Abstract
Salmonella infection represents a considerable global burden, with significant health and economic impacts. Salmonellosis is most often attributed to the consumption of contaminated foods such as poultry, beef, pork, eggs, milk, seafood, nut products, and fresh produce. Increased public awareness related to food-borne contamination resulted in greater efforts to develop more sensitive, rapid, and inexpensive methods of pathogens detection. Loop-mediated isothermal amplification (LAMP) constitutes a promising solution for rapid diagnosis of food-borne pathogens and is increasingly been applied for the specific diagnosis of different pathogens, Salmonella included. We have reviewed the application of LAMP for the specific detection of Salmonella in food matrices, compared with conventional culture techniques, and in terms of applicability, food matrices, type of assays, target genes, assay temperature, time and equipment, specificity, sensitivity, and robustness. The pros and cons of Salmonella LAMP assays are presented. The potential of LAMP for the development of new on-site diagnostics for the food and agricultural industries and its use as a routine Salmonella screening tool are discussed. Salmonella-specific LAMP assays are expected to provide a very robust, innovative, and powerful molecular diagnostic method for food safety testing services and public health authorities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
General
Salmonella are facultative, anaerobic gram-negative bacilli belonging to the Enterobacteriaceae family. The global human health impact of nontyphoidal Salmonella is high. It is estimated that it causes 93.8 million human infections, of which an estimated 80.3 million are food-borne and 155,000 deaths annually worldwide. Salmonella infection represents a considerable burden in both developing and developed countries (Tirado and Schmidt 2001; Majowicz et al. 2010; Hendriksen et al. 2011). It is estimated that in 2010 Salmonella infection in the USA cost $2.7 billion in medical bills, lost wages, and premature death, exclusive of costs associated with product recalls, disease containment and control measures, and incalculable losses to the reputations of implicated agricultural products and producers regardless of the true source of contamination (Jenkins et al. 2011).
Typically, ≥105 Salmonella cells can cause infection in humans, and as few as 15 to 20 organisms are capable of causing salmonellosis in highly susceptible hosts (Kothary and Babu 2001; Techathuvanan and D'Souza 2012).
Salmonellosis is most often attributed to the consumption of contaminated foods such as poultry, beef, pork, eggs, milk, seafood, nut products, and fresh produce (Ohtsuka et al. 2005; Foley and Lynne 2008; Wang et al. 2008a, b; Techathuvanan et al. 2010a; Chen et al. 2011; Zhang et al. 2011).
Although more than 2,500 serovars of Salmonella enterica have been identified, most human infections are caused by a limited number of serovars (Hendriksen et al. 2011). S. enterica serovar Enteritidis is one of the most frequent Salmonella strains typically associated with salmonellosis outbreaks related to eggs, poultry, and their products (Okamura et al. 2008; Techathuvanan and D'Souza 2012). Salmonella typhimurium is reported to be the most common serotype associated with swine (Techathuvanan et al. 2010a, b).
Increased public awareness related to health and economic impacts of food-borne contamination and illness has resulted in greater efforts to develop more sensitive, rapid, and inexpensive method of pathogenic microbe detection and identification (Wang et al. 2008a, b).
Classical and Molecular Methods for Salmonella Detection
To date, reference methods for bacterial detection or identification rely mainly on culture-based approaches (Francois et al. 2011). The conventional culture technique for Salmonella standardized by the International Organization for Standardization (ISO6579:2002) and the United States Food and Drug Administration includes pre-enrichment, selective enrichments, and plating on selective agar media followed by biochemical and serological tests (Okamura et al. 2008, 2009). Traditional culture-based methods for detecting Salmonella are reliable but labor-intensive, too time-consuming, demanding several days for a definitive result, and costly to meet food safety control (Wang et al. 2008a, b; Yang et al. 2010; Zhang et al. 2011). For public health and the food industry, rapid, sensitive, and specific methods to detect Salmonella in food are required (Ueda and Kuwabara 2009). Faster screening and detection methods for this pathogen have been developed and validated. Immunoassays such as enzyme-linked immunosorbent assay (ELISA) for Salmonella detection have limited use because of their low specificity (Chen et al. 2011). Moreover, rapid detection methods such as enzyme immunoassay need a high population of target pathogen (Li et al. 2009).
In recent years, molecular methods such as PCR, real-time PCR, and DNA microarray have been successfully used to detect a number of food-borne bacterial pathogens including Salmonella (Oliveira et al. 2002; Whyte et al. 2002; Aricind and Bhagwat 2003; Malorny et al. 2004, 2007, 2008; Naravaneni and Jamil 2005; Seo et al. 2006; Elizaquivel and Aznar 2008; Wattiau et al. 2008; Li et al. 2009; Lee et al. 2009; Ueda and Kuwabara 2009; Yang et al. 2010; Chen et al. 2011). Sequence-specific DNA amplification strategies can significantly decrease the time required to obtain results especially for non- or slow-growing organisms. Recent efforts in high-throughput sequencing have contributed to the elucidation of numerous genomes or specific characteristics of bacterial pathogens, enabling molecular-based detection and identification (Francois et al. 2011).
In recent years, molecular methods designed for targeting Salmonella DNA have focused on genes such as Salmonella invasion gene (invA), fimC, the ttrRSBCA locus, phoP, and other genome markers. Moreover, these genes have been targeted by conventional and quantitative real-time PCR (qPCR) technologies. Most of these molecular methods have the potential to reduce detection time to <3 days (Zhang et al. 2011). However, PCR methods often require extensive sample preparation to eliminate amplification inhibitors, are also sensitive to contamination and inhibition by the compounds present in the template material, require either laborious and time-consuming post-PCR target identification strategies, such as gel electrophoresis or amplicon sequencing, or specific labeled identification reagents built into a real-time PCR system, which are expensive (Klerks et al. 2004; Hara-Kudo et al. 2005; Okamura et al. 2008; Wang et al. 2008a, b; Francois et al. 2011). The requirement for expensive equipments and reagents is another limiting factor for their wide application (Shao et al. 2011), rendering them unfavorable for wide-scale use, particularly under field conditions (Hara-Kudo et al. 2005; Techathuvanan et al. 2010a; Yang et al. 2010; Techathuvanan and D'Souza 2012). Consequently, there is a need to develop and validate faster screening and robust diagnostic assays for this pathogen, to prevent and control the spread of Salmonella (Yang et al. 2010; Zhang et al. 2011). Sample preparation kits for detecting Salmonella by genetic methods, as well as commercial kits utilizing genetic methods have been recently recorded by Tebbs et al. (2012), in an attempt to describe the molecular technologies for Salmonella detection.
