Abstract
The magnetic molecularly imprinted polymers (MMIPs) were prepared and used for the selective separation of chlorpyrifos from rice samples. The MMIPs were synthesized by surface-imprinted polymerization, using functionalized Fe3O4 particles as magnetic cores, chlorpyrifos as template, methacrylic acid as functional monomer, and trimethylolpropane trimethacrylate as cross-linker. Magnetic particles were characterized by scanning electron microscopy, Fourier-transform infrared spectrometry, physical property measurement system, and thermogravimetric analyzer. Adsorption studies were carried out to investigate the specific binding capacity, kinetics, and recognition specificity. The kinetic property of MMIPs was well fitted to the pseudo-second-order equation. The selective recognition experiment demonstrated that MMIPs had high selectivity towards chlorpyrifos over reference compound. Chlorpyrifos extracted from the rice samples was purified with MMIPs and detected by high-performance liquid chromatography. Under the optimal conditions, the detection limit of chlorpyrifos was 0.0072 μg g−1. The relative standard deviations of intra- and interday ranging from 2.4 to 4.6 % and from 3.5 % to 7.3 % were obtained, respectively. The recoveries of chlorpyrifos at three different fortified concentrations (0.025, 2.5, and 5 μg g−1) were in the range of 81.2–92.1 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chlorpyrifos [O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate] (CPF) is an effective broad spectrum organophosphorous agricultural insecticide displaying activity against a broad range of insects and household pests (Cho et al. 2002). Agricultural, industrial, and residential uses of CPF resulted in its intentional and accidental introduction into terrestrial and aquatic ecosystem, and brought a potential health hazard to mammals. As one of the most common pesticides in food, CPF is associated with chromosomal damage and bladder cancer (Lee et al. 2004; McKellar et al. 1976; Vidal et al. 1998), many works about detection of CPF were carried out (Cherta et al. 2012; Deme et al. 2012; Omeroglu et al. 2013).
Owing to the complexity of the matrix, the analysis of food often requires efficient sample pretreatment techniques. Typically, CPF was extracted from food under shaking, and then the extracts obtained were cleaned up by liquid–liquid extraction (LLE) or solid phase extraction (SPE). However, the LLE and classic SPE may retain not only the target analytes but also other chemicals, which could interfere with the detection of the compounds of interest. The QuEChERS (quick, easy, cheap, rugged, and safe) method also had used to determine the organophosphorous (Marchis et al. 2012). It was a sample preparation technique for multiresidue analysis of pesticides in food (Anastassiades et al. 2003). The advantages of the method included high sample throughput and low amounts of solvent, glassware, and bench space required (Payá et al. 2007). But sometimes, the selectivity of this method was not satisfying, and the impurities could not be cleaned completely.
Molecularly imprinted polymers (MIPs) are stable synthetic cross-linked polymers possessing selective molecular cognition sites, which have highly specific recognition ability for target molecules or similar compounds (Feás et al. 2009; He et al. 2007, 2008; Li et al. 2008; Puoci et al. 2009; Tamayo et al. 2007; Zhang et al. 2009a). MIPs are produced by growing a polymer around the template molecules (Ai et al. 2009; Lisa and Evangelyn 2005; Schirmer and Meisel 2009; Xie et al. 2003). After polymerization, templates are removed from the polymers, leaving specific cavities that can selectively rebind template molecules in size, shape and functional groups (Alexander et al. 2006). Their most significant advantages like high stability, long life, and easy preparation have led to the development of various MIPs applications in chromatographic separation (Ou et al. 2007), chemical sensors (Kandimalla and Ju 2004; Kirsch et al. 2001; Malitesta et al. 2012 ), chiral separation (Davies et al. 2004), SPE (Mohamed et al. 2007; Urraca et al. 2007), and catalysis (Pasetto et al. 2005; Wulff 2002). During the past few years, the increasing interest and attention toward MIPs have been attributed to its predetermination, recognition, and practicability (Katz and Davis 1999).
The MIP-SPE was applied to determine organophosphate residues in strawberries (Xu et al. 2010), vegetables (Baldim et al. 2012), and water samples (Liu et al. 2010). In the studies above, disulfoton, dichlorvos, and chlorpyrifos were used as templates, respectively. Good recovery and low quantification limits were obtained in these methods. However, the drawbacks like a tedious column packing procedure, high backpressure, and a low flow rate owing to cartridge mode (Ji et al. 2009) have also restricted its widespread application.
Combining magnetism with MIPs can build a controllable rebinding process and enable magnetic separation to replace the centrifugation and filtration step in a more convenient and economical way (Zhang et al. 2009b), and the new method is easier, quicker, simpler, and more effective to perform than MIP-SPE with cartridge mode. Recently, magnetic molecularly imprinted polymers (MMIPs) have been prepared and used for the extraction and determination of pyrethroids pesticide (Guan et al. 2013), triazines pesticide (Zhang et al. 2009b), bisphenol A (Ji et al. 2009), and β-agonists (Hu et al. 2011). In our previous studies, MMIPs were used to analyze tetracyclines (Chen et al. 2009a), sulfonamides (Chen et al. 2009b), and fluoroquinolones (Chen et al. 2010) in different matrix. The results proved that target analytes can be selectively recognized. However, there were still the problems of incomplete template removal and slow binding kinetics due to limitations of synthesis methods of MMIPs.
