Abstract
Enrichment of biomass containing high lipid content is the key limiting step for the utilization of cyanobacterial feedstock in biodiesel production. This study investigated the influence of antibiotics on the biodiesel productivity of a model biodiesel-producing cyanobacterium (Synechocystis sp. PCC 6803) through a 18-day exposure test and observed that 100 ng/L of ciprofloxacin, amoxicillin, and spiramycin significantly (p < 0.05) increased biomass, chlorophyll a content, and Fv/Fm value and rETRmax value in Synechocystis sp. PCC 6803. Due to the stimulation of photosynthesis, the 18-day dry weights of the cyanobacterial cells increased from 0.354 ± 0.039 to 0.508 ± 0.048, 0.58 ± 0.028, and 0.66 ± 0.028 g/L under exposure to ciprofloxacin, amoxicillin, and spiramycin, respectively. As a stress response to antibiotics, the lipid content in Synechocystis sp. PCC 6803 increased from 14.71 to 20.92%, 20.59%, and 15.36% under exposure to ciprofloxacin, amoxicillin, and spiramycin, respectively. Due to the increase of biomass and lipid content, the lipid productivity of Synechocystis sp. PCC 6803 increased from 2.89 to 5.90, 6.63, and 5.63 mg/L/d under exposure to ciprofloxacin, amoxicillin, and spiramycin, respectively. Exposure to each target antibiotic increased the proportion of monounsaturated fatty acids in the lipid, while exposure to spiramycin reduced the proportion of saturated fatty acid. Antibiotics regulated the lipid composition of Synechocystis sp. PCC 6803 towards an increase of combustion property. This study proved that antibiotic exposure increased the lipid productivity of cyanobacteria through hormesis, which provided new insights for promoting the production of cyanobacteria-based biodiesel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to excessive consumption and increased demand of conventional fossil fuel resources, the development of alternative energy has attracted great attention. Biodiesel is a promising substitute for fossil fuel, because of its renewable and environmentally friendly features [1]. Compared with crops and animal fats, microalgae present many advantages as feedstocks for biodiesel production, including short harvesting life, fast growth rate, and high lipid content [2, 3]. Utilization of microalgae as feedstocks for biodiesel production could also avoid the occupation of farmland and reduce the emission of sulfoxide [4]. At present, eukaryotic microalgae are commonly used for the production of microalgae-based biodiesel, while less attention was paid to cyanobacteria. Cyanobacteria are a large group of fast-growing prokaryotic microalgae with great tolerance to extreme condition and variable illumination [5], which show excellent ability for the production of bioenergy, including ethanol, hydrogen, butanol, and various triglyceride lipids [6]. For instance, Vargas et al. observed a hydrogen production ability of 4.1 μmol per mg chlorophyll a per day in Anabaena sp. PCC 7120 [7]. Synechocystis sp. PCC 6803 showed a good performance in the production of both ethanol and triglycerides. Triglycerides could be further transesterified into fatty acid methyl esters (FAMEs) that have good combustion properties [8].
Cost-effective production of high-quality biodiesel is the major challenge for the commercialization of microalgae-based biodiesel [9]. Various efforts have been made to increase the lipid production ability of cyanobacteria, including the optimization of culture conditions [10], the exposure to exogenous stresses [11], the construction of mixed microalgae consortia [12,13,14], the improvement of lipid extraction and transesterification [15], as well as the application of genetic modification [16]. Antibiotics are a large group of chemicals highly effective against bacteria, which are widely used for the protection of human and animal health and the promotion of plant growth [17]. Recent studies observed that cyanobacterial cells were sensitive to antibiotic stress due to their bacterial-like structure [18]. Antibiotics are toxic to cyanobacteria at high concentrations while generate various stimulatory effects in cyanobacteria at low concentrations, including the promotion of growth, photosynthesis, and carbohydrate synthesis [19]. For instance, 100–300 ng/L of amoxicillin, ciprofloxacin, and sulfamethoxazole were found to increase growth rate and polysaccharide content as well as induce over-expression of proteins related to carbohydrate synthesis in Microcystis aeruginosa. Erythromycin was found to increase cell density and photosynthetic activity in Microcystis flos-aquae at low concentrations of 1–100 ng/L [20, 21]. The above studies suggested that antibiotic stress had the potential to simultaneously increase biomass and lipid content in cyanobacteria, which might contribute to a better biodiesel production ability. However, the influence of antibiotics on biodiesel-producing cyanobacteria has not been reported by previous studies.