LAMP Technique
In 2000, Notomi et al. developed a novel nucleic acid amplification method, designated loop-mediated isothermal amplification (LAMP). Compared with other rapid detection methods, LAMP has many advantages, such as high specificity and sensibility, simple operation, and low cost, which constitutes a potentially valuable tool for rapid diagnosis of food-borne pathogens (Shao et al. 2011).
LAMP differs from PCR in that four or six primers are used for the amplification of a single-target gene. The principle of LAMP is autocycling strand displacement DNA synthesis in the presence of Bst DNA polymerase with high strand displacement activity under isothermal conditions between 60 and 65 °C resulting in 109 copies of target DNA as well as large amount of by products within an hour (Notomi et al. 2000; Mori et al. 2001; Enosawa et al. 2003; Parida et al. 2004, 2008). The final amplification products are mixtures of many different sizes of stem–loop DNAs with several inverted repeats of the target sequence and cauliflower-like structures with multiple loops. At the end of the reaction, the presence or absence of the target DNA is judged visually by the appearance of a white magnesium pyrophosphate precipitate or by a color change on addition of a SYBR Green I to the reaction mixture. In-tube detection of DNA amplification is also possible using incorporation of other fluorescent intercalating dyes, such as EvaGreen, Hoechst 33285, ethidium bromide, P2, and SYTO9 (Niessen et al. 2013). However, gel electrophoresis may be needed to detect differences. Multiple bands observed on the agarose gel are indicative of a mixture of stem–loop DNA of varying sizes and multiple loops of DNA formed by annealing between alternately inverted repeats of the target in the same strand (cauliflower-like structures) (Notomi et al. 2000; Nagamine et al. 2002; Yang et al. 2010). The existence of nontarget DNA and inhibition in LAMP mixture are less likely to affect the result (Notomi et al. 2000; Wang et al. 2008a, b). Since it is isothermal, LAMP does not require a sophisticated temperature control device and can be performed in much simpler instruments such as a heater or water bath.
Instruments for real-time monitoring of the LAMP reaction based on turbidimetric measurements of magnesium pyrophosphate precipitated as a byproduct of the polymerization reaction are commercially available, and portable versions are also reported in the literature (Lee et al. 2008). Analogous fluorescence based real-time instruments for isothermal amplification reactions using nonspecific intercalating dyes for reporting positive reactions have been reported for LAMP (Jenkins et al. 2011; Liu et al. 2011).
Gandelman et al. (2010) reported recently that the bioluminescent monitoring using coupled conversion of inorganic pyrophosphate to ATP and the simultaneous monitoring of ATP levels using thermostable firefly luciferase provides an effective system for reporting isothermal nucleic acid amplification in real time by measuring light generated in the process of amplification in a closed tube format and by offering the potential for both quantitative and qualitative assays that are simple, fast, robust, and low cost in terms of equipment requirements (Gandelman et al. 2010).
Table 1 presents the comparative advantages and disadvantages of conventional PCR, real-time PCR, and LAMP diagnostic techniques. Different LAMP commercial kits have been developed for the rapid detection of Salmonella. The characteristics of LAMP Salmonella detection kits and selected application notes are summarized in Table 2.
LAMP Applicability
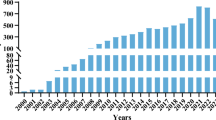
“Loop-mediated isothermal amplification” keywords search resulted in 842 publications in PubMed database (accessed on 20 May 2013), and 1,143 results in Scopus database (accessed on 20 May 2013). Figure 1 shows the annual references from 2000 (the year of first publication of LAMP assay by Notomi et al.) to 2012, regarding Salmonella-specific and general LAMP assays, according to Scopus database.
Annual references from the first LAMP publication by Notomi et al. (2000) to 2012, concerning Salmonella-specific and general LAMP assays, according to Scopus database
Both simple and real-time detection (http://loopamp.eiken.co.jp/e/index.html) has been applied for specific detection of many kinds of pathogens (Ohtsuki et al. 2008; Song et al. 2005; Kurosaki et al. 2009; Goto et al. 2007; Enosawa et al. 2003; Furuhata et al. 2005; Karanis et al. 2007; Yoneyama et al. 2007; Parida et al. 2004, 2005; Fukuda et al. 2006; Chen et al. 2008).
We have previously used the Salmonella-specific assay developed by Hara-Kudo et al. (2005) to rapidly detect Salmonella spp. strains isolated from different food matrices (Ziros et al. 2012), and are currently working on the development of virus-specific LAMP assays, as potential tools for food and environmental virology studies.
LAMP Assays for Salmonella Detection
Table 3 summarizes information collected from Salmonella-specific LAMP assays in order to provide a comprehensive overview of the type of assay, the food matrix tested, the target gene, the assay characteristics in terms of temperature, time and equipment, and the limits of detection. References are presented in chronological order.
Food Matrices
LAMP assays have been developed for the detection of Salmonella in different food matrices, including liquid egg (Hara-Kudo et al. 2005; Ohtsuka et al. 2005; Ueda and Kuwabara 2009), pork and pork processing environment (Li et al. 2009; Techathuvanan et al. 2011; Yang et al. 2010; Zhang et al. 2012), meat products (Wang et al. 2009), milk (Li et al. 2009; Shao et al. 2011; Kubota et al. 2013; Wang and Wang 2013), chicken (Okamura et al. 2008; Jenkins et al. 2011; Ravan and Yazdanparast 2012a; Zhang et al. 2012), duck (Tang et al. 2012), produce items (cantaloupe, spinach, spinach sauté and cake, tomato, cilantro, lettuce, parsley, jalapeno peppers, and raw green vegetables) (Ueda and Kuwabara 2009; Chen et al. 2011; Zhang et al. 2011, 2012), yogurt, omelet, hamburger, beef, and apple juice (Chen et al. 2011).
Type of Assays
Conventional (Notomi et al. 2000; Hara-Kudo et al. 2005; Li et al. 2009; Wang et al. 2009; Ahn et al. 2010; Yang et al. 2010; Jenkins et al. 2011; Ye et al. 2009, 2011; Kubota et al. 2013) and modified (Lu et al. 2009; Hsieh et al. 2012; Chen et al. 2011; Techathuvanan and D'Souza 2012; Tourlousse et al. 2012) Salmonella-specific LAMP assays have been reported. Modified assays included coupling of propidium monoazide (PMA) with LAMP (PMA LAMP) or ethidium monoazide (EMA) sample treatment (EMA LAMP), reverse-transcriptase (RT) LAMP, microfluidic electrochemical quantitative (MEQ LAMP), LAMP-ELISA, multiplex LAMP (mLAMP), and mLAMP coupled with restriction fragment length polymorphism assay.