The aim of this study is to develop a new method to improve the analysis of CPF in rice, which is the main food in China. In the work, MMIPs were synthesized by surface-imprinted polymerization using CPF as template molecules, methacrylic acid (MAA) as functional monomer, and trimethylolpropane trimethacrylate (TRIM) as cross-linker. Surface MIPs show many advantages, including high selectivity, more accessible sites, fast mass transfer, and binding kinetics, due to the imprinted sites mostly situated on the surface of MMIPs. The characteristics of the MMIPs and binding experiment were investigated. The polymers were used as sorbents for the separation of CPF in the extract of rice sample, followed by high-performance liquid chromatography (HPLC) analysis. The CPF was selectively isolated, and the matrix interferences were eliminated in a short time using MMIPs, which simplified the sample pretreatment procedure.
Material and Methods
Materials
Chromatographic grade methanol was obtained from Fisher (Pittsburgh, PA, USA). CPF, MAA, iron(II) chloride tetrahydrate (FeCl2·4H2O), iron(III) chloride hexahydrate (FeCl3·6H2O), 3-methacryloxypropyltrimethoxysilane (MPS), potassium persulfate (K2S2O8), polyethylene glycol (PEG 6000), and TRIM were purchased from Aladdin (Shanghai, China). Methanol, ethanol, and ethyl acetate were obtained from Kermel (Tianjin, China). Ammonia solution (25 %) and acetic acid were purchased from Guangfu (Tianjin, China). Tetraethyl orthosilicate (TEOS) was obtained from Sinopharm (Shanghai, China). The purity of analytical standard CPF was 99 %. All other chemicals employed in the study were of analytical grade. High-purity water was obtained from a Milli-Q water system (Millipore, Billerica, MA, USA).
The standard stock solution of CPF was prepared by dissolving CPF in methanol, and the concentration was 1 mg mL−1. It was stored in a refrigerator at 4 °C. Work standard solutions with concentrations from 0.1 to 200.00 μg mL−1 were daily prepared by diluting the stock solution.
Rice samples were randomly obtained from the local markets in Harbin (China). The samples were shattered and stored at room temperature. Sample processing procedure should result in analytical portions that are representative of the analytical sample (Omeroglu et al. 2013). In this study, the shattered rice samples were mixed and reduced by coning and quartering to get a homogeneous mass in sample processing procedure (Wagner et al. 2001). The principle of this method was that the sample was mixed and subsequently arranged into a flattened pile, subsequently divided into four “equal” quadrants, and repeated on two opposing quadrants until the required reduced sample mass is reached. As rice was easy to shatter and mix uniformly, 2.0 g rice was representative and used for the following sample preparation.
One sample was checked to be free of CPF by National standard method used in China (Ministry of Health of the People’s Republic of China 2008), and it was used as blank sample for calibration and validation purposes. The spiked rice samples were prepared by adding CPF standard solution into rice samples. Then, the mixture was incubated uniformly and evaporated to dryness with nitrogen gas continuously.
Instrumentation
The feature of MMIPs was observed with a scanning electron microscopy (SEM; JEM-6700F, JEOL, Tokyo, Japan). Fourier-transform infrared spectrometry (FT-IR360, Nicolet, Madison, WI, USA) was used to determine the functional groups of products. Magnetic properties were measured by a physical property measurement system (PPMS; Quantum Design Instrument, San Diego, CA, USA) at 298 K. Themogravimetric analyzer (TGA; Pyris 1, Perkin Elmer, Waltham, MA, USA) was employed to determine the thermal stability of MMIPs.
Chromatographic analysis was performed on a LC-15C liquid chromatograph (Shimadzu, Kyoto, Japan). A Zorbax SB-C18 column (250 × 4.6 mm I.D., 5 μm) was used as an analytical column (Palo Alto, CA, USA). A KQ5200E ultrasonic apparatus (Kunshan Instrument, Kunshan, China) was used for assisting extraction of CPF from rice samples. A DZKW-C thermostatic bath (Shanghai, China) was used to keep a constant temperature during the experiment of synthetic reaction. A shaking table (Shengtang, Jintan, China) was used for adsorption process.
Preparation of Polymers
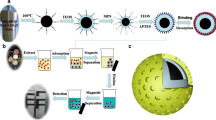
The first step was the preparation of Fe3O4 by co-precipitation method according to our previous work (Chen et al. 2009a). The Fe3O4 (0.5 g) obtained was dispersed in 100 mL of alcohol and 20 mL of water by ultrasound for 15 min, followed by the addition of 3.0 mL of ammonia solution and 2.0 mL of TEOS. The system was stirred for 24 h at 300 rpm, and Fe3O4 was encapsulated by SiO2 (Fe3O4@SiO2). The magnetic precipitates were isolated from the solvent by a permanent magnet and washed several times with water. Surface modification of Fe3O4@SiO2 was carried out by the reaction of Fe3O4@SiO2 and 0.15 mL of MPS dissolved in 100 mL of acetic acid/water (1:9, v/v). The system was stirred for 5 h at 60 °C. The magnetic particles were separated from the solvent by a permanent magnet and washed several times with water.
