Abstract
In this work, to elucidate why the acid-pretreated bamboo shows disappointingly low enzymatic digestibility comparing to the alkali-pretreated bamboo, residual lignins in acid-pretreated and kraft pulped bamboo were isolated and analyzed by adsorption isotherm to evaluate their extents of nonproductive enzyme adsorption. Meanwhile, physicochemical properties of the isolated lignins were analyzed and a relationship was established with non-productive adsorption. Results showed that the adsorption affinity and binding strength of cellulase on acid-pretreated bamboo lignin (MWLa) was significantly higher than that on residual lignin in pulped bamboo (MWLp). The maximum adsorption capacity of cellulase on MWLp was 129.49 mg/g lignin, which was lower than that on MWLa (160.25 mg/g lignin). When isolated lignins were added into the Avicel hydrolysis solution, the inhibitory effect on enzymatic hydrolysis efficiency of MWLa was found to be considerably stronger than that with MWLp. The cellulase adsorption on isolated lignins was correlated positively with hydrophobicity, phenolic hydroxyl group, and degree of condensation but negatively with surface charges and aliphatic hydroxyl group. These results suggest that the higher nonproductive cellulase adsorption and physicochemical properties of residual lignin in acid-pretreated bamboo may be responsible for its disappointingly low enzymatic digestibility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the world, China is a big producer of moso bamboo (Phyllostachys pubesescens) with a cultivation area of around 3.19 million hectares [1]. For the mature moso bamboo, it has been used as a structural material for construction, flooring, and boards and as raw material for papermaking [2]. With the increasing energy demand, bamboo has been considered as a potential feedstock for biofuel and biochemical production due to its fast growth, short renovation, easy propagation, and high polysaccharides [2, 3]. For the different age moso bamboo, the contents of glucan, xylan, and lignin in the cell wall are 22–39 %, 17–29 %, and 21–30 %, respectively [4]. Technically, these polysaccharides in the cell wall can be hydrolyzed into fermentable sugars (glucose and xylose) by enzymatic saccharification for the biofuel production.
Enzymatic saccharification of lignocellulosic materials for production of fermentable sugars has been widely investigated [5–7]. However, the recalcitrant structure of cellulose, hemicellulose, and lignin in the lignocellulosic biomass presents a significant bottleneck for saccharifying polysaccharides into monosaccharides. For lignin, the steric hindrance and nonproductive enzyme absorption are considered as two of the major inhibition factors that hinder the enzymatic saccharification of lignocellulosic materials [8, 9]. In the cell wall of lignocellulosic biomass, lignin covers the surface of cellulose and limits the accessible surface area of the cellulose available to the enzymes for saccharification. Meanwhile, lignin also can reduce the effectiveness of enzymatic saccharification by adsorbing enzyme nonproductively.
Many studies have showed that the enzymatic digestibility of lignocellulosic material has a negative correlation with its lignin content [10, 11]. One way to overcome the recalcitrance of lignin on enzymatic saccharification of biomass is to reduce its content by chemical pretreatment. During the pretreatment process, a certain amount of original lignin can be removed, simultaneously disrupting the linkages between lignin and carbohydrates and increase the surface area, leading to an excellent enzymatic hydrolysis efficiency [12]. However, the pretreated biomass still contains a certain amount of residual lignin, which also significantly affects the enzymatic digestibility of the pretreated sample by nonproductively adsorbing enzymes. It is considered that hydrophobic interaction between cellulase and lignin is the driving force that leads to cellulase nonproductive adsorbing [13, 14].
Our previous studies [15, 16] and Li et al. [2] both suggested that acid-based pretreatments are not effective for improving the enzymatic digestibility of moso bamboo, except for alkali-based pretreatments. With sulfuric acid pretreatment, even all hemicellulose and 10–18 % of lignin could be degraded under a relatively high pretreatment severity, and more pores were generated for increasing accessibility portion of the acid-pretreated bamboo, the enzymatic hydrolysis efficiencies of pretreated samples were still disappointingly low (below 25 %) [15]. For alkali pretreatment (kraft pulping), the enzymatic digestibility of the alkali-pretreated bamboo could be increased several times with the increasing degree of delignification [16]. It is speculated that the content and the nature of residual lignin in acid- and alkali-pretreated bamboo may be responsible for their different enzymatic digestibility [12, 13]. However, how the residual lignins in the acid- and alkali-pretreated bamboo affect their enzymatic digestibility have yet to be fully elucidated.
To investigate the relationship between lignin and enzymatic digestibility, how to obtain an intact lignin from the pretreated substrate is very crucial. It is proposed that cellulolytic enzymatic lignin (CEL), milled wood lignin (MWL), and protease-treated lignin (PTL) are traditionally used as a representative preparation of original lignin in substrate [12, 17, 18]. However, cellulase and proteinase are remained in the CEL and PTL after removing carbohydrates or proteins [17]. The adsorbed enzyme proteins may have blocked inhibitory sites in the lignin, which will underestimate the nonproductive adsorption of enzymes on the lignin [19]. Hence, in this study, MWL will be considered as the appropriate lignin preparation for the investigation of relationship between lignin and enzymatic digestibility of pretreated bamboo [18].
