Abstract
The key factor in lignocellulosic biorefinery is to extract maximum sugars from biomass as feedstock for the process of fermentation. The major barrier in extracting the sugars is the presence of lignin of biomass that primarily restricts the accessibility of cellulose to enzymes. Therefore, it is important to locate the presence and morphology of lignin to assess the reaction severity. In most of the severe pretreatment conditions, lignin is observed to condense and form spherical droplets on the surface of treated biomass. Such formation of pseudo-lignin differs in the action of cellulose hydrolysis from that of the initial or residual lignin content of biomass. In view of that, the present review is focused on the impact of various pretreatment techniques on the morphological changes in lignin and its subsequent redeposition or redistribution effect towards enzymatic hydrolysis process. Initially, the effect of different pretreatment methods is assessed which primarily resulted into the occurrence of lignin redeposition on the surface and interior of biomass. Thereafter, an in-depth insight is provided to understand the underlying mechanism of such formations of pseudo-lignin under severe reaction conditions. Finally, the study is concluded discussing the future recommendation to circumvent the interference of pseudo-lignin on enzymatic reactions. Hence, the present review article will not only help the readers to gain the knowledge on the formations and problems of pseudo-lignin obtained from biomass but also details the latest advancements that are involved to overcome the inhibitory effect of pseudo-lignin during the enzymatic hydrolysis of biomass.
Highlights
-
The ultrastructural morphology of pseudo-lignin in the form of droplets are discussed.
-
The various pretreatment processes that are responsible for the formation of pseudo-lignin are described.
-
An insight on the reaction mechanisms is provided for the impact of pseudo-lignin towards enzymatic hydrolysis.
-
Advances processes to overcome the inhibitory effect are comprehensively summarized.
-
Associated challenges and future recommendations are demonstrated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primarily, lignin is one of the important constituents of plant cell walls which gives them rigidity and strength, protecting them from microbial attack [1]. Hence, lignin is the major reason for biomass recalcitrance creating challenges in its hydrolysis via enzymes and thereby its biorefining process [2]. Lignin is the 3rd most abundant biopolymer available on earth after cellulose and hemicellulose, and most abundant source of aromatics available. Lignin is mostly exploited as a starting material for the production of different commercial products [3, 4]. However, the presence of lignin is also observed as a barrier towards an efficient conversion of biomass to bioenergy. In order to make the conversion processes easier, a number of different pretreatment strategies are demonstrated for the removal of substantial portions of lignin from the biomass [5]. In view of that, it was observed that few of the techniques, employing acid and hot water pretreatment significantly, disrupts the biomass network with an insignificant removal of the lignin content [6,7,8]. So, an in-depth understanding on the ultrastructural complexity of the biomass is still unclear. To comprehend on the location and nature of lignin that generally stuck with the pretreated solids, a structural investigation was carried out to track the lignin of biomass [9]. It was claimed in the study that the heterogeneous nature of lignin is due to the variable composition. Primarily, the formation and deposition of lignin in biomass typically vary based on the cell and tissue type.
Over the years, the aim of most of the research activities are objected towards identifying the nature of lignin and development of the pretreatment processes that are effective in the removal of lignin. Among other additional factors, the close interaction of xylan and cellulose of biomass typically affects the process of enzymatic hydrolysis [10]. Further, alkaline treatments involving alkaline peroxide and lime are also studied which are dedicated towards removal of lignin, while acidic pretreatments demonstrate the ability for hemicellulose removal [11, 12]. In spite of that, it is hypothesized that the pretreatments which do not participate in the process of lignin removal might influence the distribution and structure of lignin present in biomass [13]. During high-temperature-based wood pulping processes, the microscopic detection of globular formations on the periphery of pretreated biomass closely relates to the presence of lignin in wood pulp. In an another work, the appearance of droplet on the pretreated solids obtained from dilute acid pretreatment of corn stover suggests the feasibility of lignin-derived products [14]. The similar formation of droplets is also observed on the surface of lignin model compounds which were synthesized with the aid of enzyme catalysis [15].
Generally, the pretreatment process leads to produce both of the liquid and solid fractions of biomass that mostly contain lignin fragments and different forms of carbohydrates [16, 17]. The solid fraction is mostly enriched with cellulose, while the liquid phase contains several sugar-based degradation compounds such as furan compounds and organic acids like levulinic and formic acids. Now, such products undergo a series of reactions like condensation and aromatization for the formation of pseudo-lignin which acts as a barrier towards the enzymatic hydrolysis of cellulose [18, 19]. Primarily, pseudo-lignin appeared as droplets that are mainly attached over the surface of biomass and significantly contribute to the Klason lignin content of pretreated biomass. In the recent times, researchers are more focused on applying models to study the synthesis of pseudo-lignin and its structural characterization. Sannigrahi et al. [20] prepared pseudo-lignin from poplar holocellulose using sulfuric acid at 160−180 °C temperature. As a result of characterization, the polyphenolic pseudo-lignin is observed to contain different aliphatic and aromatic functional groups. Ma et al. [21] conducted X-ray photoelectron spectroscopy (XPS) of the bamboo chips which was pretreated at high temperature of 170 °C with deionized water for different time intervals. The existence of the cyclic structures and functional groups in pseudo-lignin was cross-verified with the increase in C1 category and a decrease in C2 category. He et al. [18] attempted to investigate the reaction pathways for the generation of pseudo-lignin using two different sugars as model compounds, and as a result, a drastic difference in the quantities of functional groups was noted in pseudo-lignin samples. In addition, a kinetic model of pseudo-lignin was proposed as a pathway of several reaction kinetics models involving xylan and lignin. So, a number of different research articles are available on the synthesis of pseudo-lignin and typically depend on the assumptions involved in the basic reaction pathways for the generation of pseudo-lignin.
A comprehensive detailing on the synthesis of pseudo-lignin and the underlying mechanism on how it affects the enzymatic hydrolysis of pretreated biomass is rarely available in the existing literatures [22]. Therefore, the present review article is not only focused on the nature and formations of pseudo-lignin from biomass but also details the latest advancements on its effect during enzymatic hydrolysis of biomass. In view of that, the ultrastructural morphology of pseudo-lignin in the form of droplets on the residual and pretreated biomass is exhaustively studied. Thereafter, the inhibitory effect of pseudo-lignin in enzymatic hydrolysis of biomass is discussed with an insight on reaction mechanism of the inhibition. Further, an advancement in the pretreatment processes is critically analyzed to summarize the routes like acid, hydrothermal, and steam explosion treatment that specifically leads towards the formation of pseudo-lignin. The future recommendation presents the probable strategies to circumvent the interference of pseudo-lignin on enzymatic reactions. Hence, the readers might get benefitted with the details on the formation and characterization of pseudo-lignin and its regulatory impact upon enzymatic hydrolysis of lignocellulosic biomass.