The persistence of DNA after cell death causes a major issue in aspects of medical or biological studies. The signal from the dead bacterial cells cannot be distinguished from the live cells in the conventional DNA-based detection methods. LAMP combined with the EMA treatment was applied for specific detection of viable Salmonella cells (Lu et al. 2009). Chen et al. (2011) examined a strategy for the rapid detection and quantification of viable Salmonella in produce by coupling a simple propidium monoazide sample treatment with LAMP. A multiplex loop-mediated isothermal amplification to detect food-borne pathogens was developed by Jiang et al. (2012). A multiplex LAMP-RFLP was developed and validated for simultaneous detection of Salmonella strains and Shigella strains in milk by Shao et al. (2011). RT-LAMP assays were used by Techathuvanana and D'Souza (2012) to rapidly and sensitively detect S. enterica serovar Enteritidis in liquid whole eggs (LWEs), and S. typhimurium from pork-processing environments (Techathuvanan et al. 2011) and pork (Techathuvanan et al. 2010a). A MEQ-LAMP system has been developed for the rapid, sensitive, and quantitative detection of S. enterica enterica Typhimurium DNA (Hsieh et al. 2012). A simple handheld instrument was designed to enable real-time detection of the LAMP reaction in a standard PCR tube using newly described assimilating probes as sequence-specific reporter molecules (Jenkins et al. 2011). A LAMP enzyme-linked immunosorbent assay was developed by Ravan and Yazdanparast (2012a). The LAMP-ELISA involves direct incorporation of labeled nucleotides in amplicons during the LAMP amplification process; their hybridization to specific captured oligonucleotide probes and finally detection of the captured amplicons by immunoassay technology (Ravan and Yazdanparast 2012a).
Target Genes
The invA is a gene present only in Salmonella spp. and therefore, the vast majority of the developed Salmonella-specific LAMP assays used this target for designing LAMP primers (Hara-Kudo et al. 2005; Ueda and Kuwabara 2009; Ahn et al. 2010; Jiang et al. 2012; Hsieh et al. 2012; Lu et al. 2009). Yang et al. (2010) developed a specific LAMP method utilizing the Sdf I target sequence for the detection of Salmonella enteritidis under field conditions. Okamura et al. (2008) developed a LAMP assay that amplifies fragments of O4 S. enterica-specific gene rfbJ. Specifically designed primers to target within the phoP gene, which regulates the expression of genes involved in virulence and the survival of Salmonella from destruction by macrophage, were used in a LAMP assay developed by Li et al. (2009). In the study by Zhang et al. (2012), two sets of LAMP primers were designed to specifically target the Salmonella fimY gene, a gene previously shown specificity for Salmonella detection by PCR. The conserved Salmonella serogroup D O-antigen-specific gene within rfb gene cluster, prt (rfbS) gene, was selected and used as the target for LAMP primers' design in the studies of Ravan and Yazdanparast ( 2012a, b). A HisJ-based LAMP method was developed by Zhang et al. (2012) to detect food-borne Salmonella.
Assay Temperature, Time, and Equipment
Serovar-specific S. enteritidis DNA was amplified at 65 °C in as early as 20 min in a water bath; however, the optimum reaction time was set as 40 min to ensure positive detection with a low template concentration (Yang et al. 2010). The LAMP reaction mixture was incubated for 1 h at 65 °C in a thermal cycler followed by 2 min at 80 °C to inactivate the Bst polymerase in the study by Zhang (Zhang et al. 2011). LAMP amplicons were detected by exposure to UV light, where positive reactions were visualized as bright green fluorescence (Zhang et al. 2011). Similarly, Ueda used the same time and temperature parameters, but the amplification of the gene was confirmed by real-time monitoring of the increase of turbidity produced by magnesium pyrophosphate during reaction by using a Loopamp Realtime Turbidimeter (Teramecs, Kyoto), which sequentially measured the absorbance of the reaction mixture at 650 nm (Ueda and Kuwabara 2009).
Incubation at 65 °C for 60 min using a thermal cycler was used by Hara-Kudo (2005) and Ohtsuka et al. (2005) but the LAMP amplicon was detected as a value of fluorescence (delta Rn) in real-time using an increase in fluorescence intensity from an intercalating dye, visual observation of the turbidity produced by magnesium pyrophosphate, and agarose electrophoresis (Hara-Kudo et al. 2005).
The Salmonella invA-based LAMP assay developed in the study of Chen et al. (2011) was rapid (15–40 min). In this study, to detect viable Salmonellae in produce by coupling propidium monoazide with LAMP, the reaction was carried out at 63 °C for 40 min in a real-time turbidimeter. Detection of LAMP products was also performed by adding 1 μl of 1:10 diluted original SYBR green I dye and observed immediately visually for color change (from orange to green or greenish yellow; Chen et al. 2011). Time and temperature conditions of the Salmonella invA-based LAMP assay developed by Wang and Wang (2013) were also rapid and optimized to be 40 min at 61 °C. The EMA-LAMP assay developed by Lu et al. (2009) was carried out in a total of 50 μl reaction mixture within 60 min under isothermal conditions at 65 °C.
Fluorescence caused by the unquenching of calcein during the reaction was detected using an endpoint fluorescence detector in the study of Francois et al. (2011). A simple handheld instrument was designed to enable real-time detection of the LAMP reaction in a standard PCR tube using newly described assimilating probes as sequence-specific reporter molecules. The system was validated using DNA isolated from S. enterica demonstrating accurate temperature control with little power and little overshoot of setpoint temperatures with rapid and accurate detection often in less than 30 min and within 20 min for reactions with high (>105) genome copy numbers (Jenkins et al. 2011).
The overall analysis time for the LAMP assay method developed by Li et al. (2009) was approximately 24 h. For further confirmation, LAMP products were digested after turbidity measurement with 5 units of restriction enzyme TaqI (Li et al. 2009).
Under isothermal conditions at 63 °C, ladder pattern of DNA bands could be amplified within 60 min in the presence of genomic DNAs of Salmonella and Shigella strains, which could be distinguished between Salmonella spp. and Shigella spp. simultaneously based on the different ladder pattern of DNA bands and subsequent restriction enzyme analysis. The overall analysis time was approximately 20 h including the enrichment of the bacterial cells (Shao et al. 2011).
The MEQ-LAMP chip containing a single microfluidic chamber has been used for the quantitative detection of as few as 16 copies of genomic DNA of S. enterica enterica Typhimurium in less than an hour. The reaction took place at a constant temperature of 65 °C, and it was possible to determine the initial target quantity in a manner analogous to optical real-time PCR methods (Hsieh et al. 2012).
In the study of Ravan and Yazdanparast (2012a), the LAMP–ELISA mixture was incubated for 75 min at 60 °C. LAMP reactions were also performed in a regular water bath for evaluating the reproducibility of the assay independent of the thermal cycler system (Ravan and Yazdanparast 2012a).