In order to test the above hypothesis, the influence of three commonly used antibiotics (ciprofloxacin, amoxicillin, and spiramycin) on biomass, photosynthetic activity, lipid content, and fatty acid composition in a model biodiesel-producing cyanobacterium, Synechocystis sp. PCC6803, was investigated in this study. To date, this is the first study evaluating the possibility of introducing antibiotic stress into cyanobacteria-based biodiesel production for the promotion of biodiesel productivity.
Materials and Methods
Antibiotic Exposure
Synechocystis sp. PCC 6803 was supplied by Pasteur Culture Collection of Cyanobacterial Strains (France). Ciprofloxacin (CIP, purity ≥ 98.0%), amoxicillin (AM, purity ≥ 98.7%), and spiramycin (SPI, purity ≥ 95.0%) were obtained from Dr. Ehrenstorfer, Inc. (Germany). Antibiotic stock solution (1 mg/L) was prepared by dissolving each antibiotic in methanol (Merck Co., Ltd., Germany). The stock solutions were stored in − 20 °C before use. Four test groups were prepared for evaluating the effects of antibiotics on Synechocystis sp. PCC 6803, including the CIP-treated group, the AM-treated group, the SPI-treated group, and the solvent control. In the exposure test, the cyanobacterial cells were cultivated in 250-mL flasks containing 150 mL standard BG11 medium. The initial biomass characterized by OD730 (optical density value at 730 nm) was 0.05. The stock solution of each target antibiotic was added into the culture medium at the beginning of cyanobacterial cultivation. The test concentration for each target antibiotic was 100 ng/L. Due to the photolysis and hydrolysis of antibiotics, the stock solution of each antibiotic was daily replenished into the culture medium, in order to maintain a stable exposure dose. The replenishing amount was determined in a preliminary experiment according to a previously reported method [22]. The final concentration of methanol in the culture medium was controlled below 0.005% (v/v). The BG11 medium containing cyanobacterial cells and 0.005% (v/v) methanol was prepared as the solvent control. Three replicates were prepared for each test group. Four test groups were cultured for 18 days under continuous fluorescent light at an irradiance of 2000 lx. The light:dark cycle was maintained as 16 h:8 h. The cultivation temperature was 25 °C.
Analysis of Cellular Responses
The OD730 value of the culture medium was measured every day with a TU-1810 UV–Vis spectrophotometer (Persee Co., Ltd., China). The growth rate was calculated according to the slope of the linear regression between the natural logarithm of OD730 value and the cultivation time in the exponential phase [23]. 5 mL of cyanobacterial culture medium were sampled and centrifuged at 6000×g for 10 min every 3 days using a TG-16 high-speed centrifuge (Bioridge Co., Ltd., China). The cyanobacterial cells were extracted by 90% (v/v) ethanol aqueous solution, and the supernatant was subjected to a TU-1810 UV–Vis spectrophotometer for analyzing the absorbance at 630 nm, 647 nm, 664 nm, and 750 nm. The content of chlorophyll a was calculated according to the method of Bland and expressed in μg/L [24].
The measurement of photosystem II (PSII) activity was conducted every 3 days using a Dual-PAM-100 fluorometer (Walz Co., Ltd., Germany). 5 mL of cyanobacterial cells were dark-adapted for 10 min prior to the measurement. The minimum fluorescence (F0) was obtained by applying light pulses at low frequency (1 Hz). The maximum fluorescence (Fm) was determined by applying a saturation pulse (0.6 s, 10 Hz) [25]. The maximum photochemical quantum yield of PSII (Fv/Fm) was calculated as Eq. (1):
The rapid light curve (RLC) was obtained by applying a series of 20 s light exposures with increasing photosynthetically active radiation (PAR) values [25]. The maximum relative electron transport rate (rETRmax) was defined as the value of the plateau phase in the rapid light curve.
Analysis of Total Lipid Content
At the end of the cultivation period, 50 mL cyanobacterial culture medium was sampled and centrifuged at 6000×g for 15 min. The precipitate was rinsed with distilled water and centrifuged twice to remove the inorganic salt in the medium. The pellet was frozen dried with a Scientz-10N lyophilizer (Scientz Biotechnology Co., Ltd., China) for 48 h. The dry weight of the cyanobacterial cells (M) was obtained. The dry cyanobacterial cells were ground to powder and extracted with the mixture of chloroform and methanol (2:1, v/v) according to the method of Folch [26]. The supernatant was collected by centrifugation (6000×g, 15 min), transferred to a 10 mL centrifuge tube, and mixed with 2 mL of 0.9% sodium chloride solution, and the weight of the centrifuge tube (m0) was obtained prior to use. The supernatant was removed, and the lower solution was dried under a nitrogen flow. The tubes with extracted lipid were weighed (m1), and the total lipid content and lipid productivity were calculated according to Eqs. (2) and (3), respectively.