Ultrastructural Morphology of Lignin Droplets on Biomass
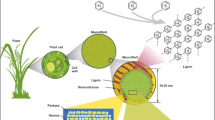
In most of the analytical studies, SEM images are primarily observed for pretreated biomass samples which show discrete droplets on the exterior of cell wall (Fig. 1). The droplets are observed on biomass samples as a result of acid pretreatment using 0.8% of H2SO4 performed in several types of reactors. Most importantly, the type of the pretreatment and reactor involved showed maximum influence on the overall features of the droplets. Moreover, Fig. 2 shows the variation in the size of droplets for multiple classes of pretreated corn stover which are identified by the morphological characteristics. The complicated network of lignin and the plant biomass matrix attribute towards such variability in the synthesis of lignin droplets. A variation in the size of droplets starting from 5 nm to 12 mm in diameter are typically observed in most of the cases. Few of the droplets are spherical with the surface touching the base, while some droplets are combined together to form a lager droplet (Fig. 2A). In another way, most of the droplets contain smooth exterior while the others show a rough surface coating (Fig. 2B). The surface of the droplets with rough exterior is hypothesized as the lignin core materials which are surrounded by the carbohydrate shell [9].
SEM image of pretreated corn stover biomass showing numerous round-shaped lignin droplets obtained after dilute acid pretreatment using 0.8% of H2SO4. Reprinted with permission from Donohoe et al. [9]
Distinct morphologies of lignin droplets on the surface of the pretreated corn stover biomass. A Small droplets combined with each other for the formation of larger droplets. B Spherical droplets with rough surface. C Lignin droplets with smooth surface. Reprinted with permission from Donohoe et al. [9]
A number of several investigations are ongoing to synthesize the composition of such droplets. It was observed that the most available surface droplets formed from pretreatments are of 20–100-nm diameter and easily isolated from the biomass by placing onto filter paper within a reaction system. Such droplet classes are helpful to provide the information on the structural composition and the reaction mechanism involved in the formation. Moreover, Fig. 2C shows that the redeposited lignin droplets are well spread over the cell wall surface. Primarily, there are three different layers in the cell wall regions that are mostly responsible to accumulate maximum number of droplets. From the SEM and TEM analyses, it was revealed that the pits are more prone to gather the droplets near to the cell wall portion of biomass. Sometimes, a pit is surrounded by a cluster of droplets while in other cases single droplets are sometimes enough to chunk the pit channel. Like pits, cell corners also exhibited a similar gathering of droplets. Sometimes, it becomes difficult to separate the cell corner from the margin of the cell as it is nearly filled with droplets. At last, the final one of droplet gathering is the delamination zone in which the lignin droplets fill the space available to them and they do not readily form spheres. In reality, the reagents and enzymes used for the movement of pretreatment solution became troublesome as the droplets tend to gather in the same regions and thereby create a problem for enzymes to access such zones. In most of the cases, the actual lignin content of the biomass does not necessarily migrate out of the wall and as a result redeposited over the cell wall surface. It was possible to track the location of lignin using staining procedure of ultra-thin sections of cell wall using KMnO4. The droplets appeared on the surfaces are actually observed with a layered pattern of droplet-like densities throughout the cell wall [9]. Therefore, it is more likely as homogenous distribution of lignin coalesces and migration towards a more localized and concentrated form of lignin. Under acidic conditions, the formation of pseudo-lignin from the degradation of carbohydrates is primarily responsible to increase the Klason lignin content of the pretreated biomass. It was observed that at the most severe pretreatment conditions, the p-dioxane-soluble Klason lignin or pseudo-lignin fraction is more than that of the isolated obtained from the starting holocellulose content of hybrid poplar biomass [20]. This was attributed that not only large amounts of pseudo-lignin are generated during the acid hydrolysis but also the additional Klason lignin is formed solely from the carbohydrates of the biomass. Such appearance of lignin in the form of spherical droplets was confirmed through the SEM images of the pretreated biomass at severe reaction conditions. Hu et al. [23] observed the formation of pseudo-lignin from both of the fractions of cellulose and holocellulose content during acid hydrolysis of poplar biomass. It was studied that different functional groups like carbonyl, carboxylic, aromatic, and aliphatic structures were present in the pseudo-lignin obtained from pretreated cellulose and holocellulose. Schmatz et al. [24] applied different combinations of pretreatment techniques prior to acid hydrolysis of sugarcane bagasse at 121 °C for 30 min. It was observed that extractive free biomass leads to reduce the formation of pseudo-lignin content during acid hydrolysis process.
Deposition of Lignin Droplets and Its Underlying Mechanism in Inhibiting Enzymatic Hydrolysis of Biomass
Unproductive Binding
Primarily, lignin is well recognized for its inhibition activity once bind with cellulase and further restricts the action of cellulase enzyme. It was observed that the addition of lignin derivatives obtained from pretreated poplar to a hydrolysis mixture negatively affect the conversion of Avicel cellulose thorough irreversible binding [25]. However, the effect of such conversion typically varied with the type of pretreatment and methods adopted for isolation of lignin from pretreated biomass. Actually, lignin depolymerization and repolymerization reactions are often occurred as a result of different pretreatment methods. Primarily, carbocations are formed as a primary intermediate product during the process of acid hydrolysis. Subsequently, the soluble phenolics are obtained from such carbocations and assumed to inhibit the hydrolysis of liquid hydrolysate [26]. Such carbocations are responsible to condensate with other aromatic rings and repolymerization of insoluble lignin [27]. During such repolymerization reactions, lignin are observed to loss few of the hydrophilic functional groups and thereby enhanced the hydrophobic interactions with cellulase enzymes [28]. During the process of pretreatment, the carbohydrates lead to form a number of different lignin like substances which are appeared as lignin-like materials and commonly termed as pseudo-lignin that typically inhibit further enzyme efficiency through unproductive binding with cellulase enzyme (Fig. 3).