Assay Sensitivity and Specificity
The detection limit of the Salmonella serovar Enteritidis-specific LAMP assay, developed by Yang et al. (2010), was four copies per microliter, being as sensitive and specific as the fluorescent quantitative real-time polymerase chain reaction (Yang et al. 2010). Comparable results of Salmonella detection were obtained by the FDA Salmonella culture method and by three molecular methods: qPCR, RT-qPCR, and LAMP, which all detected as little as 2 CFU of Salmonella cells/25 g of produce (Zhang et al. 2011).
Quantification of genomic copies by qPCR and counting CFU by classical culture-based methods result in data expressed in different units, the latter being a key feature defined by legislation. The correlation between these two targets could potentially be established to allow appropriate data interpretation. It has to be noted that the correlation between genomic copies by PCR and CFU is not straightforward and not permanent (e.g., species- and strain-based variations, growth phase-based variations, etc.). The detection of genomic copies can result in overestimation of present bacterial cells due to multiple copies of targeted genetic fragments. In addition, the challenge of the multiple chromosomes in some bacterial species can lead to several-fold overestimation of present bacterial cells (Cocolin et al. 2011).
The invA LAMP detection limit of the study of Zhang et al. (2011) was 104 CFU/reaction instead of 2 CFU/reaction reported previously, and this reduction of the detection limit was attributed by the authors to the use of visual determination by fluorescence instead of turbidimetry. Although the LAMP assay's detection limit was low in pure culture (104 invA CFU/tube), it was able to successfully detect Salmonella in all the samples that were positive by both qPCR and RT-qPCR assays. The LAMP assay detection limit was lower than those of the two qPCR methods, but the authors compared its performance in detecting Salmonella in produce with the other methods because the testing was done with enriched samples that usually contain Salmonella at levels much higher than the detection limit of the LAMP assay. All but two Salmonella strains were detected as positive by invA LAMP (99 % inclusivity). These two strains were likely to possess some nucleotide changes in the target region of the LAMP primers that precluded the amplification of that region. They were positive by qPCR using primers and probe targeting another region of the invA gene (Zhang et al. 2011).
In the study by Wang and Wang (2013), a detection limit level of 142 CFU/ml which corresponded to six to nine cells per reaction tube was reported for the LAMP assay applied to artificially contaminated raw milk samples. Comparatively, the detection level of conventional PCR was 103 CFU/ml. Data on naturally contaminated raw milk samples indicated that the LAMP method was highly specific and sensitive, giving 89.58 % concordance with the ISO 6579 reference method for the samples without enrichment and 100 % concordance for the samples after enrichment (Wang and Wang 2013). Hara-Kudo et al. (2005) described the detection limit of LAMP assay from pure culture and in liquid egg samples at 370–434 cells/ml of sample Salmonella concentration (Hara-Kudo et al. 2005). The detection limits of the first report examining the novel combination of PMA and LAMP in detecting and quantifying viable Salmonellae were 3.4–34 viable Salmonella cells in pure culture and 6.1 × 103 to 6.1 × 104 CFU/g in spiked produce samples. In comparison, PMA-PCR was up to 100-fold less sensitive. The correlation between LAMP time threshold (TT) values and viable Salmonella cell numbers was high (R 2 = 0.949–0.993) with a quantification range (102–105 CFU/reaction in pure culture and 104–107 CFU/g in produce) comparable to that of PMA in combination with PMA-qPCR (Chen et al. 2011). Based on the results of Salmonella detection in 110 samples of unpasteurized liquid eggs, the LAMP assay was the most effective method compared to a culture method and PCR. The PCR method failed to detect Salmonella in 10 % of samples while in one sample; Salmonella was detected by LAMP assay but not by the culture method (Ohtsuka et al. 2005). The detection limit of a LAMP assay for the detection of S. enterica serovar Typhi was found to be comparable to that observed using optimized home-brew qPCR assays, while the specificity of the amplification reaction remained high even at temperatures markedly different from the optimal one. Serial dilutions of purified S. Typhi DNA showed that LAMP reproducibly detected 500 fg, but failed to detect 50 fg (approximately eight genome equivalent copies; Francois et al. 2011). The probability of detection of the assay developed by Li et al. (2009) was 100 % when a Salmonella cell suspension containing 101 CFU/ml was used as a template in the LAMP assay. The lowest limit of LAMP detection was found to be 3.5 × 101 CFU/250 ml of enriched culture (Li et al. 2009). In the study by Zhang et al. (2012a), the detection limit was 16 CFU per reaction in pure culture, up to tenfold more sensitive than that of the PCR assay with the same target gene. When applied in raw food samples, the sensitivity of LAMP for the detection of Salmonella was 93.55 versus 87.10 % that tested positive using conventional PCR. The handheld system developed by Jenkins et al. (2011) could be used for quantitative determination of pathogen DNA with a limit of detection of about 76 fg—15 genome copies per reaction in purified DNA or 25 cells in DNA extracts from chicken rinsate—comparable to values obtained when running the same reaction on a commercial benchtop real-time PCR instrument. While at least one reaction nominally containing a single genome equivalent resulted in a positive amplification reaction, statistically reliable detection limits were somewhat higher and depended on the nature of the sample being analyzed (Jenkins et al. 2011). An mLAMP developed by Shao et al. (2011) allowed the detection of milk sample artificially contaminated by Salmonella and Shigella strains at initial inoculation levels of approximately 5 CFU/10 mL. The sensitivity of mLAMP was found to be 100 fg DNA/tube with genomic DNAs of Salmonella and Shigella strains, comparatively, multiplex PCR was 1 pg DNA/tube (Shao et al. 2011). A study to compare the detection sensitivity of S. typhimurium from the pork processing environment by RT-LAMP, RT-PCR, and culture-based assays showed that RT-LAMP detection for spiked carcass rinses were comparable to those of RT-PCR and cultural plating with detection limits of 1 log CFU/ml, although they were obtained significantly faster, within 24 h including pre-enrichment and enrichment (Techathuvanan et al. 2011). Improved Salmonella detection at 102 CFU/25 g for both pork chop and sausage was obtained after 10-h enrichment and 106 CFU/25 g without enrichment for both products, as reported by Techathuvanan et al. (2010b). The RT-LAMP assay, developed as a rapid screening tool for S. enterica in liquid whole eggs, showed that improved detection was obtained with increased enrichment time; after 16-h enrichment, the RT-LAMP assay could detect up to 100 to 101 CFU/25 mL of S. enteritidis, which is a comparable detection to culture-based assays (Techathuvanan and D'Souza 2012).