In this work, the residual lignins in acid-pretreated bamboo and kraft pulping bamboo were isolated and purified according to the procedure developed by Björkman [20]. The adsorption kinetics and adsorption isotherm (Langmuir isotherm) of cellulase on the two isolated lignins were investigated and compared in detail to see how much the nonproductive adsorption effect of residual lignins contributes to the enzymatic saccharification efficiencies. Finally, the surface charges, hydrophobicity, and functional groups were characterized to establish the relationship between structure characteristic and nonproductive adsorption. The goal of this work described here was to understand how the nonproductive adsorption and structural characteristic of residual lignin in pretreated bamboo affect its enzymatic digestibility.
Materials and Methods
Materials
Moso bamboo residues were collected from the stems of a 3-year-old or older bamboo (Phyllostachys heterocycla) provided by the Shaowu Bamboo Processing Factory in Fujian, China. The glucan, xylan, and lignin contents in bamboo residues were 38.22, 19.26, and 30.62 %, respectively. The commercial cellulase (C2730) and Avicel PH101 were purchased from Sigma-Aldrich Inc. (USA).
Pretreatments
In this work, to investigate the enzymatic digestibility of acid- and alkali-pretreated bamboo, medium severity conditions of sulfuric acid pretreatment and kraft pulping were chosen to pretreat bamboo residues according to our previous works [15].
Dilute sulfuric acid pretreatment was carried out in a 1 L cooking pot in an oil bath. Then, 100 g of dry bamboo residues were put into the pot with certain volume of 0.5 % (w/v) sulfuric acid solution, with a solid-to-liquid ratio of 1:10. The bamboo residues were first impregnated with the liquor at 60 °C for 30 min. After impregnation, the temperature was raised at a rate of 2 °C/min to 140 °C and maintained for 60 min. The pretreated solids were washed with distilled water (with a solid-to-liquid ratio of 1:50) to neutrality. The resulting samples were stored at 4 °C for subsequent experiments.
Kraft pulping liquors were prepared with Na2S and NaOH. The effective alkali (EA) charge (as Na2O on dry material) and the sulfidity (on Na2O basis) were 12 and 20 %, respectively. The ratio of liquid-to-solid was 6:1. Then, 100 g of dry bamboo residues were first impregnated with the liquor at 60 °C for 30 min. After impregnation, the temperature was raised at a rate of 2 °C/min to 140 °C and maintained for 60 min. The pretreated bamboo residues were washed with distilled water with a solid-to-liquid ratio of 1:50 to remove the spent chemicals. The resulting pulp was stored at 4 °C for subsequent experiments.
Isolation and Purification of Residual Lignin in Pretreated Bamboo
The residual lignins in acid-pretreated and kraft pulping bamboo were isolated according to the method proposed by Björkman [20]. A planetary ball milling (Pulverisette 7, Fritsch, Germany) was used to mill the pretreated samples. Four grams of air dry sample was subjected to 3-h milling time at 600 rpm using an 80 mL ZrO2 bowl with 25 ZrO2 balls [21]. The ball-milled meals of pretreated samples were extracted by stirring in 96 % dioxane with a solid-to-liquid ratio of 1:20 (g:mL) for 24 h, and this procedure was repeated three times with a new solvent. Then, the mixed filtrates were evaporated under vacuum at 40 °C to remove the dioxane. Finally, the solid matter was dried in a vacuum oven at 40 °C to obtain the crude lignin preparations.
The crude lignin preparations were dissolved in 90 % AcOH (20 mL/g lignin) and precipitated by dropwise addition to water (10 mL/mL 90 % AcOH). The precipitate was washed, freeze-dried, and dissolved in dichloroethane/ethanol (2:1, v/v) mixture. The solution was precipitated by dropwise addition to ether (10 mL/mL dichloroethane/ethanol), and the precipitate was filtered and washed with ether and petroleum ether, and dried to obtain the purified lignins. The isolated lignins from acid-pretreated and kraft pulping bamboo were termed MWLa and MWLp, respectively.
Enzymatic Hydrolysis of Pretreated Bamboo
Enzymatic hydrolysis of pretreated bamboo was conducted at a substrate loading of 5 % (w/v) with a cellulase loading of 20 FPU/g glucan. The enzymatic hydrolysis experiment (50 mL) was performed in a 250-mL Erlenmeyer flask at 50 °C using 50 mM citrate buffer (pH 4.8), which was shaken at 150 rpm for 48 h. Aliquot of the enzymatic saccharification was withdrawn and centrifuged for 10 min at 4000 rpm; the supernatant was subsequently filtered through a 0.22-μm syringe filter and analyzed to determine the sugar content. The enzymatic hydrolysis efficiency of pretreated sample was calculated as follows:
Enzymatic Hydrolysis of Avicel with Isolated Lignins
To investigate the effects of isolated lignins on the enzymatic hydrolysis of pure cellulose, various amount of MWLa and MWLp were added into the saccharification reaction solution with of Avicel, respectively, prior to the addition of cellulase. The amounts of lignin in the reaction substrate were 5, 10, 15, 20, and 25 %, respectively. The enzymatic hydrolysis conditions were the same as that of enzymatic hydrolysis of pretreated samples.