Measurement of Fatty Acid Methyl Ester (FAMEs)
Fatty acids in cyanobacterial cells were methylated, and the contents of fatty acid methyl ester (FAMEs) were analyzed. 2 mL of 40 g/L KOH-CH3OH was used to dissolve 10 mg dry cyanobacterial cells. After that, the mixture was homogenized with a JY92-II ultrasonic cell pulverizer (Scientz Biotechnology Co., Ltd., China) for 2 min. Then the mixture was incubated in a water bath at 75 °C for 15 min and then was cooled to room temperature. The mixture was further mixed with 2 mL of 14% BF3-CH3OH solution (Sigma-Aldrich Co., Ltd., USA) and incubated in a water bath at 75 °C for 15 min. After cooling, 1 mL saturated NaCl solution and 2 mL n-hexane (Sigma-Aldrich Co., Ltd., U.S.A.) were added to extract FAMEs. The mixed solution was centrifuged at 4000×g for 8 min. The upper hexane layer was used for the analysis of FAMEs through an Agilent 7890A-5975C gas chromatography coupled to mass spectrometer (Agilent Co., Ltd., USA) according to the method of Anahas [27].
Statistics Analysis
A repeated measured ANOVA was used to determine the differences in biomass (OD730), chlorophyll a content, and Fv/Fm and rETRmax between each antibiotic-treated group and the solvent control during the whole cultivation period. A Student’s t test was used to determine the differences in dry weights, lipid contents, lipid productivity, and FAME contents between each antibiotic-treated group and the solvent control. All the statistics analysis were conducted through the SPSS software (version 22.0).
Results
Cellular Responses to Antibiotics
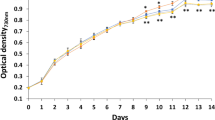
The effect of each antibiotic on the biomass of Synechocystis sp. PCC6803 was illustrated in Fig. 1. In each antibiotic-treated group, the biomass was significantly (p < 0.05) higher than that in the solvent control. The exponential growth rate of Synechocystis sp. PCC 6803 in the solvent control was 0.057 ± 0.0004 per day. The exponential growth rates in CIP-, AM-, and SPI-treated groups increased to 0.102 ± 0.002, 0.101 ± 0.002, and 0.107 ± 0.002 per day, respectively. On the last day of the culture period, the OD values in CIP-, AM-, and SPI-treated groups were 1.44-, 1.49-, and 1.52-fold higher than that in the solvent control, respectively.
The chlorophyll a content in the culture medium increased with culture time (Fig.2). Addition of antibiotics into the culture medium also significantly (p < 0.05) increased the chlorophyll a content. The highest chlorophyll a content was observed in the AM-treated group on the last day of the culture period, which was 1.76-fold higher than that in the solvent control. During the 18-day culture period, Fv/Fm and rETRmax values increased firstly and then decreased (Fig.3). This result was in accordance with the life cycle of cyanobacteria, in which cyanobacterial cells first grow slowly for adapting to new environment and then grow exponentially for rapid multiplication of biomass, followed by a stationary phase presenting low or zero growth rate. The highest Fv/Fm and rETRmax values in the solvent control were observed on the 12th day of the culture period. Compared with the solvent control, the Fv/Fm values were significantly (p < 0.05) increased by 9.8%–27.3%, 34.4%–53.4%, and 20.1%–56.4% following exposure to CIP, AM, and SPI, respectively. The rETRmax values in CIP-, AM-, and SPI-treated groups were 1.42–1.62, 1.28–1.93, and 1.26–1.91-fold higher (p < 0.05) than that in the solvent control, respectively. The above results indicated that each target antibiotic induced the stimulation of photosynthetic activity in Synechocystis sp. PCC 6803.
Influence of Antibiotics on Lipid Synthesis and FAME Composition
The dry weight, total lipid content, and the composition of FAMEs in each group are shown in Table 1. Significant increase (p < 0.05) of dry weight was observed in Synechocystis sp. PCC 6803 under exposure to each target antibiotic, which was in accordance with the responses of OD730 values. The total lipid content in the solvent control accounted for 14.71% of the dry weight. Exposure to CIP, AM, and SPI significantly increased (p < 0.05) the total lipid content, which accounted for 20.92%, 20.59%, and 15.36% of the dry weight, respectively. Furthermore, the lipid productivity of Synechocystis sp. PCC 6803 in CIP-, AM-, and SPI-treated groups was 2.04-, 2.29-, and 1.95-fold higher (p < 0.05) than that in the solvent control, respectively.