The direct and quantitative detection of as few as 16 copies of genomic DNA of S. enterica Typhimurium by a MEQ-LAMP system in less than an hour has been reported. This detection limit corresponds to 4 fg mL−1 of DNA and approximately 0.8 copies per microliter, which is approximately 7 orders of magnitude more sensitive than previously reported electrochemical detection of LAMP products (Hsieh et al. 2012).
The detection sensitivity of the DNA-based LAMP assay for Salmonella using pure culture and in situ studies using artificially and naturally contaminated egg and poultry-related samples has been previously investigated (Hara-Kudo et al. 2005; Ohtsuka et al. 2005; Okamura et al. 2008; Wang et al. 2008a, b). The LAMP assay was shown to detect Salmonella in inoculated LWEs at approximately 5.6 × 101 CFU/mL (Hara-Kudo et al. 2005), and <1 CFU/g in naturally contaminated LWE after 20-h enrichment at 37 °C in BPW (Ohtsuka et al. 2005). The detection limit of 1 CFU/cm2 of Salmonella was reported when the LAMP assay was applied to artificially contaminated eggshells after 4-h enrichment (Ye et al. 2011). The LAMP assay yielded a detection limit of 6.1 × 101 CFU/g after 1-day enrichment for Salmonella from chicken cecal droppings (Okamura et al. 2008).
Generally, LAMP has been found to have either similar or superior sensitivity over PCR. The study of Ravan and Yazdanparast (2012a) also showed that the LAMP-ELISA was more sensitive than PCR-ELISA; a positive signal was detected using 4 CFU of bacteria per tube by LAMP-ELISA, while the PCR-ELISA provided a positive signal with 50 CFU per tube (Ravan and Yazdanparast 2012a).
Assay Robustness
The detection of S. enteritidis by LAMP was not inhibited by liquid egg components and the sensitivity was very high (Hara-Kudo et al. 2005; Ohtsuka et al. 2005). When coupled with a simple PMA sample treatment, the PMA-LAMP assay developed by Chen et al. (2011) demonstrated good dead cell exclusivity (up to 108 CFU/ml in pure culture and 108 CFU/g in spiked produce).
Francois et al. (2011) evaluated the robustness of loop-mediated isothermal amplification of DNA for bacterial diagnostic applications. S. enterica serovar Typhi was used as the target organism and compared with a real-time qPCR for testing assay performance and reproducibly, as well as the impact of pH and temperature stability. This isothermal amplification method appeared to be particularly robust across 2 pH units (7.3–9.3) and temperature values (57–67 °C). The detection limit was comparable to that observed using optimized home-brew qPCR assays. The specificity of the amplification reaction remained high even at temperatures markedly different from the optimal one. Exposing reagents to the ambient temperature during the preparation of the reaction mixture as well as prolonging times for preparing the amplification reaction did not yield false-positive results. LAMP remained sensitive and specific despite the addition of untreated biological fluids such as stool or urine that commonly inhibit PCR amplification. Whereas the detection of microorganisms from whole blood or a blood-culture medium typically requires extensive sample purification and removal of inhibitors, LAMP amplification remained more sensitive than conventional qPCR when omitting such preparatory steps (Francois et al. 2011).
S. enterica serovar Enteriditis and S. enterica serovar Anatis were detected in suspect-infected ducks by LAMP, above 6.0 and 4.8 CFU/test, respectively, in pure-culture conditions, even in the existence of 0.01 g of duck liver or spleen homogenates; the detection thresholds were still achieved at 6.0 CFU per test tube (Tang et al. 2012).
The LAMP assay developed by Techathuvanan et al. (2010b) showed the detection limit of 102 CFU/25 g for all tested 24-h freeze- and cold-stressed Salmonella (at −80, −20, and 4 °C) after 3 h pre-enrichment in BPW and 12-h enrichment in TTB in pork chop samples. For pork sausages, 101 CFU/25 g of cold- and freeze-stressed Salmonella could be detected after the same enrichment procedures, while the same detection limit was obtained using standard cultural methods. The study also showed no detection when background flora was tested in both pork chops and pork sausages even after pre-enrichment followed by selective enrichment, eliminating any possible cross-reactivity, indicating the specificity of this assay (Techathuvanan et al. 2010a, b).
The Pros and Cons of LAMP Salmonella Assays
The pros of LAMP Salmonella methodology are summarized below:
-
High-throughput detection technique with high sensitivity, specificity, and simplicity.
-
Suitability for specifically detecting Salmonella under field conditions (ideally suited for on-site testing) and in laboratory settings. Ideal method of distinguishing Salmonella serovars, diagnose infections in humans and animals.
-
Elimination of the need for complicated, expensive equipment and technical training in the use of this equipment.
-
Particularly robust isothermal amplification method to: (1) physicochemical parameter modifications (pH and temperature values), (2) exposure of reagents to the ambient temperature during the preparation of the reaction mixture, (3) prolonging times for preparing the amplification reaction, (4) PCR amplification inhibitors, and (5) nontarget genomic DNA.
-
Requirement of fewer operation steps compared to PCR and real-time PCR assays.
-
More rapid method of pathogens detection compared to other molecular detection methods such as PCR and real-time PCR.
-
Multiple ways of detection of LAMP amplicons by: (1) gel electrophoresis, (2) naked eye observation of turbidity or color change, and (3) real-time turbidimeter monitoring.
-
Elimination of the need of gel electrophoresis and staining with ethidium bromide, since a large amount of DNA is synthesized by LAMP and the products can be detected simply by the presence of turbidity or fluorescence. Possibility of a closed tube procedure such as monitoring with a real-time turbidimeter to prevent cross-contamination and potentially quantify.
-
Possibility of elimination of nucleic acid extraction step, which adds time, cost, and complexity to the analysis.
-
Easily standardizable method among different laboratories.
The main drawbacks of LAMP Salmonella methodology are summarized below:
-
Reliance on the detection of DNA which can be done even after the target cells are dead. Inability to distinguish viable from dead cells (LAMP assays).
-
Technically demanding work with RNA. Moreover, some mRNA molecules can persist in dead cells for extended periods, leading to false-positive results (RT-LAMP assays).
-
Absence of internal amplification controls (IACs) and consequent limitations of monitoring the presence of inhibitors in the analyzed samples. Currently, the LAMP assays require the use of external amplification controls to eliminate false negatives.
-
Limitations for quantitative assessments and multiplexing.
-
Limitations to distinguish between the various serovars of Salmonella.
-
Open-tube procedures after amplification such as gel electrophoresis potentially act as a significant source of cross-contamination, since LAMP synthesizes a large amount of DNA (10–20 μg/25 μl reaction mixture).
-
Requirement of a higher degree of expertise for the development of primers and optimization compared to other PCR techniques.