Cellulase Adsorption Kinetics and Adsorption Isotherm on Isolated Lignins
Cellulase Adsorption Kinetics on Isolated Lignins
To measure adsorption kinetics, 0.2 g of isolated lignin (MWLa and MWLp) was suspended in 10 mL of 50 mM citrate buffer (pH 4.8) with cellulase protein content of 0.5 mg/mL. The reactions were incubated with shaking (150 rpm) for 6 h at 4 °C. Aliquots (0.5 mL) were taken at 10, 20, 30, 60, 120, 180, and 360 min during the incubation. The protein content and cellulase activity were analyzed in subsequent experiments. The adsorbed cellulase on lignin was calculated from the difference value between the initial enzyme protein content and the free enzyme protein content in supernatant.
Cellulase Adsorption Isotherm on Isolated Lignins
To determine cellulase adsorption isotherm on residual lignin, cellulase was incubated with isolated lignin (MWLa and MWLp) at 2 % (w/v) at 4 °C and 150 rpm for 4 h, as described by Lai et al. [22]. A range of cellulase concentration (0.01, 0.02, 0.04, 0.08, 0.16, 0.5, 1.0, and 2.0 mg/mL) was added in 50 mM citrate buffer with the lignin. After 4 h of incubation to reach equilibrium, the mixture was centrifuged at 4000 rpm for 20 min and the supernatant was taken for protein content analysis. The adsorbed cellulase was calculated from the difference value between the initial enzyme protein content and the free enzyme protein content in supernatant.
Cellulase adsorption on isolated lignins was characterized by Langmuir adsorption isotherms, as follows:
Г The corresponding adsorbed cellulase on the substrate (mg/g lignin)
Г max The maximum adsorption capacity (mg/g lignin)
K The Langmuir constant (mL/mg)
C The free cellulase in supernatant (mg/mL)
R The distribution coefficient (L/g)
Analysis of Sugars, Cellulase Activity, and Free Protein in Supernatant
Constituents of Pretreated and Isolated Lignin, Sugar in Supernatant
The constituents of the pretreated bamboo and isolated lignins were determined based on the procedure developed by the National Renewable Energy Laboratory for analyzing biomass materials [23]. The sugars of the constituent analysis and enzymatic saccharification were measured using a high-performance liquid chromatography (HPLC) system equipped with an Aminex HPX-87H column (300 × 7.8 mm) and a refractive index (RI) detector, and 5 mM H2SO4 solution was used as the eluent at a flow rate of 0.6 mL/min.
Determination of Activity and Protein Content of Cellulase
The activity of cellulase was determined according to the International Union of Pure and Applied Chemistry (IUPAC) standard [24].
The cellulase protein content in supernatant was determined according to the Bradford method using bovine serum albumin as the protein standard [25].
Characterization of Isolated Lignin
Surface Charge of the Lignin
Potentiometric titration was used to measure the surface charges of isolated lignins [22]. One hundred twenty milligrams of the lignin was dissolved in 10 g NaOH solution (0.1 M), then the solution was acidified by the 1 M HCl (3 g) for 10 min. After that, 30 g NaOH solution (0.1 M) was added into the solution to neutralize the acid and to re-dissolve the lignin. The lignin solution was titrated by 0.1 M HCl with the automatic titrator (AUT-701, DKK-TOA) from pH 12.0 to 2.0. Meanwhile, the lignin-free solution (blank) was prepared and titrated by the same process. The surface charges (mmol/g) on the lignin were calculated according to the equation: Q = (V blank − V sample) × M/W, where Q is the surface charges (mmol/g) of lignin, V sample and V blank are the titration volume consumed by lignin sample solution and blank solution, respectively, M is the mole concentration of HCl, and W is the weight of lignin sample.
Lignin Hydrophobicity Estimation by Rose Bengal
The hydrophobicity of the isolated lignin was estimated by measuring the distribution of Rose Bengal (hydrophobic dye) in the solution and on the lignin [22, 26]. A range of lignin concentration (2–10 g/L) was added into 50 mM citrate buffer (pH 4.8) with a constant concentration of Rose Bengal (40 mg/L). To distribute the hydrophobic dye between lignin surface and the solution, the mixture suspension was incubated at 50 °C, 150 rpm for 2 h. After incubation, the lignin (adsorbed with dye) was separated by centrifugation. The adsorbed dye in lignin was calculated by the difference between the initial dye content and the free dye content in solution. UV-Vis spectrometer (543 nm) was used to measure the free dye content in the supernatant. The partition quotient (PQ) was calculated from the ratio of the adsorbed dye over the free dye. The obtained PQ was plotted against lignin content. The slope from the linear plotting was calculated as the surface hydrophobicity of lignin (L/g).
