Abstract
A magnetic molecularly imprinted polymer (MMIP) was successfully synthesized and applied as the sorbent in the magnetic dispersive solid-phase microextraction (MDSPME) to separate and preconcentrate diazinon from real samples prior to its determination by high-performance liquid chromatography with ultraviolet detector (HPLC-UV). The MMIP was prepared by one-step surface imprinting technique using precipitation polymerization method without any use of surfactants or stabilizers. The functionalized Fe3O4 nanoparticles were used as the magnetic supporter, diazinon as the template, methacrylic acid (MAA) as the functional monomer, and ethylene glycol dimethacrylate (EGDMA) as the cross-linker. The synthesized MMIP was characterized by Fourier transform infrared (FT-IR) spectroscopy, scanning electron microscopy (SEM), X-ray diffraction (XRD) spectroscopy, and Brunauer–Emmett–Teller (BET) analysis. The selectivity study demonstrated that the MMIP had high affinity toward diazinon compared to other organophosphates (fenitrothion and chlorpyrifos). Various parameters affecting the efficiency of extraction such as sorption and desorption time, amount of sorbent, type and volume of eluting solvent, and pH were investigated and optimized. Under the optimum conditions, the calibration graph was linear over the range of 0.07–30.0 μg L−1 with the limit of detection of 0.02 μg L−1. The relative standard deviations (RSDs) at the 0.1 and 10.0 μg L−1 levels of diazinon (n = 5) were 3.8 and 2.0 %, respectively. The proposed method was successfully applied to the determination of trace amount of diazinon in tomato, cucumber, apple, and well water samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organophosphorous pesticides (OPPs) are a group of pesticides which are extensively used to control or eliminate the pest from agricultural products in order to improve the quantity and quality of foodstuffs (Kosikowska and Biziuk 2010). On the other hand, these compounds are hazardous for humans, environment, and animals due to their accumulation and potentially toxic effects on living organisms (Vasilić et al. 1999). During the past decade, the mutagenic, carcinogenic (Sanghi and Tewari 2001), cytotoxic (Wagner et al. 2005), and teratogenic (Kang et al. 2004) effects of OPPs have been proven. OPPs enter the human body through the water cycles and food chain. O,O-Diethyl O-[4-methyl-6-(propan-2-yl)pyrimidin-2-yl] phosphorothioate (diazinon) is one of the most important organophosphorous insecticides frequently used to control insects in agriculture (Drufovka et al. 2008). Diazinon acts as the inhibitor of the enzyme acetyl cholinesterase (AChE) when absorbed by human organism, and exposure to high level of this compound leads to reduction of neurotransmitter activity and causes unalterable effects on the nervous system (Maddy et al. 1986; Maroni et al. 1990). The maximum residue limit (MRL) of diazinon in food set by the Codex Alimentarious Commission and European Council (EC) regulation No 396/2005 is 0.01–5 mg kg−1 (Codex pesticides residues in food online database and Session 1997). Thus, continued monitoring and determination of diazinon by a sensitive, fast, and selective analytical procedure in food, environmental, and biological matrices are a challenging task.
Gas chromatography (GC) and high-performance liquid chromatography (HPLC) are the most commonly used instruments for determination of OPPs. However, because of the low concentration of analytes and complexity of the matrix, a sample preparation step is often required prior to their determination. Several pretreatment techniques, such as hollow-fiber-protected liquid-phase microextraction (Berhanu et al. 2008), cloud point extraction (Jia et al. 2008), dispersive liquid–liquid microextraction (Farajzadeh et al. 2011), stir bar sorptive extraction (Camino-Sánchez et al. 2013), supercritical fluid extraction (Norman and Panton 2001), matrix solid-phase dispersion (Kristenson et al. 2004), microwave-assisted extraction (Coscollà et al. 2011), solid-phase microextraction (SPME) (Volante et al. 2001), and solid-phase extraction (SPE( (Zhang et al. 2013), have been used for the extraction, cleanup, and preconcentration of diazinon from real samples before its quantification. Among these methods, SPE and SPME are the preferred sample pretreatment techniques because of their simplicity, low consumption of organic solvent, high recovery, and possibilities of preconcentration and matrix removal of trace analyte in a short time (Araújo et al. 2010; Ghaedi et al. 2014; Roosta et al. 2014; Shakerian et al. 2008). However, the selection of a proper sorbent is the key feature in the design of a SPE or SPME system, as the selectivity, simplicity of the operation, and the flexibility of the working conditions of these techniques are dependent on the sorbent material. Among different sorbents used in SPE and SPME, molecular imprinted polymers (MIPs) have attracted much attention due to their high thermal, chemical, and mechanical stability, predictable specific recognition, reusability, ease of preparation, convenience, and high selectivity in the extraction of analyte from the real samples with complex matrices (Behbahani et al. 2014; Kazemi et al. 2015; Pebdani et al. 2015; Xu et al. 2014; Yuan et al. 2012). MIPs are synthetic polymers that are prepared by the cross-linking of functional monomers in the presence of target analyte as the template molecule. After removal of the template molecule by proper eluent, the imprinted cavities are formed that are complementary in size, shape, and functionality to the analyte (Chen et al. 2011; Xu et al. 2013). However, one of the limitations of MIPs in their applications as the sorbent in SPE or SPME is their small particle sizes, which makes their separation from aqueous samples difficult. Magnetic nanoparticles (MNPs) are suitable sorbents for solid-phase extraction, because they can be quickly separated by application of an external magnetic field after they are dispersed in the solution (He et al. 2012). The selectivity of magnetic nanoparticles (MNPs) has been improved through their modification with various compounds including MIPs (Sadeghi and Aboobakri 2012). Compared with conventional MIPs, magnetic molecularly imprinted polymers (MMIPs) have the advantages of selective recognition in sorption of target molecule as well as ease of separation by use of an external magnetic field. Furthermore, the surface polymerization is improved and the number of imprinting sites of polymer is increased due to high surface-to-volume ratio of MNPs (Chang et al. 2012; Li et al. 2009; Lu et al. 2007; Ojeda and Rojas 2009). MMIPs show high selectivity, more accessible sites, fast mass transfer, and powerful anti-interference ability. MMIPs have been synthesized and used in the separation and determination of some compounds including pesticides (Chen et al. 2009; Hu et al. 2011; Ji et al. 2009; Lu et al. 2012; Pan et al. 2011; Zhang et al. 2009).