The CPF (0.5 mmol) and MAA (2.0 mmol) was dissolved in 10 mL ethanol and stored for 24 h. Then the mixture was added into a three-necked flask containing 150 mL 5 % PEG solution, the modified Fe3O4@SiO2 and 5.0 mmol TRIM. After being subjected to ultrasound for 10 min, the initiator K2S2O8 (0.1 g) was added into the system. The reaction was allowed to proceed at 60 °C and stirred at 300 rpm for 24 h. After polymerization, the product was collected by an external magnetic field. Then, the template was eluted from polymers with the mixture of methanol/acetic acid (8:2, v/v) by Soxhlet extraction until the CPF could not be detected by HPLC. Finally, the MMIPs were dried at 60 °C in vacuum. The process of preparing MMIPs is shown in Fig. 1.
The magnetic nonimprinted polymers (MNIPs) were prepared and processed similarly as above, except that the template molecules were not added. MMIPs and MNIPs could be kept and used for a long time.
Binding Experiment
The recognition property of MMIPs for CPF was studied by the static equilibrium adsorption. Each binding experiment was carried out by adding 10.0 mg of MMIPs or MNIPs in a glass tube containing 10.0 mL of CPF standard solution and shaking for some time. Then, the precipitates were collected with a magnet, and the target analyte in residual solution was detected. The amount of CPF bounded on the polymers was obtained by subtracting the free concentration from initial concentration of CPF added to the mixture.
The selectivity of the MMIPs was investigated using reference compound acephate, which was also belonging to organophosphorous.
Sample Preparation
The rice sample (2.0 g) was extracted with 10 mL ethyl acetate under ultrasound assisted for 10 min and centrifuged for 5 min at 5,000 rpm. Supernatant was collected, dried under nitrogen gas, and redissolved with 3.0 mL of 10 % aqueous methanol. Finally, the mixture was subjected to the extraction procedure by MMIPs.
Extraction Procedure
The MMIPs (5 mg) were added to above sample extract and shaken for 10 min. After that, the polymers were separated with a magnet and washed with 3.0 mL 20 % aqueous methanol. The CPF was eluted from the MMIPs with 3 × 1.0 mL (1.0 mL every time and eluted three times) of methanol/acetic acid (97:3, v/v) under ultrasound, dried with nitrogen gas, and redissolved in 0.5 mL of 85 % aqueous methanol for HPLC analysis.
The extraction conditions including amount of MMIPs, shaking time, washing solvent, elution solvent, and elution times affecting the performance of the extraction were investigated. The optimization was carried out one variable at a time. Every sample was analyzed three times.
HPLC Analysis
The analysis of CPF was performed by HPLC. The HPLC mobile phase was the 85 % aqueous methanol. The flow rate was 1.0 mL min−1. UV wavelength was set at 234 nm. The column temperature was at room temperature, and the injection volume was 20 μL. Typical chromatogram of CPF in rice samples is shown in Fig. 2.
Validation Study
Several basic analytical parameters in the method validation were evaluated. The chromatographic identification of the CPF was realized by its relative retention time. Calibration curve was obtained by plotting the peak area of the studied analyte versus the theoretical concentration of the analyte added to rice samples. The data were subjected to least squares regression analysis to provide information on linearity of the method. Limit of detection (LOD) and limit of quantification (LOQ) are considered as the minimum concentrations of analyte that can be confidently identified and quantified by the method, respectively. LOD and LOQ were estimated as the concentration of analyte producing a signal/noise ratio of 3 and 10. For the intraday assay precision and accuracy (Baldim et al. 2012), six replicates of control samples were analyzed for the same day at three different fortified concentrations of 0.025, 2.5, and 5 μg g−1. For the interday assay precision and accuracy, control samples were analyzed once a day for six sequence days at three different fortified concentrations of 0.025, 2.5, and 5 μg g−1.
Results and Discussion
Characterizations of the MMIPs
FTIR spectra of Fe3O4, Fe3O4@SiO2, and MMIPs were obtained. As can be seen from Fig. 3a, the absorption band of Fe−O bond at 574 cm−1 attributed to the Fe3O4 nanoparticles, the peaks at 583 and 591 cm−1 were also found in the spectra of Fe3O4@SiO2 and MMIPs, respectively, which further proved that Fe3O4 was embedded in MMIPs. In comparison with the infrared data of pure Fe3O4, the characteristic peak of Si−O−Si group was at 1,091 cm−1 in Fe3O4@SiO2. In MMIPs, the peak at 1,096 cm−1 was found, which indicated that SiO2 was embedded in MMIPs. In the spectra of MMIPs, the adsorption band around 3,441 cm−1, revealed the stretching vibration of O–H group in MAA, and the adsorption band around 2,959 cm–1, unveiled the telescopic vibration of C–H group in CH3. The band around 1,732 cm–1 was attributed to the C=O stretching vibration. The peak at 1,259 cm–1 indicated the existence of C-O stretching vibration in MMIPs.