NMR Spectroscopy Analysis
13C NMR was used to calculate the amount of functional groups and degree of condensation of the isolated lignins by acetylation [12, 18]. NMR spectra of the lignins were acquired on a Bruker AVANCE 600 MHz spectrometer equipped with a 5 mm BBO probe using an inverse-gated proton decoupling sequence. The acquisition parameters were according to our previous work [21]. One hundred sixty milligrams of purified lignin was dissolved in 0.5 mL DMSO-d6. Forty microliters of Chromium (III) acetylacetonate (0.01 M) was added to provide complete relaxation of all nuclei. Then, the solution was transferred to a Shigemi microtube and characterized at 25 °C. The acquisition parameters were 90° pulse width, a relaxation delay of 1.7 s, and an acquisition time of 1.2 s. A total of 20,000 scans were collected.
Results and Discussion
Enzymatic Hydrolysis of Bamboo Pretreated with Sulfuric Acid and Kraft Pulping
In this work, after pretreatment with 0.5 % (w/v) sulfuric acid at 140 °C for 60 min, the compositions of pretreated bamboo residues (APBR) were 57.14 % glucan, 4.67 % xylan, and 22.15 % lignin. After kraft pulping with 12 % EA charge and 20 % sulfidity at 140 °C for 60 min, the compositions of pretreated bamboo residues (KPBR) were 57.66 % glucan, 23.27 % xylan, and 11.71 % lignin. The enzymatic digestibility of pretreated bamboo and the amount of free cellulase (protein content and activity) in supernatant were analyzed during the enzymatic saccharification process, as shown in Fig. 1.
Figure 1a showed that the enzymatic saccharification of APBR proceeded rapidly during the first 12 h and then slowed down gradually. The enzymatic hydrolysis efficiency of APBR reached 5.61 % at 12 h. While, the efficiencies were just 7.11 and 7.94 % at 24 and 48 h, respectively. It is considered that dilute sulfuric acid pretreatment can remove hemicellulose, generating more pores in pretreated lignocellulosic materials for improving the enzymatic saccharification efficiency [11]. In this work, although the remaining hemicellulose in APBR was just 4.67 %, the enzymatic digestibility was still disappointingly low. For KPBR (Fig. 1b), 19.45 % hydrolysis efficiency was achieved at 12 h, and the efficiency was increased to 33.69 % at 48 h. These results indicated that pulped bamboo showed a better enzymatic digestibility than that pretreated by sulfuric acid. In this work, when enzymatic hydrolysis with 20 FPU/g glucan, the initial cellulase concentration in the two hydrolysis solutions were both 0.15 mg/mL (0.57 FPU/mL). For APBR, the free cellulase in hydrolysis solution was decreased in whole saccharification process; no cellulase was released into the solution. At 48 h of reaction, over 95 % of cellulase was adsorbed on the substrate, with remaining 0.005 mg/mL cellulase in the solution. For KPBR, the free cellulase in hydrolysis solution was decreased rapidly in the first 3 h, then increased gradually (3–12 h), and then decreased gradually (12–48 h), respectively. After 48 h of reaction, the cellulase protein concentration and cellulase activity in solution were 11.33 % (0.017 mg/mL) and 14.11 % (0.081 FPU/mL) of the initial value, respectively. Compared with the adsorption results, it seemed that cellulase had higher affinity on acid-pretreated bamboo residues than that on kraft pulping bamboo residues. During enzymatic hydrolysis, the amount of cellulase can be adsorbed on the cellulose and lignin in substrate. It is reported that the adsorption between cellulase and cellulose is a kind of reversible adsorption [27]. After the cellulose is saccharified by cellulase, the enzyme can release into the hydrolysis solution again [28]. Irreversible and reversible adsorption of cellulase on lignin may occur during enzymatic hydrolysis process. For the irreversible adsorption, the adsorbed cellulase on lignin is inactivated gradually. In this work, cellulose (glucan) content in these two pretreated samples was similar, and the apparent difference between APBR (22.15 % of lignin) and KPBR (11.71 % of lignin) was lignin content. During saccharification process of KPBR, cellulase in the substrate presented the status of adsorption and desorption as hydrolysis progressing. The free cellulase in APBR kept reducing as hydrolysis is progressing.
Influence of the Residual Lignins on Cellulose Hydrolysis
Residual Lignin Isolation
To determine the effect of residual lignin on the enzymatic hydrolysis of the pretreated substrates in detail, lignins in APBR and KPBR were isolated according to Björkman [20], which has also been applied to investigate the effect of residual lignin in steam-pretreated Douglas-fir on the enzymatic hydrolysis of cellulose [12]. The isolated lignins from acid-pretreated bamboo and kraft pulped bamboo were termed MWLa and MWLp, respectively. The isolation yields of MWLa and MWLp were 69.21 and 54.34 %, respectively. As shown in Table 1, two purified lignin preparations contained low amounts of carbohydrates, which had little influence on enzymatic hydrolysis in subsequent experiments. The total carbohydrates in MWLa and MWLp preparations were 3.45 and 4.23 %, respectively.