Kumar et al. [29] hydrolyzed Avicel cellulose along with pseudo-lignin, derived from xylose, to study the unproductive binding with cellulase enzyme. As a result of such enzymatic conversion, the xylose-derived pseudo-lignin exhibited an insignificant effect on the initial cellulose conversion. It is interesting to note that at prolonged hydrolysis time, the addition of small amount of pseudo-lignin showed a significant impact on cellulose conversion at any of the cellulase loadings. There is the availability of numerous research studies that are mostly based on the effect of pseudo-lignin on enzymatic hydrolysis of biomass. However, this is important to consider the adsorption behavior of both of the lignin and pseudo-lignin on the enzymes. In a recent study on acid-pretreated bamboo residues, it was observed that the pseudo-lignin exhibited similar adsorption capability to enzymes as lignin in the fast adsorption process. However, the pseudo-lignin generated from glucose took longer time in slow adsorption process than the pseudo-lignin which was generated from xylose. In addition, lignin showed faster desorption capability to enzymes than that of the pseudo-lignin [30]. Shinde et al. [31] reported that the intermediates formed from furfural and HMF ultimately condensed to form pseudo-lignin. Figure 4 represents the possible routes of the formation of pseudo-lignin obtained from the intermediates of cellulose degradation products. Most importantly, such non-specific bindings of lignin with enzymes are occurred through a number of different interactions such as hydrophobic, electrostatic, and hydrogen bonding [32,33,34]. The presence of amino acids determines the hydrophobicity of any biomass, and in addition, the hydrophobicity is also dependent on the presence of lignin [35]. It is also observed that such hydrophobicity of biomass is eventually increased in the presence of high lignin content, thus negatively affecting the process of biomass hydrolysis. Zhang et al. [34] studied the interactions of two glucanases, namely, TvEG and TrCel7A, with three different types of lignin isolated from raw aspen wood biomass, and as a result, both of the enzymes exhibited an adsorption phenomenon with the lignins due to hydrophobic interactions. However, such interactions were significantly reduced by the presence of negatively charged lignosulphonate. Apart from the hydrophobicity, another major driving force is the electrostatic interaction which is typically involved in non-productive adsorption of lignin onto cellulase. The major interactions take place among the functional groups present on the biomass with various amino acids that exist on the surface of the enzyme. It is important to state that zeta potential is an important parameter which determines the impact of electrostatic interactions with lignin and enzyme in overall attraction or repulsion between substrate and enzyme [36].
Possible routes for the generation of pseudo-lignin from the pretreatment of biomass. Reprinted with permission from [37]
Surface Blockage
A number of different articles reported that the hydrolysis of cellulose is manifested through layers of microfibrils of the surface of biomass and continues in one direction using one enzyme molecule [38]. Therefore, the inhibitory effects of lignin droplets on enzymatic hydrolysis not only occurred due to the prevention of the enzymes from proceeding along the surface of the substrate but also thorough blocking the accessibility of the enzymes into the inner layers of biomass. Hence, the blockage on the surface of biomass is primarily responsible for delaying the hydrolysis process by the lignin droplets. However, the question is raised once the drop in inhibition occurred with extended conversion of the substrate molecule which attributes towards the probability of relieving the physical barrier, imposed by such lignin droplets. One of the possible reasons was explained through the concept of traffic jam for enzyme linear movements on the cellulose surface which ultimately slows down the action of enzyme on cellulose [39]. Moreover, the other phenomenon is enzymatic deinking which is usually referred in the pulp and paper industry. As per this theory, the ink particles are assumed to be peeled off along with small fibrils from the surface of the biomass which facilitate the modification of substrate surface chemistry like hydrophobicity [40]. The analysis to detect the actual reason is one of the difficult tasks to perform. However, the experimental studies confirmed the drop off phenomenon of lignin droplets with the progress in biomass hydrolysis as the UV absorbance of enzymatic hydrolyzate increased significantly with the increase in hydrolysis time, attributing removal of more lignin droplets from the cellulose surface into the bulk liquid phase.
Major Pretreatment Processes for the Deposition of Lignin Droplets
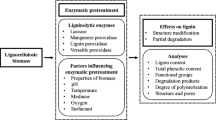
The process of pretreatments is frequently employed as an important step in the conversion of lignocellulosic biomass to bioethanol [41, 42]. Such pretreatments significantly disrupt the lignocellulosic structure and remove hemicelluloses for making the cellulose accessible towards enzymes. During this process, a portion of the carbohydrates are transformed into furfural and hydroxymethylfurfural. A number of different pretreatment processes are studied as responsible for the formation of lignin droplets or pseudo-lignin which resulted into final disposition onto the surface of biomass. Among others, acid and hydrothermal pretreatments are observed to contain significant amount of pseudo-lignin as a result of pretreatment severity. Table 1 shows different types of pretreatment processes that are responsible for the formation of pseudo-lignin on the treated biomass and their subsequent effect in the process of enzymatic hydrolysis. Among others, acid pretreatment, steam explosion pretreatment, and autohydrolysis of biomass is majorly responsible for the generation of such formations.
Acid Pretreatment
Dilute acid pretreatment was mostly employed due to its immense ability to isolate pseudo-lignin from the carbohydrate fraction of hybrid poplar biomass. This experiment was conducted in order to investigate the chemical structure of pseudo-lignin of biomass and its interaction with cellulase enzyme [23]. It was observed that due to acid-catalyzed dehydration reaction, the cellulose and hemicellulose fraction of hybrid poplar biomass underwent fragmentation, rearrangement or polymerization reactions for the formation of an acid-insoluble material referred as pseudo-lignin, made up of carbonyl, carboxylic, aromatic, and aliphatic structures. Moreover, pseudo-lignin significantly inhibits the enzymatic hydrolysis of cellulose. Therefore, dilute acid pretreatment with less severity was preferred in order to circumvent the formation of pseudo-lignin. In another experiment, Kumar et al. [29] conducted dilute acid pretreatment using Avicel and beech wood xylan at various reaction conditions. It was observed that hemicellulose-derived pseudo-lignin was formed even at moderate severities and significantly retarded cellulose hydrolysis. Due to formation of negligible amounts of pseudo-lignin at low severe dilute acid pretreatment of a xylan-Avicel mixture, enzymatic conversion of cellulose dropped significantly (> 25%) compared to that of the cellulose solely pretreated under the same reaction conditions. Xu et al. [50] conducted ethylenediamine-based pretreatment of corn stover under acidic conditions, and interestingly, a total of 27 g of lignin was obtained from both of the solid-pretreated biomass and the liquid filtrate obtained after the thermochemical pretreatment reactions. An increase of around 5.0 g of lignin after the pretreatment attributes the contribution from the generation of pseudo-lignin during thermochemical acid-based pretreatment. Huang et al. [51] used different concentrations of sulfuric acid for pretreatment of poplar saw dust, and it was observed that with the increase in acid concentration from 2 (w/w) to 4% (w/w), the formation of pseudo-lignin was also increased due to self-condensation of lignin and generation of carbohydrate degradation products under acidic conditions. Similarly, the alkali-pretreated pine biomass leads to form significant fraction of pseudo-lignin once impregnated with 3% sulfuric acid under high temperature of 200 °C for 5 min [52]. In another similar kind of work, Lin et al. [53] extracted different fractions of surface lignin from acid-pretreated bamboo residues, and with the removal of surface lignin, the yield for enzymatic hydrolysis of pretreated biomass decreased from 36.5 to 18.6%.
