAL concentration. As it showed, for the imprinted sensor, the peak currents were proportional to the concentration of SAL in the range of 9.9 × 10−9 ~8.3 × 10−7 M with the detection limit of 3.0 × 10−9 M. However, compared with the MIP/sol–gel/SWNTs/GCE, the NIP sensor gave no homologous response which can be explained by the lack of specific binding site and porous in the NIP film. The linear range and limit of quantitation of 11 β2-agonists are listed in Table 1. Besides, the calibration curves of other 10 β2-agonists were shown in figures S1 to S10 (supplementary materials).
Linear sweep voltammetry of MIP/sol–gel/SWNTs/GCE in pH 7.0 PBS after incubation in different concentrations of SAL solution for 15 min. Concentration of SAL (a to g): 9.9 × 10−9, 4.9 × 10−8, 7.9 × 10−8, 1.9 × 10−7, 4.5 × 10−7, 6.9 × 10−7, 8.3 × 10−7 M. The inset shows the calibration curves of SAL at imprinted sol–gel/SWNTs/GCE and non-imprinted sol–gel/SWNTs/GCE
Additionally, the analytical performance of the molecularly imprinted sol–gel sensor has been compared with that of other developed β2-agonists sensors reported in the literatures [28, 29, 31]. The performance features are summarized in Table 2. As can be seen, the proposed sensor exhibit a wide linear range and low detection limit for β2-agonists.
Selectivity and stability of the imprinted sensor
Selective recognition of the template molecule is an important merit for a MIP sensor. In this work, SAL was taken as an example: the selectivity of the imprinted sensor to SAL was evaluated by testing its cyclic voltammetric response of SAL or SAL in the presence of some analogues and potential interfering substances, including clenbuterol (CLE), terbutaline (TER), dopamine (DA), glucose (Glu), ascorbic acid (AA), uric acid (UA) and acetic acid (AcA). As shown in Fig. 6, the excess of interfering substances mentioned above hardly caused any significant change of peak current of SAL. The tolerance limit was taken as the maximum concentration of the foreign substances which caused an approximately ±5 % relative error in the determination. The selectivity of the imprinted sensor to other β2-agonists was shown in Fig. S11 to Fig. S20 (supplementary materials). In view of the above facts, satisfactory selectivity to β2-agonists was obtained by such a kind of sensor. This can be explained by the suitable molecular contours cavity and unique binding, which resulted from hydrogen bonds between the recognition site and β2-agonists.
Selectivity of the imprinted sol–gel/SWNTs/GCE. Peak current response of imprinted sol–gel/SWNTs/GCE incubated in (a) 5.0 × 10−7 M SAL in 0.1 mol L−1 PBS, (b) a + 7.5 × 10−5 M CLE, (c) a + 7.5 × 10−5 M TER, (d) a + 2.5 × 10−4 M DA, (e) a + 5.0 × 10−4 M Glu, (f) a + 1.0 × 10−4 M AA, (g) a + 1.0 × 10−4 M UA, (h) a + 1.0 × 10−4 M AcA. Incubation time: 15 min
The stability was evaluated for the imprinted electrode. Detailed experiments revealed that the response of β2-agonists at MIP/sol–gel/SWNTs/GCE hardly changed after the imprinted electrode was used at least 50 times with subsequent washing and measuring operations.
Sample analysis
The practical analytical utility of MIP/sol–gel/SWNTs/GCE was assessed by measurement of β2-agonists in human serum sample. SAL was taken as an example: The measurement was performed by investigating human serum samples with SAL at three different levels (20, 100 and 200 nM). For each concentration, three different samples were independently processed. On the basis of the calibration curves prepared in phosphate buffer solution, it was possible to calculate the recovery of the analyte, which ranged from 96.3 % to 100.3 % [32]. The statistical results are listed in Table 3. The excellent recoveries indicate that this method has good accuracy and great potential in the practical sample analysis.