Influence of the Isolated Lignins on Cellulose Hydrolysis
In order to assess the effect of residual lignin on pure cellulose hydrolysis, the isolated lignins were added into reaction mixture containing Avicel (PH101). The lignin content in substrate was various from 5 to 25 %. The results indicated that both MWLa and MWLp had negative effects on the enzymatic hydrolysis efficiency of Avicel. As shown in Fig. 2a, the enzymatic hydrolysis efficiency of Avicel decreased with increasing lignin content in substrate. When the substrate had same content of MWLa and MWLp, the inhibitory effect of MWLa was found to be considerably stronger than that of MWLp. For example (Fig. 2b), when the substrate contained with 20 % lignin, MWLa and MWLp addition decreased the hydrolysis efficiency of Avicel from 64.21 to 28.31 % and from 64.21 to 36.95 %, respectively. The amount of free cellulase (protein content) in supernatant was also analyzed during the enzymatic hydrolysis process (Fig. 2b). For Avicel saccharification (48 h), 42.95 % of initial cellulase was remained in the solution. However, the free cellulase in the solution of Avicel with 20 % MWLa was just 7.99 %, lower than that in the solution of Avicel with 20 % MWLp (13.79 %). In this case, as Avicel and lignin content in substrates was same, more negative effect on the enzymatic saccharification of Avicel could be the result of more significantly nonproductive adsorption from addition lignin [29]. This result was in agreement with Pan [30] that the decreasing hydrolysis efficiency of Avicel in the presence of the lignin isolated from the substrates was the result of cellulase adsorption to lignin.
Adsorption Kinetics of Cellulase on Isolated Lignins
In order to evaluate the cellulase adsorption kinetics on isolated lignin, two lignin preparations were suspended in acetate buffer (pH 4.8) with 0.5 mg/mL cellulase at 4 °C for 5 h. The adsorbed cellulase was calculated from the difference value between the initial enzyme protein content and the free enzyme protein content in solution [29]. Figure 3 showed that the equilibrium adsorption time of cellulase on MWLa required about 1 h. But cellulase absorption on MWLp needs more equilibrium time with 2 h. After 5 h, the absorbed cellulase on MWLa and MWLp was 84.39 and 91.02 % of initial cellulase content, respectively. These indicated that cellulase adsorption kinetics on MWLa was considerably different from it onto MWLp. More cellulase being adsorbed on MWLa might be attributed to a higher adsorption affinity [12, 31].
Adsorption Isotherm of Cellulase on Isolated Lignins
To characterize the cellulase adsorption capacity and affinity on residual lignin, adsorption isotherms of cellulase on MWLa and MWLp preparations were measured by incubation of cellulase with the isolated lignins at 4 °C for 4 h. The maximum adsorption capacity, affinity, and binding strength were estimated by analyzing the free cellulase present in the supernatant [22]. The results were shown in Fig. 4 and Table 2.
As shown in Fig. 4, the experimental data of enzyme adsorption fit well into Langmuir adsorption isotherm models, with R 2 > 0.98. The Langmuir adsorption isotherms revealed significant differences of cellulase adsorption on MWLa and MWLp. The maximum adsorption capacity of cellulase on MWLp was Г max = 129.49 mg/g lignin, which was lower than that on MWLa (Г max = 160.25 mg/g lignin). One probable reason for this observation was that the MWLa from acid-pretreated bamboo might have more binding sites on lignin particle surface due to the preservation of functional groups and lignin branches during pretreatment. The MWLp obtained from kraft pulping bamboo residues might possess fewer binding sites and fewer branches because a large proportion of lignin was solubilized during the pretreatment process [32].
It is reported that Langmuir constant (K) from adsorption isotherm represents an equilibrium affinity constant of cellulase on lignin, which is used to evaluate the affinity of cellulase on substrates [33]. As shown in Table 2, cellulase showed a higher affinity to MWLa (K = 2.04 mL/mg) than to MWLp (K = 1.44 mL/mg). The distribution coefficient (R), which was reported as the binding strength, was 0.33 and 0.19 L/g for MWLa and MWLp, respectively. These data indicated that residual lignin in acid-pretreated bamboo residues exhibited a higher affinity and binding strength for cellulase, suggesting again that the residual lignin in acid-pretreated bamboo residues bound more cellulase nonproductively. Hence, it is speculated that the low enzymatic hydrolysis efficiency of acid-pretreated bamboo may be due to the highly nonproductive cellulase adsorption on residual lignin, resulting in few free cellulase for enzymatic saccharification.