Steam Explosion Pretreatment
Troncoso-Ortega performed steam explosion pretreatment of E. globulus which typically intensified the enzymatic hydrolysis of the pretreated cellulose [54]. The microscopic analysis revealed a correlation between the pretreatment severity and the degree of changes of redistributed lignin. As far as the lignin redistribution is concerned, the micro- and nanodroplets of lignin are observed on the surface and the interior of the E. globulus fiber. Moreover, with respect to the chemical changes of redistributed lignin, it presented a higher degree of condensation as severity increased. These physical–chemical effects were correlated with a greater solubilization of xylans from biomass to the liquid phase, due to the action of acetyl groups of the raw material. The solubilization of the xylans could be the main phenomenon that leads to the dissociation of the carbohydrate–lignin complex, promoting the degradation of the fiber structure and the redistribution of lignin. The migration and redistribution of lignin in the residual biomass significantly improved enzymatic hydrolysis. In another work, Aurum et al. [45] conducted steam explosion pretreatment of birch stem wood at 170–200 °C temperature for 10 min of residence time for the analysis of pseudo-lignin obtained from biomass. The pyrolysis–gas chromatography–mass spectrometry analysis of the treated biomass revealed that pseudo-lignin is a humin-like substance. However, there are very few literatures available on the effect of steam explosion pretreatment for the formation of pseudo-lignin and its impact on subsequent enzymatic hydrolysis process.
Autohydrolysis
Primarily, autohydrolysis pretreatment assists to remove the lignin content in biomass with the removal of hemicelluloses. However, it was observed that autohydrolysis pretreatment is not effective for softwood lignin removal [55]. Another problem with the autohydrolysis treatment is the repolymerization reaction of lignin due to the release of acetic acid from hemicellulosic fraction of biomass. Based on the type of the biomass and reaction severity, such acidic atmosphere leads to produce carbocation ions which are responsible for the repolymerization reaction. Moreover, the carbocations are also assumed to act as intermediates for depolymerization reaction especially through the breaking of β-aryl ether linkages (Fig. 5) [56, 57]. Further from the figure, the formation of electrophilic carbocations is due to the interactions between stable C–C bonds with the electron-rich carbon atoms of the aromatic rings present in lignin. The effect of autohydrolysis was studied on both the interior and exterior surfaces of bamboo to look into the formation of droplets with an increased amount of lignin. Moreover, compared to that of the interior surface, the droplet spheres were more vibrant on the exterior surfaces. At the final stage of pretreatment, the precipitates are combined with the droplets for the formation of lignin shelter on the exterior surface of bamboo [58]. Moreover, Zhuang et al. [59] demonstrated the possibility towards the formation of pseudo-lignin during the cooling process followed by hot water pretreatment of biomass. Furthermore, a progressive deposition of 19.6 mg of cooling-induced pseudo-lignin per g of the treated wood was detected on the surface during cooling process whereas no such formation of pseudo-lignin on the treated biomass was observed once collected isothermally.
Reaction mechanisms involving lignin depolymerization and depolymerization and repolymerization reactions. a Carbocation formation through the breaking down of β-O-4 bond. b Production of enol ether. c Acid hydrolysis of enol ether. d Lignin repolymerization. Reproduced with permission from [46] from the Royal Society of Chemistry
Advancement in the Processes to Overcome the Interference Effect of Lignin Deposition During Enzymatic Hydrolysis of Biomass
Among others, acid pretreatment of lignocellulosic biomass using dilute sulfuric acid is mostly explored as an effective strategy for breaking down the intricate network of biomass and to subsequently improve the process of enzymatic saccharification [60]. However, it was observed that lignin is condensed and redeposited on the biomass surface as a result of acid pretreatment, thereby acting as an obstacle towards enzymatic access of cellulose. Schmatz et al. [24] conducted a detail study on the formation of pseudo-lignin using different structural parts of sugarcane bagasse under several process conditions, and it was observed that the extractive-free biomass leads to produce less amount of pseudo-lignin prior to the acid pretreatment compared to that of the raw biomass. In addition, co-solvent-enhanced lignocellulosic fractionation process was recently employed as an emerging pretreatment process for the removal of significant fraction of biomass lignin. The lignin is preferentially dispersed using the tetrahydrofuran (THF), water, and dilute acid manifesting towards its easy removal from carbohydrate matrix and prevents further lignin redeposition [61]. As a result, significant yield of glucose was obtained from the resulting pretreated solids due to their high digestibility. Though the removal of hemicellulose and lignin plays a significant role in obtaining higher conversion of carbohydrates from CELF-pretreated solids, the actual impact of residual lignin on the action of enzyme is yet to be explored. In view of that, Patri et al. [62] developed co-solvent-enhanced lignocellulosic fractionation (CELF) method in order to solubilize lignin and to decrease the limitations associated with acid pretreatment. It was observed that with an incubation period of 5 weeks and enzyme doses of 2 mg protein/g, an enzymatic hydrolysis of CELF-pretreated switchgrass at 7.5% (w/w) of solid loadings attributes towards glucan to glucose yield of more than 90%. In another work, different phenolic derivatives are added to investigate the mitigating effect of lignin inhibition for better enzymatic hydrolysis. Chu et al. [28] observed that among other tested conditions, addition of 2-napthol in the reaction medium significantly enhanced with 21.9% of improvement in the conversion of cellulose through minimizing the blocking effect of lignin on washed and pretreated biomass. Hu et al. [63] observed that the addition of dimethyl sulfoxide in the reaction