Future Aspects
The use of molecular biology in food microbiology is probably one of the best examples of how applied science, such as food diagnostics, gets revolutionized and driven by advances in basic sciences. Although many rapid and sensitive methods have been developed and their applications described, PCR in its different forms, remains currently the most important one. The shortcomings of the qPCR methodology in food diagnostics are still intriguing scientific attention and wide-scale efforts are made around the globe to bring PCR-based methods another step closer to overall applicability in diagnostics of food-borne pathogens (Cocolin et al. 2011).
The LAMP method is expected to provide a very robust, innovative, and powerful molecular diagnostic method for food safety testing services and public health authorities. Salmonella-specific LAMP assays have the potential for use as a routine screening tool for Salmonella contamination in the food industry and the environment and will aid to the prevention of outbreaks and product recalls.
Future trends in LAMP Salmonella assays are mentioned below:
-
Collaborative studies to assess the interlaboratory reproducibility of the developed LAMP assays.
-
Use of LAMP under field conditions by nonspecialists and in small or regional laboratories, where nucleic acid-based testing is not currently performed and equipment is limited.
-
Application of RT-LAMP or PMA-LAMP assays for routine screening (detecting and quantifying) of viable salmonellae.
-
Demonstration of multiplexing ability.
-
Improvement of the ability for the identification and discrimination of Salmonella serovars.
-
Incorporation of fluorescent dyes or molecular beacon probes to LAMP assays to observe amplification of the target gene in a real-time manner (improved detection as it concerns sensitivity and time).
-
Implementation of IACs to control the amplification results.
-
Improvement of amplification efficiency by using DNA polymerase with higher efficiency.
Importantly, LAMP has the potential to support the development of new on-site diagnostics for the food and agricultural industries. Incorporation of LAMP assays in simple, low power, handheld devices for real-time detection of the LAMP reaction will provide promising solutions for real-time on-site screening for Salmonella contamination.
References
Ahn Y-C, Cho M-H, Yoon I-K, Jung D-H, Lee E-Y, Kim J-H, Jang W-C (2010) Detection of Salmonella using the loop mediated isothermal amplification and real-time PCR. J Korean Chem Soc 54(2):215–221
Aricind A, Bhagwat AA (2003) Simultaneous detection of Escherichia coli O157:H7, Listeria monocytogenes and Salmonella strains by real-time PCR. Int J Food Microbiol 84(2):217–224
Chen H, Zhang J, Sun D, Ma L, Liu X, Cai X, Liu Y (2008) Development of reverse transcription loop-mediated isothermal amplification for rapid detection of H9 avian influenza virus. J Virol Methods 151(2):200–203
Chen S, Wang F, Beaulieu JC, Stein RE, Ge B (2011) Rapid detection of viable salmonellae in produce by coupling propidium monoazide with loop-mediated isothermal amplification. Appl Environ Microbiol 77(12):4008–4016
Cocolin L, Rajkovic A, Rantsiou K, Uyttendaele M (2011) The challenge of merging food safety diagnostic needs with quantitative PCR platforms. Trends Food Sci Technol 22(S1):S30–S38
Elizaquivel P, Aznar R (2008) A multiplex RTi-PCR reaction for simultaneous detection of Escherichia coli O157:H7, Salmonella spp. and Staphylococcus aureus on fresh, minimally processed vegetables. Food Microbiol 25(5):705–713
Enosawa M, Kageyama S, Sawai K, Wanatabe K, Notomi T, Onoe S, Mori Y, Yokomizo Y (2003) Use of loop-mediated isothermal amplification of the IS900 sequence for rapid detection of cultured Mycobacterium avium subsp. paratuberculosis. J Clin Microbiol 41(9):4359–4365
Foley SL, Lynne AM (2008) Food animal-associated Salmonella challenges: pathogenicity and antimicrobial resistance. J Anim Sci 86(14):E173–E187
Francois P, Tangomo M, Hibbs J, Bonetti EJ, Boehme CC, Notomi T, Perkins MD, Schrenzel J (2011) Robustness of a loop-mediated isothermal amplification reaction for diagnostic applications. FEMS Immunol Med Microbiol 62(1):41–48
Fukuda S, Takao S, Kuwayama M, Shimazu Y, Miyazaki K (2006) Rapid detection of norovirus from fecal specimens by real-time reverse transcription-loop-mediated isothermal amplification assay. J Clin Mircobiol 44(4):1376–1381
Furuhata K, Annaka T, Ikedo M, Fukuyama M, Yoshida S (2005) Comparison of loop-mediated isothermal amplification (LAMP) and conventional culture for the detection of Legionella species in hot spring water samples in Japan. Biocontrol Sci 10(3):117–120
Gandelman OA, Church VL, Moore CA, Kiddle G, Carne CA, Parmar S, Jalal H, Tisi LC, Murray JAH (2010) Novel bioluminescent quantitative detection of nucleic acid amplification in real-time. PLoS ONE 5(11):e14155
Goto M, Hayashidani H, Takatori K, Hara-Kudo Y (2007) Rapid detection of enterotoxigenic Staphylococcus aureus harbouring genes for four classical enterotoxins, SEA, SEB, SEC and SED, by loop-mediated isothermal amplification assay. Lett Appl Microbiol 45(1):100–107
Hara-Kudo Y, Yoshino M, Kojima T, Ikedo M (2005) Loop-mediated isothermal amplification for the rapid detection of Salmonella. FEMS Microbiol Lett 253(1):155–161
He C, Liu Z, Wang D, Sun Q, Huang J (2010) Application of LAMP to detect Salmonella in animal derived foods. Chin J Food Hyg 5:411–414 (in Chinese with English abstract)
Hendriksen RS, Vieira AR, Karlsmose S, Lo Fo Wong DM, Jensen AB, Wegener HC, Aarestrup FM (2011) Global monitoring of Salmonella serovar distribution from the world health organization global foodborne infections network country data bank: results of quality assured laboratories from 2001 to 2007. Foodborne Pathog Dis 8(8):887–900
Hsieh K, Patterson AS, Ferguson BS, Plaxco KW, Soh HT (2012) Rapid, sensitive, and quantitative detection of pathogenic DNA at the point of care through microfluidic electrochemical quantitative loop-mediated isothermal amplification. Angew Chem Int Ed 51(20):4896–4900