In the solvent control, eight fatty acids were successfully identified in cyanobacterial cells, including C15:0, C16:0, C16:1, C17:0, C18:0, C18:1, C18:2, and C18:3. Compared with the solvent control, C15:0 was not detected in the CIP-treated group, but C14:0 was detected in this group. In AM- and SPI-treated groups, C17:0 was not detected. In each test group, C16:0 and C18:2 were the major component of FAMEs. The proportion of saturated fatty acid (SFA) was significantly reduced (p < 0.05) in Synechocystis sp. PCC 6803 under exposure to SPI. The proportion of monounsaturated fatty acids (MUFA) in CIP-, AM-, and SPI-treated groups were 1.34-, 1.28-, and 1.58-fold higher than that in the solvent control, respectively. The proportion of unsaturated fatty acid (UFA) in Synechocystis sp. PCC 6803 increased significantly (p < 0.05) following exposure to CIP, AM, and SPI, respectively. The proportion of polyunsaturated fatty acid (PUFA) in Synechocystis sp. PCC 6803 was not significantly (p > 0.05) affected by antibiotic exposure.
Discussion
The total lipid productivity of microalgae is determined by biomass × lipid content. Culture of microalgae under stress conditions may facilitate lipid accumulation, while the biomass may not increase in that case. For instance, Casazza et al. observed that ultraviolet stress increased the lipid content of Chlorella sp. by 29.5%, reduced the FAME fraction by 67.3%, and caused no influence on biomass [28]. Two previous studies observed that the lipid content in Chlorella sp. increased from 11.5 to 21.8% due to the decrease of nitrogen supply in the culture medium, but the influence of nitrogen starvation on biomass was not mentioned in these studies [29, 30]. Recent studies observed that certain exogenous chemicals had the potential to simultaneously increase biomass and lipid content in biodiesel-producing microalgae. For instance, high bicarbonate concentration (HCO3− 171.2 mM) induced 37.9% increase of biomass and 32.5% increase of lipid content in Arthrospira ZJU9000, which corresponded to 82.7% increase of total lipid productivity [31]. Addition of kinetin into nitrogen-deficient culture medium led to an increase of 61.8% in the lipid productivity of Acutodesmus obliquus, compared with the microalgal cells cultured in standard BG11 medium [32]. This study proved that antibiotics also effectively increased the lipid productivity in Synechocystis sp. PCC 6803 through the increase of both biomass and lipid content.
Antibiotics were observed to generate hormesis effect in photosynthetic organisms, including plants, eukaryotic microalgae, and cyanobacteria [33]. Hormesis is a biphasic dose-dependent effect of toxicants or stressors, which is characterized by high-dose inhibition and low-dose stimulation [34]. For instance, Xie et al. found that chlortetracycline stimulated the growth of root in wheat (Triticum aestivum L.) through promoting the cell mitotic division at low concentrations of 0.125–0.5 mg/L while inhibiting the above physiological processes at high concentrations of 25–300 mg/L [35]. Liu et al. observed that amoxicillin inhibited the growth of a cyanobacterium (Microcystis aeruginosa) at high concentrations of ≥ 6.88 μg/L while stimulating cyanobacterial growth at low concentrations of 100–600 ng/L [36]. In this study, the increase of growth rate and biomass in Synechocystis sp. PCC 6803 was also supposed to be a typical hormesis effect under a moderate stress generated by antibiotics at low exposure doses. Results of previous studies and this study suggested that the growth stimulation effect of antibiotics due to hormesis was a universal phenomenon in photosynthetic organisms. In this study, all of the three target antibiotics with different antibacterial mechanisms were effective in stimulating the growth of Synechocystis sp. PCC 6803, suggesting that hormesis effects of antibiotics in cyanobacteria were not restricted by the category of antibiotics.
In photosynthetic organisms, the photosynthesis system was supposed to be the action target of antibiotics, which consequently triggered various downstream responses [37]. Direct evidence has been observed in plants. For instance, elevated root activity was coupled to increased leaf chlorophyll content in Phragmites australis exposed to 0.1–1 μg/L of ciprofloxacin, oxytetracycline, and sulfamethazine [38]. Gomes et al. observed that ciprofloxacin induced hormesis in duckweed (Lemna minor L.) through regulating photosynthesis [39]. Several studies also observed the positive correlation between photosynthetic parameters and growth parameters in eukaryotic microalgae and cyanobacteria under exposure to antibiotics. Wang et al. observed that the increase of cell density was coupled to the raise of chlorophyll a content in Microcystis aeruginosa exposed to the binary mixture of spiramycin and ampicillin at test concentrations of 300 ng/L [40]. Wan et al. [21] observed that exposure to 0.001–0.1 g/L of erythromycin increased biomass and chlorophyll a content in Microcystis flos-aquae. In this study, ciprofloxacin, amoxicillin, and spiramycin increased growth rate, chlorophyll a content, light energy conversion efficiency (Fv/Fm), and photochemical electron transport (rETRmax) in Synechocystis sp. PCC6803 at a low concentration of 100 ng/L. Increased chlorophyll a content may promote the capture of light energy, and increased photosynthetic activity may produce extra energy for cyanobacterial growth and the synthesis of cellular substances including lipid. Besides antibiotics, auxins were also found to cause hormesis in Scenedesmus sp. through the increase of photosynthetic activity and chlorophyll content, which consequently promoted growth and FAME accumulation [41]. Addition of exogenous chemicals that generate hormesis effects during the cultivation of microalgae may be a promising route for increasing the lipid productivity during the production of microalgae-based biodiesel.