mixture of dilute acid pretreatment substantially reduced the generation of pseudo-lignin. The formation of hydroxymethyl furfural was stabilized as a result of such addition of novel mixture. Therefore, the overall formation of pseudo-lignin was restricted or controlled. Thereafter, Lu et al. [48] performed enzymatic hydrolysis of elephant grass, and in the presence Trx-His-S as an additive, the alkali-pretreated biomass resulted in an enhanced production of reducing sugars. Therefore, the addition of Trx-His-S is believed to prevent the lignin-binding sites and subsequently reduce the nonproductive adsorption of cellulase onto lignin, ultimately leading towards the favorable condition for an enzymatic hydrolysis of biomass. Another tactical strategy to reduce the non-productive binding of lignin is to increase the surface negative charge of cellulase so that a repulsion force between lignin and cellulase enzyme ultimately resulted in an enhanced saccharification of biomass [64]. Furthermore, it was observed that cellulose-binding domains of cellulase enzymes derived from few fungal species typically bind to lignin along with cellulose [65]. In view of that, Rahikainen et al. [66] expressed cellulase enzyme without cellulose-binding domains and as a result, a significant reduction in non-productive binding of lignin to cellulase enzyme was observed which proved that the absence of cellulose-binding domain in cellulase enzyme significantly improved the saccharification efficiency of biomass. Likewise, moderate genetic engineering with the lignin of plant origin can also be done in order to reduce the guaiacyl monolignol content of softwood biomass which are responsible to directly inhibit the enzymatic saccharification efficiency of biomass [67]. As far as the structural modification of lignin is concerned, the hydrophobicity of phenolic hydroxyl groups is mainly responsible for the inhibition of cellulase due to the non-production adsorption onto it. Therefore, a significant reduction in the inhibitory effect of lignin was observed due to hydroxypropylation reactions of free phenolic hydroxyl groups [68]. This is an important fact that lignocellulosic conversion into the production of fermentable sugars is a costly affair, in which pretreatment and enzymatic hydrolysis include more that 70% of total cost involvement in the entire process of biofuel production. Now, the presence or absence and formation of lignin nanoparticles typically influence the overall cost shared by the process of pretreatment and enzymatic hydrolysis. In most of the biomass processings, the droplets of lignin are appeared as a result of high severity pretreatment conditions. Therefore, the selection of the pretreatment process for a particular biomass is an important criterion not only for reducing the formation of lignin droplets but also to prevent nonproductive binding of lignin with enzymes.
Challenges and Future Recommendations
As discussed, a number of various research articles are available on different aspects of pseudo-lignin. However, there are still scopes to explore for the better understanding on the formation and impact of pseudo-lignin from biomass. As far as the lignocellulosic biorefinery is concerned, it is extremely important to understand the insight on the reaction mechanisms involved in the generation of pseudo-lignin as such biomaterials are observed as inhibitory towards enzymatic hydrolysis process. An investigation to track the interaction between the spheres of pseudo-lignin and cellulases demonstrated the detrimental impact on enzymatic hydrolysis of cellulose. Moreover, this is extremely important to comprehend of what components of biomass is responsible for the formation of pseudo-lignin which will assist to reach to a decision on selection of the biomass and pretreatment techniques to overcome its obstacles in the biorefinery processes. The droplet forms of pseudo-lignin not only reduce the enzyme activity but also contributes towards enzyme deactivation manifesting the requirement of higher enzyme loadings for substantial biofuel production from biomass. The major problem with the aforementioned statement is high cost of the process associated with higher enzyme loading. One significant approach uses an acid-based mixture of THF–water co-solvents for the solubilization of lignin and to overcome the limitations associated with the pseudo-lignin generated from acid pretreatment processes. Moreover, it was also noticed that enzymatic hydrolysis of such pretreated biomass is also able to bear a high enzyme activity with substantial production of glucose. In few of the investigations, different attempts were made to utilize lignin droplets as an activator to enzymatic hydrolysis of biomass in which the presence of such spherical formation significantly improved the saccharification efficiency of the biomass.
Conclusions
Among others, dilute acid pretreatment is primarily responsible for acid-catalyzed dehydration and polycondensation reactions of the carbohydrate fractions including cellulose and hemicellulose for the generation of an acid-insoluble pseudo-lignin, which mainly consisted of a number of various functional groups like carbonyl, carboxylic, aromatic, and aliphatic structures. It is interesting to note that pseudo-lignin not only attributes towards the formation of Klason lignin but also inhibits the enzymatic hydrolysis of carbohydrates present in biomass. Therefore, it is necessary to perform dilute acid pretreatment at lower severity conditions so that the formation of pseudo-lignin can be minimized. During the process of biomass pretreatment, lignin transformed into liquid state from solid and moves across the cell wall. However, it forms round-shaped droplet on the surface of pretreated biomass and moves slowly as the result of severe pretreatment conditions. Now, the event of trafficking of the lignin droplets is usually occurred under the influence of severe pretreatment conditions which typically interferes with the saccharification of carbohydrates present in the biomass. In addition, the increase in the exposed surface area of droplets negatively affects the process of biomass saccharification. However, other important factors such as pretreatment conditions, feedstock types, and selection of the enzyme also affect as far as the real-time lignocellulosic substrate is concerned.