Physicochemical Properties of Isolated Lignins
The native characters of lignin have an effect on the nonproductive adsorption of the enzymes on lignin [34, 35]. Hence, understanding the native characters of residual lignins in acid-pretreated bamboo and pulped bamboo are of great importance to explain their different enzymatic digestibility. In this work, surface charges, hydrophobicity, and hydroxyl groups were characterized and established the relationship to the adsorption affinity and binding strength.
In general, lignin is a hydrophobic structure. Cellulase contains hydrophobic residues such as tryptophane, phenylalanine, and tyrosine. Therefore, it is considered that hydrophobicity seems to be the primary inhibitory effect of lignin that is governing cellulase unproductive binding. To measure the hydrophobicity of lignin, the methods of hydrophobic dye and contact angle are proposed. In this work, the hydrophobicity of MWLa and MWLp was determined by the hydrophobic dye (Rose Bengal), which also has been applied in the work of Lai et al. [22]. The results in Table 3 indicated that the hydrophobicity of MWLa (0.84 L/g) was higher than that of MWLp (0.55 L/g). Combining the results from adsorption isotherm, it is indicated that higher hydrophobicity of lignin results in the higher adsorption affinity and binding strength. Yang and Pan [36] also reported that the hydrophobicity of lignin had a positive correlation with adsorption affinity and binding strength. This might explain why the residual lignin in acid-pretreated bamboo (MWLa) had higher affinity constant and binding strength than those in residual lignin of kraft pulping bamboo (MWLp).
The surface charges of MWLa and MWLp were determined by potentiometric titration at pH 4.8. The results (Table 3) showed that the surface charges of MWLp was −0.54 mmol/g, higher than that of MWLa (−0.22 mmol/g). It was reported that the higher negative charges on lignin could potentially played a weak role on the nonproductive enzymes binding of lignin due to the enlarged electrostatic repulsion [37]. Hence, one reason for the lower adsorption affinity and binding strength of cellulase on kraft pulping bamboo lignin (MWLp) might be due to its higher surface charge, which could prevent the nonproductive binding [22].
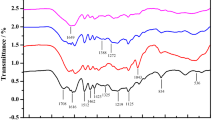
The functional groups of phenolic and aliphatic hydroxyls in lignin have shown to be another role in adsorption of enzymes on lignin by forming hydrogen bonds with carbonyl and hydroxyl group in the cellulase. Recently, Yu et al. [18] and Yang and Pan [36] both reported that the cellulase adsorption on lignin was correlated positively with phenolic hydroxyl but negatively with aliphatic hydroxyl. In this work, some of key lignin structure that have potential impact on nonproductive binding, such as the amount of functional groups and degree of condensation of two isolated lignin, were determined by 13C NMR. The spectra of MWLa and MWLp were shown in Fig. 5. The amount of hydroxyl groups (per Ar) and degree of condensation were calculated according to [12] and Yu et al. [18], and the results were listed in Table 3.
After acid pretreatment, the total aliphatic OH group in residual lignin (MWLa) was 0.96 per Ar, lower than those (1.04 per Ar) in residual lignin in kraft pulped bamboo (MWLp). For the phenolic OH groups, the amount in MWLa and MWLp were 0.71 per Ar and 0.66 per Ar, respectively. In present work, the results (for MWLa as example) also indicated higher phenolic OH amount, and lower aliphatic OH amount might result in higher adsorption affinity and binding strength, which was in agreement with the other reports [18, 36]. Table 3 showed that the degree of MWLa and MWLp were 0.51 and 0.38, respectively. Generally, the condensation reaction can generate more carbon-carbon linkages in lignin resulting in lignin being more hydrophobic. Yu et al. [18] found that higher degree of condensation of lignin was more hydrophobic, showing higher ability on cellulase adsorption. Hence, for residual lignin in acid-pretreated bamboo, the higher hydrophobicity, amount of phenolic OH, and degree of condensation might be another responsible impact factors for its higher adsorption affinity and binding strength.
In this work, although the relationship of cellulase adsorption affinity with lignin properties (surface charges, hydrophobicity, function groups, and the degree of condensation) has also been showed previously, we used this work to elucidate how the residual lignins in the acid- and alkali-pretreated bamboo affect their enzymatic digestibility and enzyme adsorption affinity on lignin. The results discussed above suggest that higher nonproductive cellulase adsorption of residual lignin in acid-pretreated bamboo may be responsible for its disappointingly low enzymatic digestibility, and the nonproductive adsorption is related to its corresponding structure properties. As the surface hydrophobicity and charge are relevant to functional groups and different lignins, how the functional groups and structure properties of different lignins affect their corresponding adsorption affinity will be done in our following work.
Conclusion
In this work, the effects of residual lignin on enzymatic hydrolysis of moso bamboo pretreated by sulfuric acid and kraft pulping were compared by enzyme adsorption and adsorption isotherm. The results from adsorption isotherm indicated that residual lignin in acid-pretreated bamboo adsorbed more cellulase than that in kraft pulping bamboo, due to the higher adsorption capacity, adsorption affinity, and binding strength. Residual lignin in acid-pretreated bamboo showed higher inhibitory effect on enzymatic digestibility of the pure cellulose. The adsorption affinity and binding strength between enzyme and lignin were related to the surface charges, hydrophobicity, amount of function group, and the degree of condensation of lignin. The higher nonproductive cellulase adsorption and physicochemical properties of residual lignin in acid-pretreated bamboo may be responsible for its disappointingly low enzymatic digestibility.