C16-C18 FAMEs are the most important chemical composition in biodiesel [42]. In this study, C16-C18 FAMEs accounted for the largest proportion of the total FAME content in the solvent control and each antibiotic-treated group. The FAME profile of the biodiesel produced by Synechocystis sp. PCC6803 was similar to that produced by Chlorella sp. [29]. Besides, the proportion of linolenic acid (C18:3) was lower than 12% in the solvent control and each antibiotic-treated group, which met the requirements of EN 14214 Standard for biodiesel [43]. The above results proved that Synechocystis sp. PCC 6803 was a qualified feedstock for biodiesel production. Treatment with antibiotic showed beneficial effects on the lipid accumulation in Synechocystis sp. PCC 6803. Lipid accumulation was supposed to be a stress response in eukaryotic microalgae and cyanobacteria under exposure to exogenous stresses. For instance, higher lipid content was observed in Scenedesmus SDEC-8 and Chlorella SDEC-18 under nitrogen-deficient conditions, possibly due to the alteration of cellular substance synthesis towards a preference of carbohydrate and a limitation of nitrogenous compounds [44]. Longworth et al. also observed that nitrogen starvation induced increased lipid accumulation in Phaeodactylum tricornutum [45]. Furthermore, the synthesis of lipid is correlated with the carbon fixation process of photosynthesis [46]. The antibiotic-induced stimulation of photosynthesis may also contribute to increased lipid accumulation.
Several studies focused on the regulation of the FAME composition in biodiesel-producing microalgae. For instance, Udayan et al. observed that addition of kinetin and gibberellic acid (GA3) during the cultivation of Nannochloropsis oceanica CASA CC201 resulted in a high proportion of MUFA and a low proportion of SFA [47]. Gao et al. found that the increase of C:N ratio slightly increased the saturation degree of FAMEs in Chlorella sp. [48]. The degree of saturation and the length of the carbon chain in the lipid affect the combustion property of microalgae-based biodiesel. A low proportion of SFA and PUFA and a high proportion of MUFA are more favorable for oxidation stability and cold flow property [49]. In this study, exposure to each target antibiotic increased the proportion of MUFA in the lipid of Synechocystis sp. PCC 6803, while exposure to spiramycin reduced the proportion of SFA. This result indicated that antibiotic exposure improved the combustion property of the biodiesel produced by Synechocystis sp. PCC 6803. Further studies were still required for exploring the action mechanisms of antibiotics in biodiesel-producing cyanobacteria during the regulation of FAME composition. Besides, the influence of more antibiotics on the lipid productivity of more biodiesel-producing cyanobacterial species deserves further investigation to fully evaluate the possibility for utilizing antibiotics in the production of cyanobacteria-based biodiesel .