References
Agrawal R, Verma A, Singhania RR, Varjani S, Di Dong C, Kumar Patel A (2021) Current understanding of the inhibition factors and their mechanism of action for the lignocellulosic biomass hydrolysis. Bioresour Technol 332:125042. https://doi.org/10.1016/j.biortech.2021.125042
Singhania RR, Ruiz HA, Awasthi MK, Dong C-D, Chen C-W, Patel AK (2021) Challenges in cellulase bioprocess for biofuel applications. Renewable Sustainable Energy Rev 151:111622. https://doi.org/10.1016/j.rser.2021.111622
Duarah P, Haldar D, Purkait MK (2020) Technological advancement in the synthesis and applications of lignin-based nanoparticles derived from agro-industrial waste residues: a review. Int J Biol Macromol 163:1828–1843. https://doi.org/10.1016/j.ijbiomac.2020.09.076
Lobato-Peralta DR, Duque-Brito E, Villafán-Vidales HI, Longoria A, Sebastian PJ, Cuentas-Gallegos AK, Arancibia-Bulnes CA, Okoye PU (2021) A review on trends in lignin extraction and valorization of lignocellulosic biomass for energy applications. J Cleaner Prod 293:126123. https://doi.org/10.1016/j.jclepro.2021.126123
Saini JK, Patel AK, Adsul M, Singhania RR (2016) Cellulase adsorption on lignin: a roadblock for economic hydrolysis of biomass. Renewable Energy 98:29–42. https://doi.org/10.1016/j.renene.2016.03.089
Haldar D, Purkait MK (2021) A review on the environment-friendly emerging techniques for pretreatment of lignocellulosic biomass: mechanistic insight and advancements. Chemosphere 264:128523. https://doi.org/10.1016/j.chemosphere.2020.128523
Meng X, Yoo CG, Pu Y, Ragauskas AJ (2022) Opportunities and challenges for flow-through hydrothermal pretreatment in advanced biorefineries. Bioresour Technol 343:126061. https://doi.org/10.1016/j.biortech.2021.126061
Singh A, Rodríguez-Jasso RM, Saxena R, Cerda RB, Singhania RR, Ruiz HA (2021) Subcritical water pretreatment for agave bagasse fractionation from tequila production and enzymatic susceptibility. Bioresour Technol 338:125536. https://doi.org/10.1016/j.biortech.2021.125536
Donohoe BS, Decker SR, Tucker MP, Himmel ME, Vinzant TB (2008) Visualizing lignin coalescence and migration through maize cell walls following thermochemical pretreatment. Biotechnol Bioeng 101(5):913–925. https://doi.org/10.1002/bit.21959
Xu H, Che X, Ding Y, Kong Y, Li B, Tian W (2019) Effect of crystallinity on pretreatment and enzymatic hydrolysis of lignocellulosic biomass based on multivariate analysis. Bioresour Technol 279:271–280. https://doi.org/10.1016/j.biortech.2018.12.096
Haldar D, Purkait MK (2020) Thermochemical pretreatment enhanced bioconversion of elephant grass (Pennisetum purpureum): insight on the production of sugars and lignin. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-020-00689-y
Rabelo SC, Amezquita Fonseca NA, Andrade RR, Maciel Filho R, Costa AC (2011) Ethanol production from enzymatic hydrolysis of sugarcane bagasse pretreated with lime and alkaline hydrogen peroxide. Biomass Bioenergy 35(7):2600–2607. https://doi.org/10.1016/j.biombioe.2011.02.042
Zeng Y, Zhao S, Wei H, Tucker MP, Himmel ME, Mosier NS, Meilan R, Ding S-Y (2015) In situ micro-spectroscopic investigation of lignin in poplar cell walls pretreated by maleic acid. Biotechnol Biofuels 8(1):126. https://doi.org/10.1186/s13068-015-0312-1
He J, Huang C, Lai C, Huang C, Li M, Pu Y, Ragauskas AJ, Yong Q (2020) The effect of lignin degradation products on the generation of pseudo-lignin during dilute acid pretreatment. Ind Crops Prod 146:112205. https://doi.org/10.1016/j.indcrop.2020.112205
Charrier AM, Lereu AL, Farahi RH, Davison BH, Passian A (2018) Nanometrology of biomass for bioenergy: the role of atomic force microscopy and spectroscopy in plant cell characterization. Fron Energy Res 6:11. https://doi.org/10.3389/fenrg.2018.00011
Huang CS, Lee HT, Li PY, Hu KC, Lan CW, Chang MJ (2019) Three-dimensional buckling analyses of cracked functionally graded material plates via the MLS-Ritz method. Thin-Walled Struct 134:189–202. https://doi.org/10.1016/j.tws.2018.10.005
Jeong S-Y, Lee E-J, Ban S-E, Lee J-W (2021) Structural characterization of the lignin-carbohydrate complex in biomass pretreated with Fenton oxidation and hydrothermal treatment and consequences on enzymatic hydrolysis efficiency. Carbohydr Polym 270:118375. https://doi.org/10.1016/j.carbpol.2021.118375
He J, Huang C, Lai C, Huang C, Li X, Yong Q (2018) Elucidation of structure-inhibition relationship of monosaccharides derived pseudo-lignin in enzymatic hydrolysis. Ind Crops Prod 113:368–375. https://doi.org/10.1016/j.indcrop.2018.01.046
Świątek K, Gaag S, Klier A, Kruse A, Sauer J, Steinbach D (2020) Acid hydrolysis of lignocellulosic biomass: sugars and furfurals formation. Catalysts 10(4):437
Sannigrahi P, Kim DH, Jung S, Ragauskas A (2011) Pseudo-lignin and pretreatment chemistry. Energy Environ Sci 4(4):1306–1310. https://doi.org/10.1039/C0EE00378F
Ma X, Yang X, Zheng X, Chen L, Huang L, Cao S, Akinosho H (2015) Toward a further understanding of hydrothermally pretreated holocellulose and isolated pseudo lignin. Cellulose 22(3):1687–1696. https://doi.org/10.1007/s10570-015-0607-1
Singhania RR, Patel AK, Raj T, Chen C-W, Ponnusamy VK, Tahir N, Kim S-H, Dong C-D (2021) Lignin valorisation via enzymes: a sustainable approach. Fuel:122608. https://doi.org/10.1016/j.fuel.2021.122608
Hu F, Jung S, Ragauskas A (2012) Pseudo-lignin formation and its impact on enzymatic hydrolysis. Bioresour Technol 117:7–12. https://doi.org/10.1016/j.biortech.2012.04.037
Schmatz AA, Salazar-Bryam AM, Contiero J, Sant’Anna C, Brienzo M (2021) Pseudo-lignin content decreased with hemicellulose and lignin removal, improving cellulose accessibility, and enzymatic digestibility. BioEnergy Res 14(1):106–121. https://doi.org/10.1007/s12155-020-10187-8
Kumar R, Wyman CE (2009) Access of cellulase to cellulose and lignin for poplar solids produced by leading pretreatment technologies. Biotechnol Prog 25(3):807–819. https://doi.org/10.1002/btpr.153