According to our literature survey, some molecular imprinted polymers for diazinon have been synthesized and applied as the sorbent in SPE and SPME of this pestiside (Bayat et al. 2015; Wang et al. 2013). The MIPs are usually prepared by two protocols, namely, free-radical polymerization and sol-gel process. The sol-gel method is a two-stage, time-consuming, and difficult process which requires surfactant or stabilizer that can remain adsorbed to the surface of the polymer, interfering with the selective binding of the target to the imprinted materials. The free radical polymerization includes bulk polymerization, suspension polymerization, emulsion polymerization, seed polymerization, and precipitation polymerization (Chen et al. 2011). Among these methods, the precipitation polymerization has the advantages of one-step synthesis without the need of surfactant or stabilizer and produces high-quality, uniform, and spherical imprinted particles and requires no crushing and sieving step (Tse Sum Bui and Haupt 2010). However, it has the drawback of the use of higher amounts of porogen solvents (Garcia et al. 2011). Recently, Zare et al. (2015) synthesized a MMIP based on Fe3O4@PEG for diazinon, using sol-gel method. In this work, a new MMIP for diazinon based on Fe3O4@SiO2 nanoparticles is synthesized by precipitation method. The sorbent was then used in the development of a simple, selective, and relatively fast magnetic dispersive solid-phase microextraction (MDSPME) method for the separation, cleanup, and preconcentration of trace amounts of diazinon from various matrices. The preconcentrated analyte was determined by high-performance liquid chromatography with ultraviolet detector (HPLC-UV). The synthesized MMIP was characterized by Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM), and the conditions of extraction and preconcentration were optimized by the univariable method. Finally, the applicability of the method in the determination of diazinon in tomato, cucumber, apple, and well water samples was studied.
Material and Methods
Reagents and Chemicals
Organophosphorus pesticides (OPPs), diazinon, fenitrothion, and chlorpyrifos were purchased from Sigma-Aldrich Company (St. Louis, MO, USA, www.sigmaaldrich.com). Stock standard solution of each OPP (1000 mg L−1) was prepared in methanol and was stored at −4 °C. Working solutions were prepared daily from the stock solutions by serial dilutions with methanol. Methacrylic acid (MAA), ethylene glycol dimethacrylate (EGDMA), ferric chloride hexahydrate (FeCl3·6H2O), ferrous chloride tetrahydrate (FeCl2·4H2O), tetraethyl orthosilicate (TEOS), chloroform, phosphoric acid, acetic acid, boric acid, 2-propanol, ammonia solution (25 %, w/w), analytical-grade anhydrous magnesium sulfate (MgSO4), sodium chloride (NaCl), HPLC-grade acetonitrile (ACN), and methanol were obtained from Merck Company (Darmstadt, Germany, www.merck-chemicals.com). 2,2-Azobisisobutyronitrile (AIBN) was purchased from the ACROS Company (Acros, USA, www.acros.com). High-purity water (18.2 MΩ cm, Millipore Milli-Q water purification system, Bedford, MA, USA) was used for the preparation of all aqueous solutions. Filtering of all solutions was carried out using a Millipore 0.45-μm-pore-size membrane (Bedford, MA, USA).
To prepare the Britton–Robinson buffer solution, 100 mL of a solution of phosphoric, acetic, and boric acids, each 0.2 mol L−1, was prepared and the pH was adjusted to 9.0 with sodium hydroxide.