The magnetic properties of Fe3O4, Fe3O4@SiO2, and MMIPs were studied by PPMS. Figure 3b showed the magnetic hysteresis loops of Fe3O4, Fe3O4@SiO2, and MMIPs. There was a similar general shape of these three curves, being symmetrical about the origin. The figure illustrated that the materials could respond magnetically to an external magnetic field, and this response vanished upon the removal of the magnetic field. The saturation magnetization of Fe3O4, Fe3O4@SiO2, and MMIPs were 57.86, 21.13, and 10.15 emu g−1, respectively. As shown in the inserted photograph of Fig. 3b, without the external magnetic field, a brown homogeneous dispersion existed; with the external magnetic field, the brown particles were attracted to the wall of vial.
The SEM image of the MMIPs is shown in Fig. 3c. The amount of Fe3O4 encapsulated in MMIPs was measured through TGA. As shown in Fig. 3d, when the temperature was slowly increased to 200 °C, the flat curve might be water loss of 5 %. From 200 to 600 °C, the polymer particles accounted for the significant mass loss 78 %, which might be due to decomposition of organic matter in MMIPs. The remaining mass was attributed to the thermal resistance of Fe3O4 particles, and their content in the MMIPs was 17 wt%.
Isothermal Absorption Experiment
The isothermal absorption experiment was carried out at the concentration of 1–200 μg mL−1. The amount of CPF adsorbed by the polymers was calculated as:
where Q (mg g−1) is the amount of CPF adsorbed on per gram of polymers, C i (μg mL−1) is the initial concentration of CPF, C f (μg mL−1) is the final concentration of CPF, V (mL) is the total volume of the adsorption mixture, and m (mg) is the mass of the polymers. As shown in Fig. 4a, CPF adsorbed on the polymers increased with increase in the initial concentration of CPF. Furthermore, the MMIPs showed significantly higher CPF loading than the MNIPs. In order to further study the binding properties of MMIPs, Scatchard analysis was performed. The equation of Scatchard analysis can be expressed as follows (Chen et al. 2009a):
where K d (μg mL−1) is the dissociation constant of binding sites, Q max (mg g−1) is the maximum amount of apparent binding, C (μg mL−1) is the equilibrium concentration of CPF in solution, Q is the same as Eq. (1). Scatchard graph was plotted based on Eq. (2). As can be seen from Fig. 4b, the relationship between Q/C and Q could be expressed using two straight lines, which illustrated that two kinds of different binding sites existed in MMIPs. For the two kinds of binding sites, the linear regression equation for the left part of the cure was Q/C = −0.0983Q + 1.4534 when the initial concentration was in the range of 1–100 μg mL−1; K d and Q max were 10.17 mg L−1 and 14.79 mg g−1, respectively. The linear regression equation for the right part of the cure was Q/C = −0.0085Q + 0.6623 when the initial concentration was in the range of 100–200 μg mL−1, K d and Q max were 117.65 mg L−1 and 77.92 mg g−1, respectively.
While for MNIPs, as shown in Fig. 4c, the relationship between Q/C and Q could be expressed using a liner equation. The equation was Q/C = −0.0042Q + 0.1120 when the initial concentration was in the range of 1–200 μg mL−1. K d and Q max were 238.10 mg L−1 and 26.67 mg g−1, respectively.
Kinetics Adsorption Experiment
Kinetics adsorption experiment shown in Fig. 5a was carried out. In this study, two of the most widely used kinetic models, i.e., pseudo-first-order equation and pseudo-second-order equation were used to research the adsorption kinetic behaviors of CPF onto MMIPs and MNIPs. The pseudo-first-order kinetic model is expressed by the following equation (Chen and Wang 2006):
where Q eq and Q t are the amount of CPF adsorbed (mg g−1) at equilibrium and time t (min), respectively. K 1 is the equilibrium rate constant of pseudo-first-order sorption (min−1). Q e is the theoretic adsorption capacity of the pseudo-first-order kinetic model. The values of K 1 and Q e can be calculated from the slope and intercept of the linear line plotted in versus t. The binding of CPF to the MMIPs and MNIPs were analyzed by pseudo-first-order kinetic model and described in Fig. 5b. The linear regression equations of the curves were as follows:
Equations (4) and (5) referred to MMIPs and MNIPs, respectively.
Another kinetic model is pseudo-second-order model, which is expressed as follow (Sheng et al. 2009):
where K 2 is the equilibrium rate constant of pseudo-second-order adsorption (g mg−1 min−1). Q e is the theoretic adsorption capacity of the pseudo-second-order kinetic model. The slope and intercept of the plot of t/Q t versus t are used to calculate K 2 and Q e . The binding of CPF to the MMIPs and MNIPs were analyzed by pseudo-second-order kinetic model and described in Fig. 5c. By calculating, the equations were as follows:
Equations (7) and Eq. (8) referred to MMIPs and MNIPs, respectively.