Zhai R, Hu J, Saddler JN (2018) Minimizing cellulase inhibition of whole slurry biomass hydrolysis through the addition of carbocation scavengers during acid-catalyzed pretreatment. Bioresour Technol 258:12–17. https://doi.org/10.1016/j.biortech.2018.02.124
Chiranjeevi T, Mattam AJ, Vishwakarma KK, Uma A, Peddy VCR, Gandham S, Ravindra Velankar H (2018) Assisted single-step acid pretreatment process for enhanced delignification of rice straw for bioethanol production. ACS Sustainable Chem Eng 6(7):8762–8774. https://doi.org/10.1021/acssuschemeng.8b01113
Chu Q, Tong W, Wu S, Jin Y, Hu J, Song K (2021) Modification of lignin by various additives to mitigate lignin inhibition for improved enzymatic digestibility of dilute acid pretreated hardwood. Renewable Energy 177:992–1000. https://doi.org/10.1016/j.renene.2021.06.048
Kumar R, Hu F, Sannigrahi P, Jung S, Ragauskas AJ, Wyman CE (2013) Carbohydrate derived-pseudo-lignin can retard cellulose biological conversion. Biotechnol Bioeng 110(3):737–753. https://doi.org/10.1002/bit.24744
He J, Huang C, Lai C, Jin Y, Ragauskas A, Yong Q (2020) Investigation of the effect of lignin/pseudo-lignin on enzymatic hydrolysis by Quartz Crystal Microbalance. Ind Crops Prod 157:112927. https://doi.org/10.1016/j.indcrop.2020.112927
Shinde SD, Meng X, Kumar R, Ragauskas AJ (2018) Recent advances in understanding the pseudo-lignin formation in a lignocellulosic biorefinery. Green Chem 20(10):2192–2205. https://doi.org/10.1039/C8GC00353J
Huang Y, Sun S, Huang C, Yong Q, Elder T, Tu M (2017) Stimulation and inhibition of enzymatic hydrolysis by organosolv lignins as determined by zeta potential and hydrophobicity. Biotechnol Biofuels 10(1):162. https://doi.org/10.1186/s13068-017-0853-6
Sun S, Huang Y, Sun R, Tu M (2016) The strong association of condensed phenolic moieties in isolated lignins with their inhibition of enzymatic hydrolysis. Green Chem 18(15):4276–4286. https://doi.org/10.1039/C6GC00685J
Zhang Y, Jiang X, Wan S, Wu W, Wu S, Jin Y (2020) Adsorption behavior of two glucanases on three lignins and the effect by adding sulfonated lignin. J Biotechnol 323:1–8. https://doi.org/10.1016/j.jbiotec.2020.07.013
Strobel KL, Pfeiffer KA, Blanch HW, Clark DS (2015) Structural insights into the affinity of Cel7A carbohydrate-binding module for lignin. J Biol Chem 290(37):22818–22826. https://doi.org/10.1074/jbc.M115.673467
Nakagame S, Chandra RP, Kadla JF, Saddler JN (2011) Enhancing the enzymatic hydrolysis of lignocellulosic biomass by increasing the carboxylic acid content of the associated lignin. Biotechnol Bioeng 108(3):538–548. https://doi.org/10.1002/bit.22981
Sheng Y, Lam SS, Wu Y, Ge S, Wu J, Cai L, Huang Z, Le QV, Sonne C, Xia C (2021) Enzymatic conversion of pretreated lignocellulosic biomass: a review on influence of structural changes of lignin. Bioresour Technol 324:124631. https://doi.org/10.1016/j.biortech.2020.124631
Igarashi K, Uchihashi T, Koivula A, Wada M, Kimura S, Okamoto T, Penttilä M, Ando T, Samejima M (2011) Traffic jams reduce hydrolytic efficiency of cellulase on cellulose surface. Science 333(6047):1279–1282. https://doi.org/10.1126/science.1208386
Xu F, Ding H (2007) A new kinetic model for heterogeneous (or spatially confined) enzymatic catalysis: contributions from the fractal and jamming (overcrowding) effects. Appl Catal A 317(1):70–81. https://doi.org/10.1016/j.apcata.2006.10.014
Ibarra D, Concepción Monte M, Blanco A, Martínez AT, Martínez MJ (2012) Enzymatic deinking of secondary fibers: cellulases/hemicellulases versus laccase-mediator system. J Ind Microbiol Biotechnol 39(1):1–9. https://doi.org/10.1007/s10295-011-0991-y
Aparicio E, Rodríguez-Jasso RM, Pinales-Márquez CD, Loredo-Treviño A, Robledo-Olivo A, Aguilar CN, Kostas ET, Ruiz HA (2021) High-pressure technology for Sargassum spp biomass pretreatment and fractionation in the third generation of bioethanol production. Bioresour Technol 329:124935. https://doi.org/10.1016/j.biortech.2021.124935
Chablé-Villacis R, Olguin-Maciel E, Toledano-Thompson T, Alzate-Gaviria L, Ruiz HA, Tapia-Tussell R (2021) Enzymatic hydrolysis assisted with ligninocellulolytic enzymes from Trametes hirsuta produced by pineapple leaf waste bioconversion in solid-state fermentation. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-01851-w
Espirito Santo M, Rezende CA, Bernardinelli OD, Pereira N, Curvelo AAS, deAzevedo ER, Guimarães FEG, Polikarpov I (2018) Structural and compositional changes in sugarcane bagasse subjected to hydrothermal and organosolv pretreatments and their impacts on enzymatic hydrolysis. Ind Crops Prod 113:64–74. https://doi.org/10.1016/j.indcrop.2018.01.014
Jung S, Trajano HL, Yoo CG, Foston MB, Hu F, Tolbert AK, Wyman CE, Ragauskas AJ (2018) Topochemical understanding of lignin distribution during hydrothermal flowthrough pretreatment. ChemistrySelect 3(32):9348–9352. https://doi.org/10.1002/slct.201801837
Aarum I, Devle H, Ekeberg D, Horn SJ, Stenstrøm Y (2018) Characterization of pseudo-lignin from steam exploded birch. ACS Omega 3(5):4924–4931. https://doi.org/10.1021/acsomega.8b00381
Pielhop T, Larrazábal GO, Studer MH, Brethauer S, Seidel C-M, Rudolf von Rohr P (2015) Lignin repolymerisation in spruce autohydrolysis pretreatment increases cellulase deactivation. Green Chem 17(6):3521–3532. https://doi.org/10.1039/C4GC02381A
Huang C, He J, Min D, Lai C, Yong Q (2016) Understanding the nonproductive enzyme adsorption and physicochemical properties of residual lignins in moso bamboo pretreated with sulfuric acid and kraft pulping. Appl Biochem Biotechnol 180(8):1508–1523. https://doi.org/10.1007/s12010-016-2183-8
Lu X, Li C, Zhang S, Wang X, Zhang W, Wang S, Xia T (2019) Enzymatic sugar production from elephant grass and reed straw through pretreatments and hydrolysis with addition of thioredoxin-His-S. Biotechnol Biofuels 12(1):297. https://doi.org/10.1186/s13068-019-1629-y