References
Huang, C., He, J., Li, X., Min, D., & Yong, Q. (2015). Facilitating the enzymatic saccharification of pulped bamboo residues by degrading the remained xylan and lignin–carbohydrates complexes. Bioresource Technology, 192, 471–477.
Li, Z., Jiang, Z., Fei, B., Cai, Z., & Pan, X. (2014). Comparison of bamboo green, timber and yellow in sulfite, sulfuric acid and sodium hydroxide pretreatments for enzymatic saccharification. Bioresource Technology, 151, 91–99.
Xin, D., Yang, Z., Liu, F., Xu, X., & Zhang, J. (2015). Comparison of aqueous ammonia and dilute acid pretreatment of bamboo fractions: structure properties and enzymatic hydrolysis. Bioresource Technology, 175, 529–536.
Yang, Z., Zhang, M., Xin, D., Wang, J., & Zhang, J. (2014). Evaluation of aqueous ammonia pretreatment for enzymatic hydrolysis of different fractions of bamboo shoot and mature bamboo. Bioresource Technology, 173, 198–206.
Zhang, J., Wang, X., Chu, D., He, Y., & Bao, J. (2011). Dry pretreatment of lignocellulose with extremely low steam and water usage for bioethanol production. Bioresource Technology, 102(6), 4480–4488.
Galbe, M., & Zacchi, G. (2012). Pretreatment: the key to efficient utilization of lignocellulosic materials. Biomass and Bioenergy, 46, 70–78.
Han, Q., Jin, Y., Jameel, H., Chang, H. M., Phillips, R., & Park, S. (2015). Autohydrolysis pretreatment of waste wheat straw for cellulosic ethanol production in a co-located straw pulp mill. Applied Biochemistry and Biotechnology, 175(2), 1193–1210.
Tu, M., Chandra, R. P., & Saddler, J. N. (2007). Recycling cellulases during the hydrolysis of steam exploded and ethanol pretreated lodgepole pine. Biotechnology Progress, 23(5), 1130–1137.
Kumar, L., Arantes, V., Chandra, R., & Saddler, J. (2012). The lignin present in steam pretreated softwood binds enzymes and limits cellulose accessibility. Bioresource Technology, 103(1), 201–208.
Studer, M. H., DeMartini, J. D., Davis, M. F., Sykes, R. W., Davison, B., Keller, M., & Wyman, C. E. (2011). Lignin content in natural Populus variants affects sugar release. Proceedings of the National Academy of Sciences, 108(15), 6300–6305.
Yu, Z., Jameel, H., Chang, H. M., & Park, S. (2011). The effect of delignification of forest biomass on enzymatic hydrolysis. Bioresource Technology, 102(19), 9083–9089.
Nakagame, S., Chandra, R. P., Kadla, J. F., & Saddler, J. N. (2011). The isolation, characterization and effect of lignin isolated from steam pretreated Douglas-fir on the enzymatic hydrolysis of cellulose. Bioresource Technology, 102(6), 4507–4517.
Wang, W., Zhu, Y., Du, J., Yang, Y., & Jin, Y. (2015). Influence of lignin addition on the enzymatic digestibility of pretreated lignocellulosic biomasses. Bioresource Technology, 181, 7–12.
Li, Y., Sun, Z., Ge, X., & Zhang, J. (2016). Effects of lignin and surfactant on adsorption and hydrolysis of cellulases on cellulose. Biotechnology for Biofuels, 9(1), 1.
Huang, C., Chu, Q., Xie, Y., Li, X., Jin, Y., Min, D., & Yong, Q. (2015). Effect of kraft pulping pretreatment on the chemical composition, enzymatic digestibility, and sugar release of moso bamboo residues. BioResources, 10(1), 240–255.
Huang, C., He, J., Wang, Y., Min, D., & Yong, Q. (2015). Associating cooking additives with sodium hydroxide to pretreat bamboo residues for improving the enzymatic saccharification and monosaccharides production. Bioresource Technology, 193, 142–149.
Kumar, R., & Wyman, C. E. (2009). Access of cellulase to cellulose and lignin for poplar solids produced by leading pretreatment technologies. Biotechnology Progress, 25(3), 807–819.
Yu, Z., Gwak, K. S., Treasure, T., Jameel, H., Chang, H. M., & Park, S. (2014). Effect of lignin chemistry on the enzymatic hydrolysis of woody biomass. ChemSusChem, 7(7), 1942–1950.
Palonen, H., Tjerneld, F., Zacchi, G., & Tenkanen, M. (2004). Adsorption of Trichoderma reesei CBH I and EG II and their catalytic domains on steam pretreated softwood and isolated lignin. Journal of Biotechnology, 107(1), 65–72.