Different kinetic parameters were shown in Table 1. The best-fit model was selected based on the linear regression correlation coefficient (R 2) values. All the experimental data of CPF adsorption onto MMIPs and MNIPs showed better compliance with pseudo-second-order kinetic model in terms of higher R 2 values and closer values between Q e and Q eq.
Selective Adsorption for CPF
The static distribution coefficient (δ), separation factor (α), and relative separation factor (β) were used to evaluate the selectivity of MMIPs (Tan et al. 2008).
where C p and C s are the adsorbed and unadsorbed concentration, respectively. δ reflects the adsorption capacity. The bigger is δ, the stronger is the adsorption capacity.
where δ 1 and δ 2 are the static distribution coefficients of CPF and acephate. The parameter α embodies the selectivity.
where α 1 and α 2 are the separation factors of MMIPs and MNIPs, respectively. The parameter β characterized the selective difference between MMIPs and MNIPs. The bigger is β, the stronger is the selectivity.
The data of the selectivity are listed in Table 2. According to the values of δ, a marked preference of CPF was observed for MMIPs while there was a little difference in rebinding acephate and CPF for MNIPs. The value of β was 6.179, confirming again that MMIPs had a greater selectivity than the MNIPs. This might have resulted from the imprinting effect. During the preparation of MMIPs, the template of CPF was incorporated into organic networks. After the removal of CPF, specific binding sites in a predetermined orientation were formed, whereas the MNIPs had no such imprinted cavities and specific binding sites.
Extraction Conditions
The extraction conditions were optimized by analyzing the rice samples. According to the National standard method used in China, ethyl acetate was chosen as extraction solvent for the extraction of CPF from rice samples (Ministry of Health of the People’s Republic of China 2008). The extraction solvent volume and extraction time were optimized, and the results showed that CPF can be extracted from the rice by 10 mL ethyl acetate assisted with ultrasound for 10 min. After the extraction of CPF from rice, 10 % aqueous methanol was used for rebinding CPF. This is because the result of experiment indicated that complete rebinding of CPF could be obtained with this solvent.
The Amount of MMIPs
During the absorption procedure, the MMIPs were used to rebind the CPF. The optimal amount of sorbents required getting efficient recovery was investigated. The amounts of MMIPs ranging from 1 to 6 mg were applied. Figure 6a showed that the adsorption capacity of MMIPs increased with increase in dosage in <5 mg. Five milligrams MMIPs almost enabled to get complete recovery of CPF.
The recycling of the sorbent was then studied, and the results showed that the sorbent could be used at least five times with the satisfied recoveries in the range of 83.6–90.5 %.
Shaking Time
The experimental results indicated that the shaking time had an effect on the adsorption of target analyte. The adsorption process of CPF must have enough time to approach equilibrium to obtain satisfactory recovery. In this study, the shaking time ranging from 1 to 15 min were investigated. The result is shown in Fig. 6b, which showed that 10 min was sufficient to achieve satisfactory adsorption, and the increase in the shaking time beyond 10 min could not improve the recoveries.
Washing Solvent
Due to the rice samples contained complex matrix components, a washing step was required to remove the interfering compounds from sample matrixes without desorbing the target analyte. Different percentages of aqueous methanol were used as the washing solution. As shown in Fig. 6c, the recoveries of CPF decreased when methanol was more than 20 % in the washing solution. Finally, 3 mL 20 % aqueous methanol was used as washing solution.
Elution Solvent
Three different organic solvents (ethanol, methanol, and acetonitrile), acetic acid/methanol (1:99, 3:97 and 5:95, v/v), acetic acid/ethanol (1:99, 3:97, and 5:95, v/v), acetic acid/acetonitrile (1:99, 3:97, and 5:95, v/v) were used as elution solvents to investigate their impacts on the process of extraction. As can be seen from Fig. 6d, different solvents showed different elution efficiency. The lower recovery of CPF was obtained using organic solvents without acetic acid. In the study, acetic acid/methanol (3:97, v/v) was the choice of elution solvent.
Elution Times
In order to obtain the best elution efficiency, elution times were investigated. One milliliter elution solvent was used every time for eluting CPF. The result, shown in Fig. 6e, indicated that CPF was almost eluted from the MMIPs when the polymers were eluted three times.
Method Validation
The linear range of CPF was 0.025–10 μg g−1 and the correlation coefficient was 0.999. The LOD and LOQ of CPF obtained in this study were 0.0072 and 0.024 μg g−1, respectively. The maximum residue limit (MRL) of CPF established by China (Ministry of Health of the People’s Republic of China 2005) and Codex (Codex Alimentarious Commission 2010) were 0.1 and 0.05 mg kg−1, respectively. Compared with them, the value of LOD proved that the method was sensitive.