Jenkins DM, Kubota R, Dong J, Li Y, Higashiguchi D (2011) Handheld device for real-time, quantitative, LAMP-based detection of Salmonella enterica using assimilating probes. Biosens Bioelectron 30(1):255–260
Jiang K, Lv Q, Zhang D, Gao X, Zhao Y, Li M, Liu L, Wu J, Lu X, Luo C (2012) A novel, sensitive, accurate multiplex loop-mediated isothermal amplification method for detection of Salmonella spp., Shigella spp. and Staphylococcus aureus in food. J Food Agric Environ 10(3&4):252–256
Karanis P, Thekisoe O, Kiouptsi K, Ongerth J, Igarashi I, Inoue N (2007) Development and preliminary evaluation of a loop-mediated isothermal amplification procedure for sensitive detection of cryptosporidium oocysts in fecal and water samples. Appl Environ Microbiol 73(17):5660–5662
Klerks MM, Zijlstra C, van Bruggen AH (2004) Comparison of real-time PCR methods for detection of Salmonella enterica and Escherichia coli O157:H7, and introduction of a general internal amplification control. J Microbiol Methods 59(3):337–349
Kothary MH, Babu US (2001) Infective dose of foodborne pathogens in volunteers: a review. J Food Saf 21(1):49–73
Kubota R, Labarre P, Weigl BH, Li Y, Haydock P, Jenkins DM (2013) Molecular diagnostics in a teacup: non-instrumented nucleic acid amplification (NINA) for rapid, low cost detection of Salmonella enterica. Chin Sci Bull 58(10):1162–1168
Kurosaki Y, Sakuma T, Fukuma A, Fujinami Y, Kawamoto K, Kamo N, Makino S-I, Yasuda J (2009) A simple and sensitive method for detection of Bacillus anthracis by loop-mediated isothermal amplification. J Appl Microbiol 107(6):1947–1956
Lee S-Y, Huang J-G, Chuang T-L, Sheu J-C, Chuang Y-K, Holl M, Meldrum DR, Lee C-N, Lin C-W (2008) Compact optical diagnostic device for isothermal nucleic acids amplification. Sensors Actuators B Chem 133(2):493–501
Lee K, Iwata T, Shimizu M, Taniguchi T, Nakadai A, Hirota Y, Hayashidani H (2009) A novel multiplex PCR assay for Salmonella subspecies identification. J Appl Microbiol 107(3):805–811
Li XF, Zhang S, Zhang HW, Zhang LH, Tao HT, Yu J, Zheng WJ, Liu CH, Lü D, Xiang R, Liu Y (2009) A loop-mediated isothermal amplification method targets the phoP gene for the detection of Salmonella in food samples. Int J Food Microbiol 13(3):252–258
Liu B, Zhang L, Zhu X, Shi C, Chen J, Liu W, He X, Shi X (2011) PCR identification of Salmonella serogroups based on specific targets obtained by comparative genomics. Int J Food Microbiol 144(3):511–508
Lu Y, Yang W, Shi L, Li L, Alam MJ, Guo S, Miyoshi S (2009) Specific detection of viable Salmonella cells by an ethidium monoazide-loop mediated isothermal amplification (EMA-LAMP) method. J Health Sci 55(5):820–824
Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O'Brien SJ, Jones TF, Fazil A, Hoekstra RM (2010) The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis 50(6):882–889
Malorny B, Paccassoni E, Fach P, Bunge C, Martin A, Helmuth R (2004) Diagnostic real-time PCR for detection of Salmonella in food. Appl Environ Microbiol 70(12):7046–7052
Malorny B, Bunge C, Helmuth R (2007) A real-time PCR for the detection of Salmonella enteritidis in poultry meat and consumption eggs. J Microbiol Methods 70(2):245–251
Malorny B, Lofstrom C, Wagner M, Kramer N, Hoorfar J (2008) Enumeration of Salmonella bacteria in food and feed samples by real-time PCR for quantitative microbial risk assessment. Appl Environ Microbiol 74(5):1299–1304
Mori Y, Nagamine K, Tomita N, Notomi T (2001) Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Commun 289(1):150–154
Nagamine K, Hase T, Notomi T (2002) Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol Cell Probes 16(3):223–229
Naravaneni R, Jamil K (2005) Rapid detection of food-borne pathogens by using molecular techniques. J Med Microbiol 54(1):51–54
Niessen L, Luo J, Denschlag C, Vogel RF (2013) The application of loop-mediated isothermal applification (LAMP) in food testing for bacterial pathogens and fungal contaminants. Food Microbiol 36(2):191–206
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28(12):e63
Ohtsuka K, Yanagawa K, Takatori K, Hara-Kudo Y (2005) Detection of Salmonella enterica in naturally contaminated liquid eggs by loop-mediated isothermal amplification, and characterization of Salmonella isolates. Appl Environ Microbiol 71(11):6730–6735
Ohtsuki R, Kawamoto K, Kato Y, Shah MM, Ezaki T, Makino S-I (2008) Rapid detection of Brucella spp. by the loop-mediated isothermal amplification method. J Appl Microbiol 104(6):1815–1823
Okamura M, Ohba Y, Kikuchi S, Suzuki A, Tachizaki H, Takehara K, Ikedo M, Kojima T, Nakamura M (2008) Loop-mediated isothermal amplification for the rapid, sensitive, and specific detection of the O9 group of Salmonella in chickens. Vet Microbiol 132(1–2):197–204
Okamura M, Ohba Y, Kikuchi S, Takehara K, Ikedo M, Kojima T, Nakamura M (2009) Rapid, sensitive, and specific detection of the O4 group of Salmonella enterica by loop-mediated isothermal amplification. Avian Dis 53(2):216–221
Oliveira SD, Santos LR, Schuch DMT, Silva AB, Salle CTP, Canal CW (2002) Detection and identification of salmonellas from poultry-related samples by PCR. Vet Microbiol 87(1):25–35
Parida M, Posadas G, Inoue S, Hasebe F, Morita K (2004) Real time reverse transcription loop-mediated isothermal amplification for rapid detection of West Nile virus. J Clin Microbiol 42(1):257–263
Parida M, Horioke K, Ishida H, Dash PK, Saxena P, Jana AM, Islam MA, Inoue S, Hosaka N, Morita K (2005) Rapid detection and differentiation of dengue virus serotypes by a real-time reverse transcription-loop-mediated isothermal amplification assay. J Clin Microbiol 43(6):2895–2903
Parida M, Sannarangaiah S, Dash PK, Rao PV, Morita K (2008) Loop mediated isothermal amplification (LAMP): a new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev Med Virol 18(6):407–421
Ravan H, Yazdanparast R (2012a) Development and evaluation of a loop-mediated isothermal amplification method in conjunction with an enzyme-linked immunosorbent assay for specific detection of Salmonella serogroup D. Anal Chim Acta 733:64–70
Ravan H, Yazdanparast R (2012b) Development of a new loop-mediated isothermal amplification assay for prt (rfbS) gene to improve the identification of Salmonella serogroup D. World J Microbiol Biotechnol 28(5):2101–2106