Apparatus
HPLC analyses were performed using a Sykam high-performance liquid chromatograph (Eresing, Germany), equipped with a vacuum degasser, a S2100 quaternary pump, ODS C18 column (250 mm × 4.6 mm i.d., 5 μm), and a UV/Vis detector (Sykam, S3210, Germany) set to 245 nm. A personal computer equipped with a Clarity program for LC was used to process chromatographic data. The sample loop volume was 20.0 μL and the mobile-phase composition was a mixture of (70:15:15, v/v/v) ACN–methanol–water with a flow rate of 1 mL min−1. The column temperature was 25 °C, and a 25-μL LC microsyringe (ILS, Stutzerbach, Germany) was used for sample injection. The synthesized sorbent was characterized by scaning electron microscope (SEM, JEOL, JSM-7001 F, Japan) and Fourier transform infrared (FT-IR; Thermo Scientific, Nicolet iS10, USA). Ultraviolet analyses were carried out using a Cary 100 UV-visible spectrophotometer (Varian, Australia). Nitrogen sorption isotherms were obtained at 77 K with a Monosorb Autosorb (Monosorb Autosorb, Quantachrome, USA). X-ray diffraction (XRD) pattern was recorded using a Rigaku D-max XRD equipped with Cu Kα radiation (Canada, USA). The pH measurements were carried out using a pH meter (AZ 86502 pH meter, Mainland China) equipped with a combined glass-calomel electrode. Besides, an ultrasonic bath (Elmasonic, Singen/Htw, Germany) and a mechanical stirrer (Shengtang, Jintan, China) were also used throughout this study.
Preparation of Magnetic Imprinted Polymer
Preparation of Magnetic Nanoparticles
The Fe3O4 nanoparticles were prepared using chemical co-precipitation method (Wang et al. 2009). Briefly, 3.15 g FeCl3·6H2O and 1.15 g FeCl2·4H2O were dissolved in 50.0 mL of deoxygenated water with vigorous stirring at 80 °C. Then, 7.0 mL of ammonia solution (25 %, w/w) was added drop-wise to the clear yellow solution which was stirred at 300 rpm under nitrogen gas. In this stage, the solution turned black and the nanoparticles (NPs) were formed. Then, the black mixture was stirred vigorously for 30 min at 80 °C until the crystallization of NPs was completed. The precipitate was then collected by a magnet, washed repeatedly with deionized water, and finally dried under vacuum for the further use.
Synthesis of Fe3O4@SiO2
The Fe3O4 NPs (0.5 g) were dispersed in 80.0 mL of 2-propanol and 10.0 mL of water in an ultrasound bath for 15 min. Then, 6.0 mL of ammonia solution (25 % w/w) and 2.0 mL of TEOS were added, respectively. The mixture was stirred for 12 h at the room temperature, and the Fe3O4@SiO2 NPs formed were separated by an external magnetic field. They were then thoroughly washed with ethanol and water and dried under vacuum.
Preparation of Magnetic Molecular Imprinted Nanoparticles of Diazinon
Diazinon (0.2 mmol) as the template molecule and MAA (2.0 mmol) as the functional monomer were dissolved in 20.0 mL of chloroform in a 250-mL round bottom flask and was kept at −4 °C for 2 h. The mixture was then placed in a shaker and was mixed at 300 rpm for 1 h at room temperature until a preassembly solution was formed. Five hundred milligrams of Fe3O4@SiO2 and 20.0 mmol of EGDMA were dissolved in 30.0 mL of chloroform by sonication, and the mixture was added to the preassembly solution purged with nitrogen in an ice bath. After being subjected to ultrasonic waves for 15 min, 20.0 mg of the AIBN as the initiator was added to the mixture. The reaction was then carried out at 60 °C for 24 h under stirring. After the polymerization was completed, the synthesized magnetic polymer was separated from the mixture by a magnet. The diazinon molecules were then leached by washing the sorbent consecutively in soxhlet apparatus with methanol/acetic acid (9:1, v/v), and the removal of template molecule was monitored by measuring its absorption by UV spectrophotometry (at 245 nm) until the eluent was free of diazinon. Finally, the MMIP was washed with methanol and dried overnight under vacuum at 60 °C. For comparison, magnetic nonmolecularly imprinted polymer (MNMIP) was prepared under the same experimental conditions but without the target molecule.
Extraction Procedure
The pH of 5.0 mL of the standard or sample solution containing proper amount of diazinon was adjusted to 9.0 upon addition of 2.0 mL of Britton–Robinson (BR) buffer solution. Subsequently, 10.0 mg of the prepared MMIP was added to the solution, and the mixture was stirred by a mechanical stirrer for 15 min. Then, the sorbent was separated with a magnet and the decantation of the supernatant solution. The preconcentrated diazinon was eluted from the MMIP with 0.2 mL of (9:1, v/v) methanol/acetic acid by sonical agitation for 15 min. The sorbent was then separated by an external magnetic field, and the supernatant extract was introduced to HPLC for the analysis.
Preparation of Real Samples
Fruit Samples
The modified QUECHERS procedure (Baldim et al. 2012; Hassanzadeh et al. 2010) was used for digestion of samples. Thus, a 15.0-g portion of the thoroughly homogenized fruit samples (tomato, cucumber, or apple) was accurately weighed and transferred into a 50-mL polypropylene centrifuge tube. Then, 15.0 mL acetonitrile was added, the screw cap was closed, and the tube was vortexed vigorously for 10 min. After that, 1.0 g of NaCl and 4.0 g of anhydrous MgSO4 were added and the shaking process was repeated for 2 min. The tube was then centrifuged at 4000 rpm for 3 min, and the supernatant was collected by filtering the mixture through a membrane filter (0.45 μm). The acetonitrile was evaporated and the solution was concentrated to 1 mL with a gentle stream of ultra-pure nitrogen gas. The resulting solution was diluted to 25 mL with a Britton–Robinson (BR) buffer solution (pH = 9.0), and 5.0 mL of it was subjected to the developed MDSPME.