Conclusions
This study introduced antibiotics into the culture medium of the biodiesel-producing cyanobacterium Synechocystis sp. PCC 6803 and observed a significant increase of lipid productivity under antibiotic exposure. Ciprofloxacin, amoxicillin, and spiramycin effectively promoted cyanobacterial growth, photosynthesis, and lipid accumulation in Synechocystis sp. PCC 6803 and changed the composition of FAMEs towards an increase of unsaturation degree, which may improve the combustion property of the biodiesel. Antibiotics had a promising potential to be used as a positive regulator in the cultivation of cyanobacteria for biodiesel production.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Sun J, Xiong X, Wang M, Du H, Li J, Zhou D, Zuo J (2019) Microalgae biodiesel production in China: a preliminary economic analysis. Renew Sust Energ Rev 104:296–306. https://doi.org/10.1016/j.rser.2019.01.021
Chen J, Li J, Dong W, Zhang X, Tyagi RD, Drogui P, Surampalli RY (2018) The potential of microalgae in biodiesel production. Renew Sust Energ Rev 90:336–346. https://doi.org/10.1016/j.rser.2018.03.073
Mubarak M, Shaija A, Suchithra TV (2019) Flocculation: an effective way to harvest microalgae for biodiesel production. Journal of Environmental Chemical Engineering 7:103221. https://doi.org/10.1016/j.jece.2019.103221
Mathimani T, Baldinelli A, Rajendran K, Prabakar D, Matheswaran M, Pieter Van Leeuwen R, Pugazhendhi A (2019) Review on cultivation and thermochemical conversion of microalgae to fuels and chemicals: process evaluation and knowledge gaps. J Clean Prod 208:1053–1064. https://doi.org/10.1016/j.jclepro.2018.10.096
Garlapati D, Chandrasekaran M, Devanesan A, Mathimani T, Pugazhendhi A (2019) Role of cyanobacteria in agricultural and industrial sectors: an outlook on economically important byproducts. Appl Microbiol Biot 103:4709–4721. https://doi.org/10.1007/s00253-019-09811-1
Mathimani T, Pugazhendhi A (2019) Utilization of algae for biofuel, bio-products and bio-remediation. Biocatalysis and Agricultural Biotechnology. 17:326–330. https://doi.org/10.1016/j.bcab.2018.12.007
Vargas SR, Santos PVD, Zaiat M, Calijuri MDC (2018) Optimization of biomass and hydrogen production by Anabaena sp. (UTEX 1448) in nitrogen-deprived cultures. Biomass Bioenergy 111:70–76. https://doi.org/10.1016/j.biombioe.2018.01.022
Deshmukh S, Kumar R, Bala K (2019) Microalgae biodiesel: a review on oil extraction, fatty acid composition, properties and effect on engine performance and emissions. Fuel Process Technol 191:232–247. https://doi.org/10.1016/j.fuproc.2019.03.013
Chung Y, Lee J, Chung C (2017) Molecular challenges in microalgae towards cost-effective production of quality biodiesel. Renew Sust Energ Rev 74:139–144. https://doi.org/10.1016/j.rser.2017.02.048
Anto S, Mukherjee SS, Muthappa R, Mathimani T, Deviram G, Kumar SS, Verma TN, Pugazhendhi A (2020) Algae as green energy reserve: technological outlook on biofuel production. Chemosphere. 242:125079. https://doi.org/10.1016/j.chemosphere.2019.125079
Ramesh Kumar B, Deviram G, Mathimani T, Duc PA, Pugazhendhi A (2019) Microalgae as rich source of polyunsaturated fatty acids. Biocatalysis and Agricultural Biotechnology 17:583–588. https://doi.org/10.1016/j.bcab.2019.01.017
Sharma J, V Kumar, S S Kumar, S K Malyan, T Mathimani, N R Bishnoi, and A Pugazhendhi (2020) Microalgal consortia for municipal wastewater treatment – lipid augmentation and fatty acid profiling for biodiesel production. Journal of Photochemistry and Photobiology B: Biology. 202: 111638. https://doi.org/10.1016/j.jphotobiol.2019.111638
Sharma J, Kumar SS, Bishnoi NR, Pugazhendhi A (2018) Enhancement of lipid production from algal biomass through various growth parameters. J Mol Liq 269:712–720. https://doi.org/10.1016/j.molliq.2018.08.103
Sharma J, Kumar SS, Bishnoi NR, Pugazhendhi A (2019) Screening and enrichment of high lipid producing microalgal consortia. J Photochem Photobiol B Biol 192:8–12. https://doi.org/10.1016/j.jphotobiol.2019.01.002
Ortiz-Martínez VM, Andreo-Martínez P, García-Martínez N, Ríos APDL, Hernández-Fernández FJ, Quesada-Medina J (2019) Approach to biodiesel production from microalgae under supercritical conditions by the PRISMA method. Fuel Process Technol 191:211–222. https://doi.org/10.1016/j.fuproc.2019.03.031