Seo KH, Valentin-Bon IE, Brackett RE (2006) Detection and enumeration of Salmonella enteritidis in homemade ice cream associated with outbreak: comparison of conventional and real-time PCR methods. J Food Prot 69(3):639–643
Shao Y, Zhu S, Jin C, Chen F (2011) Development of multiplex loop-mediated isothermal amplification-RFLP (mLAMP-RFLP) to detect Salmonella spp. and Shigella spp. in milk. Int J Food Microbiol 148(2):75–79
Song T, Toma C, Nakasone N, Iwanaga M (2005) Sensitive and rapid detection of Shigella and enteroinvasive Escherichia coli by a loop-mediated isothermal amplification method. FEMS Microbiol Lett 243(1):259–263
Tang T, Cheng A, Wang M, Li X, He Q, Jia R, Zhu D, Chen X (2012) Development and clinical verification of a loop-mediated isothermal amplification method for detection of Salmonella species in suspect infected ducks. Poult Sci 91(4):979–986
Tebbs RS, Wong LY, Brzoska P and Petrauskene OV (2012) Molecular technologies for Salmonella detection. In: Annous BA and Gurtler JB (eds). Salmonella—distribution, adaptation, control measures and molecular technologies. ISBN 978-953-51-0661-6. InTech. DOI: 10.5772/2470
Techathuvanan C, D'Souza DH (2012) Reverse-transcriptase loop-mediated isothermal amplification as a rapid screening/monitoring tool for Salmonella enterica detection in liquid whole eggs. J Food Sci 77(4):M200–M205
Techathuvanan C, Draughon FA, D'Souza DH (2010a) Loop-mediated isothermal amplification (LAMP) for the rapid and sensitive detection of Salmonella typhimurium from pork. J Food Sci 75(3):M165–M172
Techathuvanan C, Draughon FA, D'Souza DH (2010b) Real-time reverse transcriptase PCR for the rapid and sensitive detection of Salmonella typhimurium from pork. J Food Prot 73(3):507–514
Techathuvanan C, Draughon FA, D'Souza DH (2011) Comparison of reverse transcriptase PCR, reverse transcriptase loop-mediated isothermal amplification, and culture-based assays for Salmonella detection from pork processing environments. J Food Prot 74(2):294–301
Tirado C, Schmidt K (2001) WHO surveillance programme for control of foodborne infections and intoxications: results and trends across greater Europe. J Infect 43(1):80–84
Tourlousse DM, Ahmad F, Stedtfeld RD, Seyrig G, Tiedje JM, Hashsham SA (2012) A polymer microfluidic chip for quantitative detection of multiple water- and foodborne pathogens using real-time fluorogenic loop-mediated isothermal amplification. Biomed Microdevices 14(4):769–778
Ueda S, Kuwabara Y (2009) The rapid detection of Salmonella from food samples by loop-mediated isothermal amplification (LAMP). Biocontrol Sci 14(2):73–76
Wang Y-Z, Wang D-G (2013) Development and evaluation of a loop-mediated isothermal amplification (LAMP) method for detecting foodborne Salmonella in raw milk. Adv Mater Res 647:577–582
Wang D, Huo G, Wang F, Li Y, Ren D (2008a) Drawback of loop-mediated isothermal amplification. Afr J Food Sci 2:83–86
Wang L, Shi L, Alam MJ, Geng Y, Li L (2008b) Specific and rapid detection of foodborne Salmonella by loop-mediated isothermal amplification method. Food Res Int 41(1):69–74
Wang Y, Zhang W, Yuan Y, Ma X, Zhang H, Su X, Wang Z, Ma B (2009) Study on loop-mediated isothermal amplification assay for detection of Salmonella in meat products. 3rd International Conference on Bioinformatics and Biomedical Engineering (ICBBE 2009): 1–6
Wattiau P, Weijers T, Andreoli P, Schliker C, Veken HV, Maas HM, Verbruggen AJ, Heck ME, Wannet WJ, Imberechts H, Vos P (2008) Evaluation of the Premi Test Salmonella, a commercial low-density DNA microarray system intended for routine identification and typing of Salmonella enterica. Int J Food Microbiol 123(3):293–298
Whyte P, Mc Gill K, Collins JD, Gormley E (2002) The prevalence and PCR detection of Salmonella contamination in raw poultry. Vet Microbiol 89(1):53–60
Yang JL, Ma GP, Yang R, Yang SQ, Fu LZ, Cheng AC, Wang MS, Zhang SH, Shen KF, Jia RY, Deng SX, Xu ZY (2010) Simple and rapid detection of Salmonella serovar Enteritidis under field conditions by loop-mediated isothermal amplification. J Appl Microbiol 109(5):1715–1723
Ye Y, Wang B, Huang F, Song Y, Yan H, Alam MJ, Yamasaki S, Shi L (2011) Application of in situ loop-mediated isothermal amplification method for detection of Salmonella in foods. Food Control 22(2–3):438–444
Yoneyama T, Kiyohara T, Shimasaki N, Kobayashi G, Ota Y, Notomi T, Totsuka A, Wakita T (2007) Rapid and real-time detection of hepatitis A virus by reverse transcription loop-mediated isothermal amplification assay. J Virol Methods 145(2):162–168
Ye Y, Yamasaki S, Lei S (2009) In situ loop-mediated isothermal amplification technology for rapid detection of food-borne Salmonella. Food Ferment Ind 35:137–141
Zhang G, Brown EW, González-Escalona N (2011) Comparison of real-time PCR, reverse transcriptase real-time PCR, loop-mediated isothermal amplification, and the FDA conventional microbiological method for the detection of Salmonella spp. in produce. Appl Environ Microbiol 77(18):6495–6501
Zhang L, Pan ZM, Geng SZ, Chen X, Liu ZY, Zhao F, Jiao XA (2012) A loop-mediated isothermal amplification method targets the HisJ gene for the detection of foodborne Salmonella. Eur Food Res Technol 234(6):1055–1062
Zhu SM, Wu JJ, Xu C, Qu J, Cheng W, Chen FS (2008) Rapid detection of Salmonella spp. by loop-mediated isothermal amplification method. Mod Food Sci Technol 24(7):725–730
Ziros PG, Kokkinos PA, Papanotas K, Vantarakis A (2012) Loop-mediated isothermal amplification (LAMP) for the detection of Salmonella spp. isolated from different food types. J Microbiol Biotechnol Food Sci (JMBFS) 2(1):152–161
Conflict of Interest
Kokkinos P.A. declares that he has no conflict of interest. Ziros P.G. declares that he has no conflict of interest. Bellou M. declares that she has no conflict of interest. Vantarakis A. declares that he has no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kokkinos, P.A., Ziros, P.G., Bellou, M. et al. Loop-Mediated Isothermal Amplification (LAMP) for the Detection of Salmonella in Food. Food Anal. Methods 7, 512–526 (2014). https://doi.org/10.1007/s12161-013-9748-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-013-9748-8