The relative standard deviations (RSDs) of intra- and interday ranging from 2.4 to 4.6 % and 3.5 to 7.3 % were obtained, respectively. In all three fortified levels, recovery of CPF was in the range of 81.2–92.1 %. SANCO provided that RSD must be below 20 % and the recovery should be in the range of 70–120 % (SANCO 2011). RSD and recovery in the work satisfied the requirements of SANCO, which illustrated that the method was feasible.
The analytical results obtained by the proposed method were compared with those obtained by the methods used in the literatures for analyzing CPF (Gelsomino et al. 1997; Hou et al. 2013; Tanaka et al. 2007; Viana et al. 1996; Zhang et al. 2010, 2012). The results presented in Table 3 indicated that the proposed method provided similar sensitivity, recovery, and precision. However, the sample preparation time and consumption of solvent were decreased in our method. Moreover, MMIPs prepared in this work have selective molecular cognition sites, which can adsorb CPF selectively. The magnetic polymers adsorbing analytes were easily collected by an external magnetic field without any additional centrifugation or filtration, which makes separation easier and faster. The dosage of sorbent in this study (5 mg per 2.0 g sample) was lower than that of other studies. It indicates that the MMIPs have high adsorption ability for CPF.
Application to Real Samples
To demonstrate the application of the method, three rice samples were detected. CPF residues at detectable levels were not found in these samples. The recovery of CPF was studied by analyzing the spiked samples under the optimized conditions. The level of CPF added into the samples was 2.5 μg g−1. The following steps were according to the procedure described in experiment. The recoveries of CPF obtained were 87.7 ± 4.2 %, which indicated good accuracy of the method.
Conclusion
In the study, MMIPs were synthesized using CPF as template. The obtained MMIPs were characterized by SEM, FTIR, PPMS, and TGA. The high adsorption capacity and good selectivity were obtained for CPF with MMIPs. The equilibrium data of MMIPs was well described by Scatchard analysis. The adsorption kinetics was fitted to the mechanism of the pseudo-second-order equation. The method based on coupling of MMIPs extraction with HPLC detection was successfully applied in analyzing CPF in rice samples. The MMIPs not only provided a convenient separation but also overcame problems of low selectivity and high operating cost.
References
Ai KL, Liu YL, Lu LH (2009) Hydrogen-bonding recognition-induced color change of gold nanoparticles for visual detection of melamine in raw milk and infant formula. J Am Chem Soc 131:9496–9497
Alexander C, Andersson HS, Andersson LI, Ansell RJ, Kirsch N, Nicholls IA, Mahony JO, Whitcombe MJ (2006) Molecular imprinting science and technology: a survey of the literature for the years up to and including 2003. J Mol Recognit 19:106–180
Anastassiades M, Lehotay SJ, Štajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86:412–431
Baldim IM, Souza MCDE, Souza JCJDC, Figueiredo EC, Martins I (2012) Application of the molecularly imprinted solid-phase extraction to the organophosphate residues determination in strawberries. Anal and Bioanal Chem 404:1959–1966
Chen C, Wang X (2006) Adsorption of Ni(II) from aqueous solution using oxidized multiwall carbon nanotubes. Ind Eng Chem Res 45:9144–9149
Chen LG, Liu J, Zeng QL, Wang H, Yu AM, Zhang HQ, Ding L (2009a) Preparation of magnetic molecularly imprinted polymer for the separation of tetracycline antibiotics from egg and tissue samples. J Chromatogr A 1216:3710–3719
Chen LG, Zhang XP, Sun L, Xu Y, Zeng QL, Wang H, Xu HY, Yu AM, Zhang HQ, Ding L (2009b) Fast and selective extraction of sulfonamides from honey based on magnetic molecularly imprinted polymer. J Agric Food Chem 57:10073–10080
Chen LG, Zhang XP, Xu Y, Du XB, Sun X, Sun L, Wang H, Zhao Q, Yu AM, Zhang HQ, Ding L (2010) Determination of fluoroquinolone antibiotics in environmental water samples based on magnetic molecularly imprinted polymer extraction followed by liquid chromatography–tandem mass spectrometry. Anal Chim Acta 662:31–38
Cherta L, Beltran J, López F, Hernández F (2012) Application of fast gas chromatography–mass spectrometry in combination with the QuEChERS method for the determination of pesticide residues in fruits and vegetables. Food Anal Methods. doi:10.1007/s12161-012-9524-1
Cho CM, Mulchandani A, Chen W (2002) Bacterial cell surface display of organophosphorus hydrolase for selection screening of improved hydrolysis of organophosphate nerve agents. Appl Environ Microbiol 68:2026–2030