Water Samples
Water samples were filtered through 0.45-μm Millipore filter; the pH was adjusted to 9.0 by BR buffer solution and was subjected to the developed MDSPME procedure for quantification of analyte.
Results and Discussion
Characterization of the MMIP
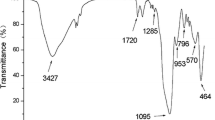
The synthesized sorbent was characterized by FT-IR spectroscopy. For this purpose, the spectra of Fe3O4, Fe3O4@SiO2, diazinon MMIP (before and after leaching), and diazinon magnetic nonimprinted polymer (MNIP) were recorded (Fig. 1). The absorption band of about 591 cm−1 in Fig. 1a is related to the Fe–O bond of Fe3O4; the appearance of peaks around 800, 950, and 1093 cm−1 in Fig. 1b is attributed to the stretching of Si–O, Si–O–H, and Si–O–Si, respectively, indicating the silanization of Fe3O4 nanoparticles. The peak at 1158 cm−1 in Fig. 1c–e indicates that SiO2 is embedded in MMIPs and MNIPs. The strong absorption bands around 1732 and 1259 cm−1 in Fig. 1c–e are assigned to C=O stretching vibration and C–O symmetric stretching vibration of ester (EGDMA), respectively, which display the successful polymerization process. The band around 3400–3600 cm−1 (Fig. 1c–e) corresponds to O–H stretching and is alike in MMIP after leaching and MNIP, whereas its intensity is lowered in unleached MMIP. This phenomenon can be related to the formation of hydrogen bond between template molecule (diazinon) and hydroxyl functional group of the sorbent in unleached MMIP which after removal of the template molecule, the hydroxyl group becomes free and so its intensity is increased.
The surface characterization of leached and unleached MMIP sorbent was investigated by scanning electron microscopy (SEM). As SEM image demonstrated (Fig. 2a), the sorbent is made of spherical nanoparticles with the average particle size of ∼70 nm in radius. Thus, the prepared sorbent can be classified as a nano-size selective sorbent for the extraction of trace amounts of diazinon. The pore size of diazinon imprinted polymer is in the nano-range (Fig. 2b), and comparison of the leached and unleached MMIP images indicates that the removal of the template molecule from the polymer results in an increase in its porosity.
The additional information about the surface area, pore volume, and mean pore diameter of the imprinted polymer was obtained by Brunauer–Emmett–Teller (BET) analysis. The BET surface area, total pore volume, and average pore diameter for MMIP were calculated to be 90.6 m2/g, 0.27 cm3/g, and 11.9 nm, respectively. These data confirm the formation of polymer with nano-pore size and the existence of imprinting effect in polymer. High total pore volume of MMIP results in higher extraction capacity of sorbent. Furthermore, the XRD patterns of MMIP (Fig. 3) show the eight characteristic reflection peaks of Fe3O4 with the miller indices of (220), (311), (400), (422), (333), (440), and (622) (JCPDS Card 79-0417) indicating the right synthesis of MMIP.
Optimization of the Extraction Procedure
Effect of pH
The pH of loading solution can affect the interaction of diazinon and sorbent through protonation/deprotonation of functional groups of the specific binding sites of the sorbent as well as the target molecules. Thus, the influence of pH on the extraction efficiency was investigated by varying the pH of sample solution in the range of 5.0–10.0 using suitable buffer. As it is demonstrated (Fig. 4), the peak area increased as the pH was increased from 5.0 to 9.0 and then decreased by further increase in pH. Thus, the pH of 9.0 was selected for all subsequent experiments. This observation can be related to the fact that diazinon is hydrolyzed under acidic or highly alkali conditions (pH >10) (Zare et al. 2015). Thus, as the cavities of the polymer were imprinted with the neutral form of diazinon, its hydrolyzed form cannot completely fit to the cavities. A similar behavior has been reported before (Baldim et al. 2012; Bayat et al. 2015)
Effect of Type and Volume of Eluent
The nature and volume of elution solvent have an important effect on the desorption of analyte from the MMIP and the efficiency of the preconcentration procedure. In this study, the capability of several solvents including methanol, acetic acid, mixture of methanol/acetonitrile (9:1, v/v), and mixture of methanol/acetic acid (9:1, v/v) in desorbing the analyte from the sorbent was examined. The peak area related to these eluents was as follows: methanol (5622 mv), acetic acid (5136 mv), methanol containing 10.0 % v/v acetonitrile (6135 mv), and methanol containing 10.0 % v/v acetic acid (7727 mv). According to the results, the mixture of methanol/acetic acid (9:1, v/v) was chosen as the eluent. The most likely explanation can be that acetic acid competes with analyte for the binding site which weakens the hydrogen bond between analyte and MMIP whereas methanol has high affinity for analyte through the formation of strong hydrogen bond which makes it the most effective eluent (Cacho et al. 2009; He et al. 2007; Peng-Ju et al. 2007).