Conclusions
In this work, an electrochemical imprinted sensor for the determination of 11 β2-agonists was constructed via stepwise modification of SWNTs and a sol–gel imprinted film on GCE. The excellent performance of the imprinted sol–gel/SWNTs/GCE towards β2-agonists can be ascribed to the SWNTs functional layer with electrochemical catalytic activities and the imprinted film with plentiful selective binding sites. It offers advantages by its easy preparation, high sensitivity and low detection limits. With high selectivity and sensitivity, the electrochemical sensor had great potential application in the real samples analysis.
References
Virant FS (1997) In: Weiler JM (ed) Allergic and respiratory disease in sports medicine. Marcel Dekker Inc, New York, pp 65–66
Mitchell GA, Dunnavan G (1998) Illegal use of beta-adrenergic agonists in the United States. J Anim Sci 76:208
Martinez NJF (1990) Food poisoning related to consumption of illicit β-agonist in liver. Lancet 336:1311
Sirichai S, Khanatharana P (2008) Rapid analysis of clenbuterol, salbutamol, procaterol, and fenoterol in pharmaceuticals and human urine by capillary electrophoresis. Talanta 76:1194
Esquisabel A, Herandez RM, Gascon AR, Igartua M, Calvo B (1997) Determination of Salbutamol enantiomers by high performance capillary electrophoresis and its applicationto dissolution assays. J Pharm Biomed Anal 16:357
Lau JHW, Khoo CS, Murby JE (2004) Determination of clenbuterol, salbutamol, and cimaterol in bovine retina by electrospray ionization-liquid chromatography-tandem mass spectrometry. J AOAC Int 87:31
Saleh MI, Koh YM, Tan SC, Aishah AL (2000) Clean-up, detection and determination of salbutamol in human urine and serum. Analyst 125:1569
Black SB, Hansson RC (1999) Determination of salbutamol and detection of other β-agonists in human postmortem whole blood and urine by GC-MS-SIM. J Anal Toxicol 23:113
Couper FJ, Drummer OH (1996) Gas chromatographic-mass spectrometric determination of β2-agonists in postmortem blood: application in forensic medicine. J Chromatogr B 685:265
Deveaux M, Kintz P, Goulle JP, Bessard J, Pépin G, Gosset D (2000) The hair analysis proficiency testing program of the French Society of Analytical Toxicology. Forensic Sci Int 107:389
Basavaiah K, Prameela HC (2003) Three useful bromimetric methods for the determination of salbutamol sulfate. Anal Bioanal Chem 376:879
Mohamed GG, Khalil SM, Zayed MA, Ei-Hamid El-Shall MA (2002) 2,6-Dichloroquinone chlorimide and 7,7,8,8-tetracyanoquinodimethane reagents for the spectrophotometric determination of salbutamol in pure and dosage forms. J Pharm Biomed Anal 28:1127
Loss JR II, Orzechowski RF, Hock RS (2000) Measurement of albuterol in guinea pig serum by high performance liquid chromatography with fluorescence detection. Biomed Chromatogr 14:1
Sheu SY, Lei YC, Tai YT, Chang TH, Kuo TF (2009) Screening of salbutamol residues in swine meat and animal feed by an enzyme immunoassay in Taiwan. Anal Chim Acta 654:148
Ventura R, González G, Smeyers MT, Torre R, Segura J (1998) Screening procedure for β-adrenergic drugs in sports drug testing by immunological methods. J Anal Toxicol 22:127
Wang JP, Pan MF, Fang GZ, Wang S (2009) Preparation of a novel molecularly imprinted polymer by a sol–gel process for on-line solid-phase extraction coupled with high performance liquid chromatography to detect trace enrofloxacin in fish and chicken samples. Microchim Acta 166:295
Xie CG, Gao S, Guo QB, Xu K (2010) Electrochemical sensor for 2,4-dichlorophenoxy acetic acid using molecularly imprinted polypyrrole membrane as recognition element. Microchim Acta 169:145