Codex Alimentarious Commission (CAC) (2010) Pesticide residues in food and feed. http://www.codexalimentarius.net/pestres/data/pesticides/details.html?id=17
Davies MP, Biasi VD, Perrett D (2004) Approaches to the rational design of molecularly imprinted polymers. Anal Chim Acta 504: 7–14
Deme P, Azmeera T, Kanjilal S, Jonnalagadda P, Upadhyayula VS (2012) LC-MS/MS determination of organophosphorus pesticide residues in coconut water. Food Anal Methods doi:10.1007/s12161-012-9523-2
Feás X, Seijas JA, Vázquez-Tato MP, Regala P, Cepeda A, Fente C (2009) Syntheses of molecularly imprinted polymers: Molecular recognition of cyproheptadine using original print molecules and azatadine as dummy templates. Anal Chim Acta 631:237–244
Gelsomino A, Petrovičová B, Tiburtini S, Magnani E, Felici1 M (1997) Multiresidue analysis of pesticides in fruits and vegetables by gel permeation chromatography followed by gas chromatography with electron-capture and mass spectrometric detection. J Chromatogr A 782:105–122
Guan WS, Lei JR, Wang X, Zhou Y, Lu CC, Sun SF (2013) Selective recognition of beta-cypermethrin by molecularly imprinted polymers based on magnetite yeast composites. J Appl Polym Sci doi:10.1002/APP.38879
He C, Long Y, Pan J, Li K, Liu F (2007) Application of molecularly imprinted polymers to solid-phase extraction of analytes from real samples. J Biochem Bioph Meth 70:133–150
He CY, Long YY, Pan JL, Li KA, Liu F (2008) A method for coating colloidal particles with molecularly imprinted silica films. J Mater Chem 18:2849–2854
Hou X, Han M , Dai X, Yang X, Yi S (2013) A multi-residue method for the determination of 124 pesticides in rice by modified QuEChERS extraction and gas chromatography–tandem mass spectrometry. Food Chem 138:1198–1205
Hu YL, Li YW, Liu RJ, Tan W, Li GK (2011) Magnetic molecularly imprinted polymer beads prepared by microwave heating for selective enrichment of β-agonists in pork and pig liver samples. Talanta 84:462–470
Ji YS, Yin JJ, Xu ZG, Zhao CD, Huang HY, Zhang HX, Wang CM (2009) Preparation of magnetic molecularly imprinted polymer for rapid determination of bisphenol A in environmental water and milk samples. Anal Bioanal Chem 395:1125–1133
Kandimalla VB, Ju HX (2004) Molecular imprinting: a dynamic technique for diverse applications in analytical chemistry. Anal Bioanal Chem 380:587–605
Katz A, Davis ME (1999) Investigations into the mechanisms of molecular recognition with imprinted polymers. Macromolecules 32: 4113–4121
Kirsch N, Hart JP, Bird DJ, Luxton RW, Mccalley DV (2001) Towards the development of molecularly imprinted polymer based screen-printed sensors for metabolites of PAHs. Analyst 126:1936–1941
Lee WJ, Blair A, Hoppin JA, Lupin JH, Ruseicki JA, Sandler DP, Dosemei A, Alavanza MC (2004) Cancer incidence among pesticide applicators exposed to chlorpyrifos in the agricultural health study. J Natl Cancer Inst 96:1781–1789
Li Y, Yang T, Qi X, Qiao Y, Deng A (2008) Development of a group selective molecularly imprinted polymers based solid phase extraction of malachite green from fish water and fish feed samples. Anal Chim Acta 624:317–325
Lisa MK, Evangelyn CA (2005) A molecularly imprinted polymer on indium tin oxide and silicon. Biosens Bioelectron 20:2163–2167
Liu J, Yang M, Huai LF (2010) Molecularly imprinted solid-phase extraction for chlorpyrifos determination in water samples. Adv Mater Res 113–116:947–951
Malitesta C, Mazzotta E, Picca RA, Poma A, Chianella I, Piletsky SA (2012) MIP sensors - the electrochemical approach. Anal Bioanal Chem 402:1827–1846
Marchis D, Ferro GL, Brizio P, Squadrone S, Abete MC (2012) Detection of pesticides in crops: A modified QuEChERS approach. Food Control 25:270–273
McKellar RL, Dishburger HJ, Rice JR, Craig LF, Pennington J (1976) Residues of chlorpyrifos, its oxygen analog and 3,5,6-trichloro-2-pyridinol in milk and cream from cows fed chlorpyrifos. J Agric Food Chem 24:283–286
Ministry of Health of the People’s Republic of China (2005) Maximum residue limits for pesticides in food (GB 2763-2005), Beijing
Ministry of Health of the People’s Republic of China (2008) Determination of organophosphorus pesticides residues in unpolished rice (GB/T 5009.207-2008), Beijing
Mohamed R, Richoz-Payot J, Gremaud E, Mottier P, Yilmaz E, Tabet JC, Guy PA (2007) Advantages of molecularly imprinted polymers LC-ESI-MS/MS for the selective extraction and quantification of chloramphenicol in Milk-Based matrixes. Comparison with a classical sample preparation. Anal Chem 79:9557–9565
Omeroglu PY, Ambrus Á, Boyacioglu D (2013) Estimation of sample processing uncertainty of large-size crops in pesticide residue analysis. Food Anal Methods 6:238–247