The effect of the percentage of acetic acid in methanol was then studied by varying its amount from 0.0 to 20.0 %. It was found that an increase in the percentage of acetic acid of eluent up to 10.0 % causes an increase in the analyte signal which leveled off by further increase in the amount of acetic acid. Thus, the mixture of methanol/acetic acid (9:1, v/v) was capable of effective desorption of diazinon from the sorbent and was employed as the eluent in further studies.
The volume of eluent has an important role in the preconcentration factor of the procedure. The preconcentration factor will increase by decrease in the eluent volume, but it may decrease the recovery of the analyte from the sorbent. In order to optimize the eluent volume, different volumes of eluent in the range of 0.1–0.5 mL were evaluated, and it was found that 0.2 mL of eluent is adequate for complete desorption of diazinon which was selected as the optimum volume of eluent.
Effect of Sorption and Desorption Time
The sorption process must be long enough to guarantee the establishment of equilibrium between the samples and sorbent to obtain favorable extraction yield, but it must be short enough to have reasonable speed of analysis. Thus, in order to obtain the optimum extraction time, defined as the required time for mixing the sorbent and solution, different shaking times from 2.0 to 25 minutes were tested. The results (Fig. 5) showed that the peak area increases with an increase in extraction time up to 15 min and then becomes constant at higher shaking time. So, 15 min was selected as the optimum sorption time for the following experiments.
The effect of desorption times of analyte from the MMIP was also investigated in the range of 2.0–20 min. The results indicated that desorption time of 15 min is adequate for the complete removal of analyte from the sorbent.
Effect of Ionic Strength
The effect of the ionic strength on the extraction of diazinon was evaluated by performing the procedure in samples containing various amounts of NaCl (0.0–2.0 mol L−1). The results showed that the presence of salt has no significant effect on the recovery of analyte. Thus, the method is suitable for extraction of diazinon from saline samples such as seawater.
The Amount of MMIP
The mass of the sorbent must be sufficient for the complete and quantitative extraction of the target molecules. The effect of the amount of MMIP (4.0 to 20.0 mg) on the extraction of diazinon from 5.0 mL of sample was investigated. The results demonstrated that 8.0 mg of the sorbent is adequate for the quantitative extraction of the analyte. The use of a higher amount of sorbent had no significant effect on the recovery of diazinon. Therefore, 10.0 mg of sorbent was applied for subsequent studies.
Effect of Sample Volume
The sample volume is one of the most important parameters affecting the preconcentration factor of the method. The effect of volume of sample on the enhancement factor was studied. For this purpose, the extraction of 0.25 μg of diazinon from different aqueous volumes (2.5–30.0 mL) was investigated. The results (Fig. 6) showed that with up to 5 mL of aqueous phase, the peak area was constant and then decreased with the further increase in the sample volume. So, a sample volume of 5.0 mL was selected as the optimum for further studies. The preconcentration factor defined as the ratio of the optimum sample volume to the eluent volume was found to be 25.
Evaluation of Sorbent Capacity and Selectivity
For determination of the sorption capacity, 10.0 mg of MMIP was dispersed in 5.0 mL of diazinon solution (60 mg L−1) under optimum conditions of pH. After the sample was shaken for 25 min, the sorbent was separated by a magnet and the concentration of diazinon in the supernatant solution was determined by spectrophotometery. The capacity of MMIP for the diazinon was determined from the differences in the amount of analyte in the initial and final solution. The capacity of the MMIP for diazinon was found to be 24.6 mg g−1.
The sorption selectivity of synthesized MMIP and its corresponding MNIP for diazinon was studied by extraction of analyte from a binary mixture of diazinon and the organophosphate pesticides with similar molecular structure, namely, fenitrothion and chlorpyrifos. The results (Fig. 7) show that the MMIP has higher sorption affinity for the template molecule than MNIP, and the sorption affinity of MMIP for diazinon is remarkably greater than for the other examined organophosphate pesticides even with similar structures to diazinon. The high selectivity of MMIP toward diazinon can be attributed to the effective imprinting process of the synthetic sorbent.
Adsorption selectivity of the MMIP and MNIP for diazinon, fenitrothion, and chlorpyrifos. Conditions: pesticides concentration, 50 μg L−1; pH, 9; eluent, 0.2 mL of methanol/acetic acid (9:1, v/v); sorption time, 15 min; desorption time, 15 min; amount of the sorbent, 10 mg; sample volume, 5.0 mL; n = 3
Analytical Characteristic of the Proposed Method
The calibration graph was constructed by plotting the peak area against the concentrations of diazinon in initial solution using least squares regression analysis. Three replicate injections of the same standard solution of diazinon were done, and the results were averaged. The calibration graph exhibited linearity over the range of 0.07–30.0 μg L−1 of diazinon with the equation of A = 1530.3 C + 164.5 (r 2 = 0.9996) where C is the concentration of diazinon in μg L−1, and A is the peak area. The limit of detection (LOD) and the limit of quantification (LOQ) defined as 3S b/m and 10S b/m (where S b is the standard deviation of the blank and m is the slope of the calibration curve) were found to be 0.02 and 0.07 μg L−1, respectively. The relative standard deviations (RSDs) for five replicate measurements at the 0.1 and 10.0 μg L−1 levels of diazinon were 3.8 and 2.0 %, respectively. The enhancement factor defined as the ratio of the slope of calibration curves with and without preconcentration was found to be 19.4.