Wan G, Zhang Q, Li M, Jia Z, Guo C, Luo B, Wang S, Min D (2019) How pseudo-lignin Is generated during dilute sulfuric acid pretreatment. J Agric Food Chem 67(36):10116–10125. https://doi.org/10.1021/acs.jafc.9b02851
Xu L, Zhang J, Zong Q-J, Wang L, Xu T, Gong J, Liu Z-H, Li B-Z, Yuan Y-J (2022) High-solid ethylenediamine pretreatment to fractionate new lignin streams from lignocellulosic biomass. Chem Eng J 427:130962. https://doi.org/10.1016/j.cej.2021.130962
Huang Y, Chu Q, Tong W, Wu S, Jin Y, Hu J, Song K (2021) Carbocation scavenger assisted acid pretreatment followed by mild alkaline hydrogen peroxide (AHP) treatment for efficient production of fermentable sugars and lignin adsorbents from hardwood biomass. Ind Crops Prod 170:113737. https://doi.org/10.1016/j.indcrop.2021.113737
Das P, Stoffel RB, Area MC, Ragauskas AJ (2019) Effects of one-step alkaline and two-step alkaline/dilute acid and alkaline/steam explosion pretreatments on the structure of isolated pine lignin. Biomass Bioenergy 120:350–358. https://doi.org/10.1016/j.biombioe.2018.11.029
Lin W, Yang J, Zheng Y, Huang C, Yong Q (2021) Understanding the effects of different residual lignin fractions in acid-pretreated bamboo residues on its enzymatic digestibility. Biotechnol Biofuels 14(1):143. https://doi.org/10.1186/s13068-021-01994-y
Troncoso-Ortega E, Castillo RDP, Reyes-Contreras P, Castaño-Rivera P, Teixeira Mendonça R, Schiappacasse N, Parra C (2021) Effects on lignin redistribution in Eucalyptus globulus fibres pre-treated by steam explosion: a microscale study to cellulose accessibility. Biomolecules 11 (4). https://doi.org/10.3390/biom11040507
Pielhop T, Larrazábal GO, Rudolf von Rohr P (2016) Autohydrolysis pretreatment of softwood – enhancement by phenolic additives and the effects of other compounds. Green Chem 18(19):5239–5247. https://doi.org/10.1039/C6GC01447J
Voitl T, Nagel MV, von Rohr PR (2010) Analysis of products from the oxidation of technical lignins by oxygen and H3PMo12O40 in water and aqueous methanol by size-exclusion chromatography. Holzforschung 64(1):13–19. https://doi.org/10.1515/hf.2010.006
Chu Q, Tong W, Wu S, Jin Y, Hu J, Song K (2021) Eco-friendly additives in acidic pretreatment to boost enzymatic saccharification of hardwood for sustainable biorefinery applications. Green Chem 23(11):4074–4086. https://doi.org/10.1039/D1GC00738F
Ma XJ, Cao SL, Lin L, Luo XL, Chen LH, Huang LL (2013) Surface characterizations of bamboo substrates treated by hot water extraction. Bioresour Technol 136:757–760. https://doi.org/10.1016/j.biortech.2013.03.120
Zhuang J, Wang X, Xu J, Wang Z, Qin M (2017) Formation and deposition of pseudo-lignin on liquid-hot-water-treated wood during cooling process. Wood Sci Technol 51(1):165–174. https://doi.org/10.1007/s00226-016-0872-7
Lorenci Woiciechowski A, Dalmas Neto CJ, de Souza P, Vandenberghe L, de Carvalho Neto DP, Novak Sydney AC, Letti LAJ, Karp SG, Zevallos Torres LA, Soccol CR (2020) Lignocellulosic biomass: acid and alkaline pretreatments and their effects on biomass recalcitrance – Conventional processing and recent advances. Bioresour Technol 304:122848. https://doi.org/10.1016/j.biortech.2020.122848
Patri AS, Mostofian B, Pu Y, Ciaffone N, Soliman M, Smith MD, Kumar R, Cheng X, Wyman CE, Tetard L, Ragauskas AJ, Smith JC, Petridis L, Cai CM (2019) A multifunctional cosolvent pair reveals molecular principles of biomass deconstruction. J Am Chem Soc 141(32):12545–12557. https://doi.org/10.1021/jacs.8b10242
Patri AS, Mohan R, Pu Y, Yoo CG, Ragauskas AJ, Kumar R, Kisailus D, Cai CM, Wyman CE (2021) THF co-solvent pretreatment prevents lignin redeposition from interfering with enzymes yielding prolonged cellulase activity. Biotechnol Biofuels 14(1):63. https://doi.org/10.1186/s13068-021-01904-2
Hu F, Ragauskas A (2014) Suppression of pseudo-lignin formation under dilute acid pretreatment conditions. RSC Adv 4(9):4317–4323. https://doi.org/10.1039/C3RA42841A
Zhou Z, Ju X, Chen J, Wang R, Zhong Y, Li L (2021) Charge-oriented strategies of tunable substrate affinity based on cellulase and biomass for improving in situ saccharification: a review. Bioresour Technol 319:124159. https://doi.org/10.1016/j.biortech.2020.124159
Singh A, Patel AK, Adsul M, Mathur A, Singhania RR (2017) Genetic modification: a tool for enhancing cellulase secretion. Biofuel Res J 4(2):600–610. https://doi.org/10.18331/brj2017.4.2.5
Rahikainen JL, Martin-Sampedro R, Heikkinen H, Rovio S, Marjamaa K, Tamminen T, Rojas OJ, Kruus K (2013) Inhibitory effect of lignin during cellulose bioconversion: The effect of lignin chemistry on non-productive enzyme adsorption. Bioresour Technol 133:270–278. https://doi.org/10.1016/j.biortech.2013.01.075
Ko JK, Um Y, Park YC, Seo JH, Kim KH (2015) Compounds inhibiting the bioconversion of hydrothermally pretreated lignocellulose. Appl Microbiol Biotechnol 99(10):4201–4212. https://doi.org/10.1007/s00253-015-6595-0
Yang Q, Pan X (2016) Correlation between lignin physicochemical properties and inhibition to enzymatic hydrolysis of cellulose. Biotechnol Bioeng 113(6):1213–1224. https://doi.org/10.1002/bit.25903
Acknowledgements
The authors DH and PD are thankful to Karunya Institute of Technology and Sciences (Deemed University), Coimbatore, India, for providing support for this study and to develop knowledge towards subsequent research works.
Author information
Authors and Affiliations
Contributions
The idea for the article was conceived by DH and RRS; DH and PD performed the literature search and data analysis; the first draft was prepared by DH and PD; RRS, AKP, and CDD were also involved in the draft preparation and critically revised the work.
Corresponding authors
Ethics declarations
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Haldar, D., Dey, P., Patel, A.K. et al. A Critical Review on the Effect of Lignin Redeposition on Biomass in Controlling the Process of Enzymatic Hydrolysis. Bioenerg. Res. 15, 863–874 (2022). https://doi.org/10.1007/s12155-021-10374-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-021-10374-1