Björkman, A. (1954). Isolation of lignin from finely divided wood with neutral solvents. Nature, 174, 1057–1058.
Huang, C., He, J., Du, L., Min, D., & Yong, Q. (2016). Structural characterization of the lignins from the green and yellow bamboo of bamboo culm (Phyllostachys pubescens). Journal of Wood Chemistry and Technology, 36(3), 157–172.
Lai, C., Tu, M., Li, M., & Yu, S. (2014). Remarkable solvent and extractable lignin effects on enzymatic digestibility of organosolv pretreated hardwood. Bioresource Technology, 156, 92–99.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., & Crocker, D. (2011). Determination of structural carbohydrates and lignin in biomass, laboratory analytical procedure (LAP), technical report NREL/TP-510-42618, National Renewable Energy Laboratory (NREL). U.S. Dept. of Energy: Golden, CO..
Ghose, T. K. (1987). Measurement of cellulase activities. Pure and Applied Chemistry, 59(2), 257–268.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities utilizing the principle of protein dye binding. Analytical Biochemistry, 72(1–2), 248–254.
Gessner, A., Waicz, R., Lieske, A., Paulke, B. R., Mäder, K., & Müller, R. H. (2000). Nanoparticles with decreasing surface hydrophobicities: influence on plasma protein adsorption. International Journal of Pharmaceutics, 196(2), 245–249.
Taniguchi, M., Kobayashi, M., & Fujii, M. (1989). Properties of a reversible soluble–insoluble cellulase and its application to repeated hydrolysis of crystalline cellulose. Biotechnology and Bioengineering, 34(8), 1092–1097.
Yang, B., & Wyman, C. E. (2006). BSA treatment to enhance enzymatic hydrolysis of cellulose in lignin containing substrates. Biotechnology and Bioengineering, 94(4), 611–617.
Tu, M., Pan, X., & Saddler, J. N. (2009). Adsorption of cellulase on cellulolytic enzyme lignin from lodgepole pine. Journal of Agricultural and Food Chemistry, 57(17), 7771–7778.
Pan, X. (2008). Role of functional groups in lignin inhibition of enzymatic hydrolysis of cellulose to glucose. Journal of Biobased Materials and Bioenergy, 2(1), 25–32.
Yu, H., Li, X., Zhang, W., Sun, D., Jiang, J., & Liu, Z. (2015). Hydrophilic pretreatment of furfural residues to improve enzymatic hydrolysis. Cellulose, 22(3), 1675–1686.
Nonaka, H., Kobayashi, A., & Funaoka, M. (2013). Lignin isolated from steam-exploded eucalyptus wood chips by phase separation and its affinity to Trichoderma reesei cellulase. Bioresource Technology, 140, 431–434.
Tomme, P., Boraston, A., McLean, B., Kormos, J., Creagh, A. L., Sturch, K., & Kilburn, D. G. (1998). Characterization and affinity applications of cellulose-binding domains. Journal of Chromatography B: Biomedical Sciences and Applications, 715(1), 283–296.
Shevchenko, S. M., Chang, K., Dick, D. G., Gregg, D. J., & Saddler, J. N. (2001). Structure and properties of lignin in softwoods after SO2-catalyzed steam explosion and enzymatic hydrolysis. Cell Chemistry Technology, 35(5–6), 487–502.
Zhu, M. Q., Wen, J. L., Su, Y. Q., Wei, Q., & Sun, R. C. (2015). Effect of structural changes of lignin during the autohydrolysis and organosolv pretreatment on Eucommia ulmoides Oliver for an effective enzymatic hydrolysis. Bioresource Technology, 185, 378–385.
Yang, Q., & Pan, X. (2016). Correlation between lignin physicochemical properties and inhibition to enzymatic hydrolysis of cellulose. Biotechnology and Bioengineering, 113, 1213–1224.
Wang, Z., Zhu, J. Y., Fu, Y., Qin, M., Shao, Z., Jiang, J., & Yang, F. (2013). Lignosulfonate-mediated cellulase adsorption: enhanced enzymatic saccharification of lignocellulose through weakening nonproductive binding to lignin. Biotechnology for Biofuels, 6, 156.
Acknowledgments
The research was supported by the National Natural Science Foundation of China (31570561) and the Natural Science Foundation of Jiangsu Province (BK20150874). The authors thank the Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD) and the Doctorate Fellowship Foundation of Nanjing Forestry University for supporting the work presented in this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Juan He contributed equally to this work.
Rights and permissions
About this article
Cite this article
Huang, C., He, J., Min, D. et al. Understanding the Nonproductive Enzyme Adsorption and Physicochemical Properties of Residual Lignins in Moso Bamboo Pretreated with Sulfuric Acid and Kraft Pulping. Appl Biochem Biotechnol 180, 1508–1523 (2016). https://doi.org/10.1007/s12010-016-2183-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2183-8