Analysis of Real Samples
The proposed method was applied to the determination of diazinon in different samples including tomato, cucumber, apple, and water samples. Three replicates of each sample were prepared and analyzed according to the given procedure. The accuracy of the method was examined through the recovery experiments by spiking the samples with different concentration levels of diazinon. The results of this study are summarized in Table 1 and show that the recoveries of the spiked samples are good (96.0–104.0 %). Thus, the method is suitable for determination of diazinon in the sample type examined.
Comparison of Developed Method with Others
The analytical performance of the developed MDSPME was compared with the other published methods for the determination of diazinon (Table 2). The results illustrate that the developed method has a wider linear dynamic range, lower detection limit, and a precision comparable with other reported methods. This is due to the surface imprinting conditions for magnetic imprinting polymerization that caused the molecular recognition system to be built on the surface of supported substrate resulting in the formation of a sorbent with faster mass transfer, higher binding capacity, and lower diffusion barrier. Furthermore, in comparison to MMIP prepared by Zare et al. (2015), the sorbent was synthesized by the precipitation polymerization method which produced high-quality, selective, uniform, and spherical imprinted particles resulting in the improvement of its efficiency.
Conclusions
A MMIP was synthesized by one-step surface imprinting technique using precipitation polymerization for the selective solid-phase extraction of diazinon prior to its determination by high-performance liquid chromatography with ultraviolet detector. The synthesized MMIP was characterized by Fourier transform infrared (FT-IR) spectroscopy, scanning electron microscopy (SEM), X-ray diffraction (XRD) spectroscopy, and Brunauer–Emmett–Teller (BET) analysis. Under the optimized conditions, the sorbent exhibited good chemical and physical stability, high capacity, and high selectivity toward template molecule; eliminated the centrifugation or filtration step; and, at the same time, accelerated the separation of sorbent from bulk solution by the use of an external magnetic field. The developed method was demonstrated to be rapid, accurate, and efficient for the determination of trace amounts of diazinon in tomato, cucumber, apple, and water samples.
References
Araújo CS, Alves VN, Rezende HC, Coelho NM (2010) Development of a flow system for the determination of low concentrations of silver using Moringa oleifera seeds as biosorbent and flame atomic absorption spectrometry. Microchem J 96:82–85
Bagheri H, Ayazi Z, Sistani H (2011) Chemically bonded carbon nanotubes on modified gold substrate as novel unbreakable solid phase microextraction fiber. Microchim Acta 174:295–301
Baldim IM, de Oliveira Souza MC, da Cunha Souza JCJ, Figueiredo EC, Martins I (2012) Application of the molecularly imprinted solid-phase extraction to the organophosphate residues determination in strawberries. Anal Bioanal Chem 404:1959–1966
Bayat M, Hassanzadeh-Khayyat M, Mohajeri SA (2015) Determination of diazinon pesticide residue in tomato fruit and tomato paste by molecularly imprinted solid-phase extraction coupled with liquid chromatography analysis. Food Anal Methods 8:1034–1041
Behbahani M, Salarian M, Bagheri A, Tabani H, Omidi F, Fakhari A (2014) Synthesis, characterization and analytical application of Zn (II)-imprinted polymer as an efficient solid-phase extraction technique for trace determination of zinc ions in food samples. J Food Compos Anal 34:81–89
Berhanu T, Megersa N, Solomon T, Jönsson JÅ (2008) A novel equilibrium extraction technique employing hollow fibre liquid phase microextraction for trace enrichment of freely dissolved organophosphorus pesticides in environmental waters. Int J Environ Anal Chem 88:933–945
Codex pesticides residues in food online database CAC, 22nd, Session (1997). http://www.codexalimentarius.net/mrls/pestdes/jsp/pest_qe.jsp (accessed on 09.01.2011)
Cacho C, Turiel E, Pérez-Conde C (2009) Molecularly imprinted polymers: an analytical tool for the determination of benzimidazole compounds in water samples. Talanta 78:1029–1035
Camino-Sánchez F, Zafra-Gómez A, Ruiz-García J, Vílchez J (2013) Screening and quantification of 65 organic pollutants in drinking water by stir bar sorptive extraction-gas chromatography-triple quadrupole mass spectrometry. Food Anal Methods 6:854–867
Chang L, Chen S, Li X (2012) Synthesis and properties of core-shell magnetic molecular imprinted polymers. Appl Surf Sci 258:6660–6664
Chen L, Liu J, Zeng Q, Wang H, Yu A, Zhang H, Ding L (2009) Preparation of magnetic molecularly imprinted polymer for the separation of tetracycline antibiotics from egg and tissue samples. J Chromatogr A 1216:3710–3719