Yan J, Kuang Y, Gui X, Han X, Yan Y (2019) Engineering a malic enzyme to enhance lipid accumulation in Chlorella protothecoides and direct production of biodiesel from the microalgal biomass. Biomass Bioenergy 122:298–304. https://doi.org/10.1016/j.biombioe.2019.01.046
Liu X, Guo X, Liu Y, Lu S, Xi B, Zhang J, Wang Z, Bi B (2019) A review on removing antibiotics and antibiotic resistance genes from wastewater by constructed wetlands: performance and microbial response. Environ Pollut 254:112996. https://doi.org/10.1016/j.envpol.2019.112996
Maul JD, Schuler LJ, Belden JB, Whiles MR, Lydy MJ (2006) Effects of the antibiotic ciprofloxacin on stream microbial communities and detritivorous macroinvertebrates. Environ Toxicol Chem 25:1598–1606. https://doi.org/10.1897/05-441R.1
Sendra M, Moreno-Garrido I, Blasco J, Araújo CVM (2018) Effect of erythromycin and modulating effect of CeO2 NPs on the toxicity exerted by the antibiotic on the microalgae Chlamydomonas reinhardtii and Phaeodactylum tricornutum. Environ Pollut 242:357–366. https://doi.org/10.1016/j.envpol.2018.07.009
Liu Y, Chen S, Zhang J, Gao B (2016) Growth, microcystin-production and proteomic responses of Microcystis aeruginosa under long-term exposure to amoxicillin. Water Res 93:141–152. https://doi.org/10.1016/j.watres.2016.01.060
Wan J, Guo P, Peng X, Wen K (2015) Effect of erythromycin exposure on the growth, antioxidant system and photosynthesis of Microcystis flos-aquae. J Hazard Mater 283:778–786. https://doi.org/10.1016/j.jhazmat.2014.10.026
Xu M, Huang H, Li N, Li F, Wang D, Luo Q (2019) Occurrence and ecological risk of pharmaceuticals and personal care products (PPCPs) and pesticides in typical surface watersheds, China. Ecotox Environ Safe 175:289–298. https://doi.org/10.1016/j.ecoenv.2019.01.131
Le Henry M, Charton M, Alignan M, Maury P, Luniov A, Pelletier I, Pontalier P, Binder BM, Vaca-Garcia C, Chervin C (2017) Ethylene stimulates growth and affects fatty acid content of Synechocystis sp. PCC 6803. Algal Res 26:234–239. https://doi.org/10.1016/j.algal.2017.07.032
Bland E, Angenent LT (2016) Pigment-targeted light wavelength and intensity promotes efficient photoautotrophic growth of cyanobacteria. Bioresour Technol 216:579–586. https://doi.org/10.1016/j.biortech.2016.05.116
Fu W, Li P, Wu Y (2012) Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci Hortic 135:45–51. https://doi.org/10.1016/j.scienta.2011.12.004
Folch J, Lees M, Slonane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Anahas AMP, Muralitharan G (2015) Isolation and screening of heterocystous cyanobacterial strains for biodiesel production by evaluating the fuel properties from fatty acid methyl ester (FAME) profiles. Bioresour Technol 184:9–17. https://doi.org/10.1016/j.biortech.2014.11.003
Casazza AA, Ferrari PF, Aliakbarian B, Converti A, Perego P (2015) Effect of UV radiation or titanium dioxide on polyphenol and lipid contents of Arthrospira (Spirulina) platensis. Algal Res 12:308–315 https://doi.org/10.1016/j.algal.2015.09.012
Chi NTL, Duc PA, Mathimani T, Pugazhendhi A (2019) Evaluating the potential of green alga Chlorella sp. for high biomass and lipid production in biodiesel viewpoint. Biocatalysis and Agricultural Biotechnology. 17:184–188. https://doi.org/10.1016/j.bcab.2018.11.011
Anto S, Pugazhendhi A, Mathimani T (2019) Lipid enhancement through nutrient starvation in Chlorella sp. and its fatty acid profiling for appropriate bioenergy feedstock. Biocatalysis and Agricultural. Biotechnology. 20:101179. https://doi.org/10.1016/j.bcab.2019.101179
Lu H, J Cheng, Y Zhu, K Li, J Tian, and J Zhou (2019) Responses of Arthrospira ZJU9000 to high bicarbonate concentration (HCO3−: 171.2 mM): how do biomass productivity and lipid content simultaneously increase? Algal Research. 41: 101531. https://doi.org/10.1016/j.algal.2019.101531
Renuka N, Guldhe A, Singh P, Ansari FA, Rawat I, Bux F (2017) Evaluating the potential of cytokinins for biomass and lipid enhancement in microalga Acutodesmus obliquus under nitrogen stress. Energ Convers Manage 140:14–23. https://doi.org/10.1016/j.enconman.2017.02.065
Agathokleous E, Kitao M, Calabrese EJ (2019) Hormesis: a compelling platform for sophisticated plant science. Trends Plant Sci 24:318–327. https://doi.org/10.1016/j.tplants.2019.01.004
Agathokleous E, Calabrese EJ (2019) Hormesis: the dose response for the 21st century: the future has arrived. Toxicology. 425:152249. https://doi.org/10.1016/j.tox.2019.152249