Lin CI, Joseph AK, Chang CK, Wang YC, Lee YD (2003) Synthesis of molecular imprinted organic–inorganic hybrid polymer binding caffeine. Anal Chim Acta 481:175
Umpleby RJ II, Baxter SC, Rampey AM, Rushton GT, Chen Y, Shimizu KD (2004) Characterization of the heterogeneous binding site affinity distributions in molecularly imprinted polymers. J Chromatogr B 804:141
Holthoff EL, Bright FV (2007) Molecularly templated materials in chemical sensing. Anal Chim Acta 594:147
Hoshina K, Horiyama S, Matsunaga H, Haginaka J (2009) Molecularly imprinted polymers for simultaneous determination of antiepileptics in river water samples by liquid chromatography–tandem mass spectrometry. J Chromatogr A 1216:4957
Kala R, Gladis JM, Rao TP (2004) Preconcentrative separation of erbium from Y, Dy, Ho, Tb and Tm by using ion imprinted polymer particles via solid phase extraction. Anal Chim Acta 518:143
Tan J, Wang HF, Yan XP (2009) A fluorescent sensor array based on ion imprinted mesoporous silica. Biosens Bioelectron 24:3316
Marx S, Zaltsman A, Turyan I (2004) Parathion sensor based on molecularly imprinted sol–gel films. Anal Chem 76:120
Lakshmi D, Bossi A, Whitcombe MJ, Chianella I, Fowler SA, Subrahmanyam S, Piletska EV, Piletsky SA (2009) Electrochemical sensor for catechol and dopamine based on a catalytic molecularly imprinted polymer-conducting polymer hybrid recognition element. Anal Chem 81:3576
Feng L, Liu YJ, Tan YY, Hu JM (2004) Biosensor for the determination of sorbitol based on molecularly imprinted electrosynthesized polymers. Biosens Bioelectron 19:1513
Huang CY, Tsai TC, Thomas JL, Lee MH, Liu BD, Lin HY (2009) Urinalysis with molecularly imprinted poly(ethylene-co-vinyl alcohol) potentiostat sensors. Biosens Bioelectron 24:2611
Zhou HJ, Zhang ZJ, He DY, Xiong Y (2005) Flow through chemiluminescence sensor using molecularly imprinted polymer as recognition elements for detection of salbutamol. Sens Actuators B 107:798
Zhao C, Jin GP, Chen LL, Li Y, Yu B (2011) Preparation of molecular imprinted film based on chitosan/nafion/nano-silver/poly quercetin for clenbuterol sensing. Food Chem 129:595
Andrea P, Miroslav S, Silvia S, Stanislav M (2001) A solid binding matrix/molecularly imprinted polymer-based sensor system for the determination of clenbuterol in bovine liver using differential-pulse voltammetry. Sens Actuators B 76:286
Liang RN, Gao Q, Qin W (2012) Potentiometric sensor based on molecularly imprinted polymers for rapid determination of clenbuterol in pig urine. Chin J Anal Chem 40:354
Mi Q, Wang ZW, Chai CY, Zhang J, Zhao B, Chen CY (2011) Multilayer structured immunosensor based on a glassy carbon electrode modified with multi-wall carbon nanotubes, polythionine, and gold nanoparticles. Microchim Acta 173:459
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 21075029), the Natural Science Fund for Creative Research Groups of Hubei Province of China (No. 2011CDA111), and the Program for Excellent Youth Scholars of Innovative Research Team by Hubei Provincial Department of Education (T201101).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 3458 kb)
Rights and permissions
About this article
Cite this article
Xu, W., Liu, P., Guo, C. et al. Electrochemical sensor based on a carbon nanotube-modified imprinted sol–gel for selective and sensitive determination of ß2-agonists. Microchim Acta 180, 1005–1011 (2013). https://doi.org/10.1007/s00604-013-1020-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-013-1020-9