Chen L, Xu S, Li J (2011) Recent advances in molecular imprinting technology: current status, challenges and highlighted applications. Chem Soc Rev 40:2922–2942
Coscollà C, Castillo M, Pastor A, Yusà V (2011) Determination of 40 currently used pesticides in airborne particulate matter (PM 10) by microwave-assisted extraction and gas chromatography coupled to triple quadrupole mass spectrometry. Anal Chim Acta 693:72–81
Drufovka K, Danevčič T, Trebše P, Stopar D (2008) Microorganisms trigger chemical degradation of diazinon. Int Biodeterior Biodegrad 62:293–296
Farajzadeh MA, Bahram M, Vardast MR, Bamorowat M (2011) Dispersive liquid-liquid microextraction for the analysis of three organophosphorus pesticides in real samples by high performance liquid chromatography-ultraviolet detection and its optimization by experimental design. Microchim Acta 172:465–470
Garcia R, Cabrita MJ, Freitas AMC (2011) Application of molecularly imprinted polymers for the analysis of pesticide residues in food—a highly selective and innovative approach. Am J Anal Chem 2:16–25
Ghaedi M, Ansari A, Habibi M, Asghari A (2014) Removal of malachite green from aqueous solution by zinc oxide nanoparticle loaded on activated carbon: kinetics and isotherm study. J Ind Eng Chem 20:17–28
Hassanzadeh N, Bahramifar N, Esmaili‐Sari A (2010) Residue content of carbaryl applied on greenhouse cucumbers and its reduction by duration of a pre‐harvest interval and post‐harvest household processing. J Sci Food Agric 90:2249–2253
He C, Long Y, Pan J, Li K, Liu F (2007) Application of molecularly imprinted polymers to solid-phase extraction of analytes from real samples. J Biochem Biophys Methods 70:133–150
He Z, Liu D, Li R, Zhou Z, Wang P (2012) Magnetic solid-phase extraction of sulfonylurea herbicides in environmental water samples by Fe3O4@dioctadecyl dimethyl ammonium chloride@silica magnetic particles. Anal Chim Acta 747:29–35
Hu Y, Li Y, Liu R, Tan W, Li G (2011) Magnetic molecularly imprinted polymer beads prepared by microwave heating for selective enrichment of β-agonists in pork and pig liver samples. Talanta 84:462–470
Ji Y, Yin J, Xu Z, Zhao C, Huang H, Zhang H, Wang C (2009) Preparation of magnetic molecularly imprinted polymer for rapid determination of bisphenol A in environmental water and milk samples. Anal Bioanal Chem 395:1125–1133
Jia G, Lv C, Zhu W, Qiu J, Wang X, Zhou Z (2008) Applicability of cloud point extraction coupled with microwave-assisted back-extraction to the determination of organophosphorous pesticides in human urine by gas chromatography with flame photometry detection. J Hazard Mater 159:300–305
Kang HG, Jeong SH, Cho JH, Kim DG, Park JM, Cho MH (2004) Chlropyrifos-methyl shows anti-androgenic activity without estrogenic activity in rats. Toxicology 199:219–230
Kazemi E, Shabani AMH, Dadfarnia S (2015) Synthesis and characterization of a nanomagnetic ion imprinted polymer for selective extraction of silver ions from aqueous samples. Microchim Acta 182:1025–1033
Kosikowska M, Biziuk M (2010) Review of the determination of pesticide residues in ambient air. TrAC Trends Anal Chem 29:1064–1072
Kristenson E, Shahmiri S, Slooten C, Vreuls R, Brinkman UT (2004) Matrix solid-phase dispersion micro-extraction of pesticides from single insects with subsequent GC–MS analysis. Chromatographia 59:315–320
Li L, He X, Chen L, Zhang Y (2009) Preparation of core‐shell magnetic molecularly imprinted polymer nanoparticles for recognition of bovine hemoglobin. Chem Asian J 4:286–293
Lu AH, Salabas EL, Schüth F (2007) Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed 46:1222–1244
Lu F, Li H, Sun M, Fan L, Qiu H, Li X, Luo C (2012) Flow injection chemiluminescence sensor based on core–shell magnetic molecularly imprinted nanoparticles for determination of sulfadiazine. Anal Chim Acta 718:84–91
Maddy K, Knaak J, Gibbons D (1986) Monitoring the urine of pesticide applicators in California for residues of chlordimeform and its metabolites 1982–1985. Toxicol Lett 33:37–44
Maroni M, Catenacci G, Galli D, Cavallo D, Ravazzani G (1990) Biological monitoring of human exposure to acephate. Arch Environ Contam Toxicol 19:782–788
Norman KN, Panton SH (2001) Supercritical fluid extraction and quantitative determination of organophosphorus pesticide residues in wheat and maize using gas chromatography with flame photometric and mass spectrometric detection. J Chromatogr A 907:247–255
Ojeda CB, Rojas FS (2009) Separation and preconcentration by dispersive liquid–liquid microextraction procedure: a review. Chromatographia 69:1149–1159
Pan J, Xu L, Dai J, Li X, Hang H, Huo P, Li C, Yan Y (2011) Magnetic molecularly imprinted polymers based on attapulgite/Fe3O4 particles for the selective recognition of 2, 4-dichlorophenol. Chem Eng J 174:68–75
Pebdani AA, Shabani AMH, Dadfarnia S, Khodadoust S (2015) Solid phase microextraction of diclofenac using molecularly imprinted polymer sorbent in hollow fiber combined with fiber optic-linear array spectrophotometry. Spectrochim Acta, Part A 147:26–30