Xie X, Zhou Q, He Z, Bao Y (2010) Physiological and potential genetic toxicity of chlortetracycline as an emerging pollutant in wheat (Triticum aestivum L.). Environ Toxicol Chem 29:922–928. https://doi.org/10.1002/etc.79
Liu Y, Chen X, Zhang J, Gao B (2015) Hormesis effects of amoxicillin on growth and cellular biosynthesis of Microcystis aeruginosa at different nitrogen levels. Microb Ecol 69:608–617. https://doi.org/10.1007/s00248-014-0528-9
Liu B, Nie X, Liu W, Snoeijs P, Guan C, Tsui MTK (2011) Toxic effects of erythromycin, ciprofloxacin and sulfamethoxazole on photosynthetic apparatus in Selenastrum capricornutum. Ecotox Environ Safe 74:1027–1035. https://doi.org/10.1016/j.ecoenv.2011.01.022
Liu L, Liu Y, Liu C, Wang Z, Dong J, Zhu G, Huang X (2013) Potential effect and accumulation of veterinary antibiotics in Phragmites australis under hydroponic conditions. Ecol Eng 53:138–143. https://doi.org/10.1016/j.ecoleng.2012.12.033
Gomes MP, Gonçalves CA, de Brito JCM, Souza AM, Da Silva Cruz FV, Bicalho EM, Figueredo CC, Garcia QS (2017) Ciprofloxacin induces oxidative stress in duckweed (Lemna minor L.): implications for energy metabolism and antibiotic-uptake ability. J Hazard Mater 328:140–149. https://doi.org/10.1016/j.jhazmat.2017.01.005
Wang Z, Chen Q, Hu L, Wang M (2018) Combined effects of binary antibiotic mixture on growth, microcystin production, and extracellular release of Microcystis aeruginosa: application of response surface methodology. Environ Sci Pollut R 25:736–748. https://doi.org/10.1007/s11356-017-0475-3
Dao G, Wu G, Wang X, Zhuang L, Zhang T, Hu H (2018) Enhanced growth and fatty acid accumulation of microalgae Scenedesmus sp. LX1 by two types of auxin. Bioresour Technol 247:561–567. https://doi.org/10.1016/j.biortech.2017.09.079
Sierra-Cantor JF, Guerrero-Fajardo CA (2017) Methods for improving the cold flow properties of biodiesel with high saturated fatty acids content: a review. Renew Sust Energ Rev 72:774–790. https://doi.org/10.1016/j.rser.2017.01.077
EN E S. (2004) Automotive fuels-fatty acid methyl esters (FAME) for diesel engines-requirements and test methods
Yu Z, Pei H, Jiang L, Hou Q, Nie C, Zhang L (2018) Phytohormone addition coupled with nitrogen depletion almost tripled the lipid productivities in two algae. Bioresour Technol 247:904–914. https://doi.org/10.1016/j.biortech.2017.09.192
Longworth J, Wu D, Huete-Ortega M, Wright PC, Vaidyanathan S (2016) Proteome response of Phaeodactylum tricornutum, during lipid accumulation induced by nitrogen depletion. Algal Res 18:213–224. https://doi.org/10.1016/j.algal.2016.06.015
Li T, Gargouri M, Feng J, Park J, Gao D, Miao C, Dong T, Gang DR, Chen S (2015) Regulation of starch and lipid accumulation in a microalga Chlorella sorokiniana. Bioresour Technol 180:250–257. https://doi.org/10.1016/j.biortech.2015.01.005
Udayan A, Kathiresan S, Arumugam M (2018) Kinetin and Gibberellic acid (GA3) act synergistically to produce high value polyunsaturated fatty acids in Nannochloropsis oceanica CASA CC201. Algal Res 32:182–192. https://doi.org/10.1016/j.algal.2018.03.007
Gao F, Yang H, Li C, Peng Y, Lu M, Jin W, Bao J, Guo Y (2019) Effect of organic carbon to nitrogen ratio in wastewater on growth, nutrient uptake and lipid accumulation of a mixotrophic microalgae Chlorella sp. Bioresour Technol 282:118–124. https://doi.org/10.1016/j.biortech.2019.03.011
Adu-Mensah D, Mei D, Zuo L, Zhang Q, Wang J (2019) A review on partial hydrogenation of biodiesel and its influence on fuel properties. Fuel 251:660–668. https://doi.org/10.1016/j.envpol.2019.112996
Funding
This study was funded by the National Natural Science Foundation of China (51679130) and the Fundamental Research Funds of Shandong University (2017WLJH35).
Author information
Authors and Affiliations
Contributions
Investigation and original draft writing were performed by Mengwen Cui. Ying Liu was responsible for methodology and project administration. Jian Zhang was responsible for supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
All authors were informed and consented to the manuscript publishment.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Cui, M., Liu, Y. & Zhang, J. The Variation of Growth Rate, Photosynthetic Activity, and Biodiesel Productivity in Synechocystis sp. PCC 6803 under Antibiotic Exposure. Bioenerg. Res. 13, 955–962 (2020). https://doi.org/10.1007/s12155-020-10114-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10114-x