Ou J, Li X, Feng S, Dong J, Dong X, Kong L, Ye M, Zou H (2007) Preparation and evaluation of a molecularly imprinted polymer derivatized silica monolithic column for capillary electrochromatography and capillary liquid chromatography. Anal Chem 79:639–646
Pasetto P, Maddock SC, Resmini M (2005) Synthesis and characterisation of molecularly imprinted catalytic microgels for carbonate hydrolysis. Anal Chim Acta 542:66–75
Payá P, Anastassiades M, Mack D, Sigalova I, Tasdelen B, Oliva J, Barba A (2007) Analysis of pesticide residues using the quick easy cheap effective rugged and safe (QuEChERS) pesticide multiresidue method in combination with gas and liquid chromatography and tandem mass spectrometric detection. Anal Bioanal Chem 389:1697–1714
Puoci F, Cirillo G, Curcio M, Parisi OI, Spizzirri UG, Picci N (2009) New restricted access materials combined to molecularly imprinted polymers for selective recognition/release in water media. Eur Polym J 45:1634–1640
SANCO (Directorate General for Health and Consumer Affairs) (2011) Method validation and quality control procedures for pesticide residues analysis in food and feed. SANCO 12495/2011.http://ec.europa.eu/food/plant/protection/pesticides/docs/qualcontrol_en.pdf
Schirmer C, Meisel H (2009) synthesis and evaluation of molecularly imprinted polymers (MIP) with affinity for the polypeptide nisin. Food Anal Methods 2:257–263
Sheng G, Wang S, Hu J, Lu Y, Li J, Dong Y, Wang X (2009) Adsorption of Pb(II) on diatomite as affected via aqueous solution chemistry and temperature. Colloids Surf A 339:159–166
Tamayo FG, Turiel E, Martín-Esteban A (2007) Molecularly imprinted polymers for solid-phase extraction and solid-phase microextraction: Recent developments and future trends. J Chromatogr A 1152:32–40
Tan CJ, Wang S, Bai RB, Tong YW (2008) Defining the interactions between proteins and surfactants for nanoparticle surface imprinting through miniemulsion polymerization. Chem Mater 20:118–127
Tanaka T, Hori T, Asada T, Oikawa K, Kawata K (2007) Simple one-step extraction and cleanup by pressurized liquid extraction for gas chromatographic–mass spectrometric determination of pesticides in green leafy vegetables. J Chromatogr A 1175:181–186
Urraca JL, Moreno-Bondi MC, Hall AJ, Sellergren B (2007) Direct extraction of penicillin G and derivatives from aqueous samples using a stoichiometrically imprinted polymer. Anal Chem 79:695–701
Viana E, Molto JC, Font G (1996) Optimization of matrix solid-phase dispersion method for the analysis of pesticide residues in vegetables. J Chromatogr A 754:437–444
Vidal JLM, González FJE, Galera MM, Cano MLC (1998) Diminution of chlorpyrifos and chlorpyrifos oxon in tomatoes and green beans grown in greenhouses. J Agric Food Chem 46:1440–1444
Wagner G, Desaules A, Muntau H, Theocharopoulos S, Quevauviller P (2001) Harmonisation and quality assurance in pre-analytical steps of soil contamination studies-conclusions and recommendations of the CEEM soil project. Sci Total Environ 264:103–117
Wulff G (2002) Enzyme-like catalysis by molecularly imprinted polymers. Chem Rev 102:1–27
Xie JC, Chen LR, Li CX, Xu XJ (2003) Precise gas chromatography with retention time locking in comprehensive toxicological screening for drugs in blood. J Chromatogr B 788:233–242
Xu Z, Fang G, Wang S (2010) Molecularly imprinted solid phase extraction coupled to high-performance liquid chromatography for determination of trace dichlorvos residues in vegetables. Food Chem 119:845–850
Zhang W, Qin L, He X W, Li WY, Zhang YK (2009a) Novel surface modified molecularly imprinted polymer using acryloyl-β-cyclodextrin and acrylamide as monomers for selective recognition of lysozyme in aqueous solution. J Chromatogr A 1216:4560–4567
Zhang Y, Liu RJ, Hu YL, Li GK (2009b) Microwave heating in preparation of magnetic molecularly imprinted polymer beads for trace triazines analysis in complicated samples. Anal Chem 81:967–976
Zhang C, Zhang X, Tian Z, He D, Liu X (2010) Degradation of chlorpyrifos and fipronil in rice from farm to fork and risk assessment. Agr Sci China 9:754–763
Zhang X, Shen Y, Yu X, Liu X (2012) Dissipation of chlorpyrifos and residue analysis in rice, soil and water under paddy field conditions. Ecotox Environ Safe 78:276–280
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 21205010) and Fundamental Research Funds for the Central Universities (no. DL10DB01).
Conflict of Interest
Guifu Ma and Ligang Chen declare that they have no conflicts of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, G., Chen, L. Determination of Chlorpyrifos in Rice Based on Magnetic Molecularly Imprinted Polymers Coupled with High-Performance Liquid Chromatography. Food Anal. Methods 7, 377–388 (2014). https://doi.org/10.1007/s12161-013-9636-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-013-9636-2