Peng-Ju W, Jun Y, Qing-De S, Yun G, Xiao-Lan Z, Ji-Bao C (2007) Rapid removal of template from molecularly imprinted polymers by accelerated solvent extraction. Chin J Anal Chem 35:484–488
Ravelo‐Pérez LM, Hernández‐Borges J, Ángel Rodríguez‐Delgado M (2008) Multiwalled carbon nanotubes as solid‐phase extraction materials for the gas chromatographic determination of organophosphorus pesticides in waters. J Sep Sci 31:3612–3619
Roosta M, Ghaedi M, Shokri N, Daneshfar A, Sahraei R, Asghari A (2014) Optimization of the combined ultrasonic assisted/adsorption method for the removal of malachite green by gold nanoparticles loaded on activated carbon: experimental design. Spectrochim Acta, Part A 118:55–65
Sadeghi S, Aboobakri E (2012) Magnetic nanoparticles with an imprinted polymer coating for the selective extraction of uranyl ions. Microchim Acta 178:89–97
Sanagi MM, Salleh S, Ibrahim WAW, Naim AA, Hermawan D, Miskam M, Hussain I, Aboul-Enein HY (2013) Molecularly imprinted polymer solid-phase extraction for the analysis of organophosphorus pesticides in fruit samples. J Food Compos Anal 32:155–161
Sanghi R, Tewari V (2001) Monitoring of pesticide residues in summer fruits and vegetables from Kanpur. India Bull Environ Contam Toxicol 67:587–593
Shakerian F, Dadfarnia S, Shabani AMH, Rohani M (2008) MultiSimplex optimization of on-line sorbent proconcentration and determination of iron by FI-AAS and microcolumn of immobilized ferron. Talanta 77:551–555
Tse Sum Bui B, Haupt K (2010) Molecularly imprinted polymers: synthetic receptors in bioanalysis. Anal Bioanal Chem 398:2481–2492
Vasilić Ž, Štengl B, Drevenkar V (1999) Dimethylphosphorus metabolites in serum and urine of persons poisoned by malathion or thiometon. Chem Biol Interact 119:479–487
Volante M, Galarini R, Miano V, Cattaneo M, Pecorelli I, Bianchi M, Marinoni M, Cossignani L, Damiani P (2001) A SPME-GC-MS approach for antivarroa and pesticide residues analysis in honey. Chromatographia 54:241–246
Wagner E, McMillan S, Plewa M (2005) Cytotoxicity of organophosphorus ester (OP) insecticides and cytotoxic synergism of 2-acetoxyacetylaminofluorene (2AAAF) in Chinese hamster ovary (CHO) cells. Bull Environ Contam Toxicol 75:329–334
Wang X, Wang L, He X, Zhang Y, Chen L (2009) A molecularly imprinted polymer-coated nanocomposite of magnetic nanoparticles for estrone recognition. Talanta 78:327–332
Wang Y-L, Gao Y-L, Wang P-P, Shang H, Pan S-Y, Li X-J (2013) Sol–gel molecularly imprinted polymer for selective solid phase microextraction of organophosphorous pesticides. Talanta 115:920–927
Xu S, Lu H, Zheng X, Chen L (2013) Stimuli-responsive molecularly imprinted polymers: versatile functional materials. J Mater Chem C 1:4406–4422
Xu S, Guo C, Li Y, Yu Z, Wei C, Tang Y (2014) Methyl parathion imprinted polymer nanoshell coated on the magnetic nanocore for selective recognition and fast adsorption and separation in soils. J Hazard Mater 264:34–41
Yuan L, Ma J, Ding M, Wang S, Wu X, Li Y, Ma K, Zhou X, Li F (2012) Preparation of estriol–molecularly imprinted silica nanoparticles for determining oestrogens in milk tablets. Food Chem 131:1063–1068
Zare F, Ghaedi M, Daneshfar A, Ostovan A (2015) Magnetic molecularly imprinted polymer for the efficient and selective preconcentration of diazinon before its determination by high‐performance liquid chromatography. J Sep Sci 38:2797–2803
Zhang Y, Liu R, Hu Y, Li G (2009) Microwave heating in preparation of magnetic molecularly imprinted polymer beads for trace triazines analysis in complicated samples. Anal Chem 81:967–976
Zhang L, Wang Y, Sun C, Yang S, He H (2013) Simultaneous determination of organochlorine, organophosphorus, and pyrethroid pesticides in bee pollens by solid-phase extraction cleanup followed by gas chromatography using electron-capture detector. Food Anal Methods 6:1508–1514
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was not funded by any organization.
Conflict of Interest
Alireza Bazmandegan-Shamili declares that he has no conflict of interest. Shayessteh Dadfarnia declares that she has no conflict of interest. Ali Mohammad Haji Shabani declares that he has no conflict of interest. Mahboubeh Saeidi declares that she has no conflict of interest. Masoud Rohani Moghadam declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants and animal subjects.
Informed consent
Not applicable to this study.
Rights and permissions
About this article
Cite this article
Bazmandegan-Shamili, A., Dadfarnia, S., Haji Shabani, A.M. et al. High-Performance Liquid Chromatographic Determination of Diazinon after Its Magnetic Dispersive Solid-Phase Microextraction Using Magnetic Molecularly Imprinted Polymer. Food Anal. Methods 9, 2621–2630 (2016). https://doi.org/10.1007/s12161-016-0456-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0456-z