Abstract
In this study, a steam gasification with a dual fluidized bed reactor is constructed using a commercial process simulator and validated by experimental data to investigate the behaviors of raw and torrefied spruce wood during the conversion process. Effects of torrefaction, gasification temperature, and steam-to-biomass ratio on the performance of spruce gasification are examined. Main gasification indicators including product gas composition and heating value as well as cold gas efficiency are investigated. Simulation results show that both the H2 and CO2 contents in the product gas are reduced with increasing the gasification temperature or decreasing the steam-to-biomass ratio. On the other hand, the CO content shows an opposite trend. In addition, increasing the gasification temperature or decreasing the steam-to-biomass ratio enhances the heating value of the product gas but reduces the cold gas efficiency. Compared with the raw feedstock, the torrefied spruce offers lower H2 but higher CO content in the product gas at the same gasification condition. Nevertheless, gasification of the torrefied spruce always results in higher cold gas heating value and efficiency than that of the raw spruce. The increased values are up to 0.46 MJ/Nm3 for the heating value and 5.96% for the efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gasification is a thermochemical conversion of biomass into product gas at high temperatures and in partial oxidation conditions. Recently, biomass gasification is becoming an important process for bioenergy deployment worldwide because the product of gasification has higher versatility for further applications than other common conversion routes [1,2,3,4]. The product gas can be used directly for heat and power generation [1, 2] or employed for liquid fuels and chemical productions [3, 4]. Common oxidizing agents for gasification are oxygen/air, steam, and carbon dioxide. Among the agents, carbon dioxide offers the lowest reactivity. If air is employed for gasification, the process can be operated autothermally but the product gas is diluted with a large amount of nitrogen and thus reduces its heating value. The use of oxygen instead of air can solve this problem, but separation of oxygen from air is costly. On the other hand, gasification in steam offers higher hydrogen content and lower tar yield in the product gas [5, 6]. Nevertheless, steam gasification requires external heat for endothermic reactions.
Dual fluidized bed (DFB) reactor can overcome the abovementioned problem of steam biomass gasification by employing two separate reactors: a combustor and a gasifier [7]. In the combustor, where fluidizing agent is air, part of char is burnt to heat the bed material (e.g., sand) which is then transferred to the gasifier, where the remaining char is gasified in the presence of steam as gasifying agent. Heat for endothermic gasification reactions is provided by the heat from the hot bed material, and thereafter, the cold bed material is circulated to the combustor. A demonstration of the DFB gasification technology is adopted from Kern et al. [7] and shown in Fig. 1. Since the technology was developed at the Vienna University of Technology and successfully demonstrated in Güssing and Oberwart [7], several researches have been conducted to understand the effects of different biomass feedstocks, bed materials, and operating conditions on the performance of the DFB gasification process [7,8,9].
Demonstration of dual fluidized bed reactor (adopted from Kern et al. [7])
Apart from the advantages as a green conversion route, gasification of biomass is however coupled with some problems. Biomass feedstock has high moisture content and low energy density; thus, biomass gasification normally offers a final product gas with a low heating value. In addition, the formation of condensable tar in gasifiers may occur due to thermal instability of the biomass. More importantly, fuels with high O/C ratio like biomass result in low gasification efficiencies [10]. Because of the aforementioned drawbacks, gasification of biomass as sole feedstock is challenging in practice. Indeed, biomass and coal can be blended and co-fed in a co-gasification process [11,12,13]. However, utilization of coal may lead to other issues relating to environmental impacts. A solution for biomass gasification without employment of coal is an additional pretreatment of the feedstock via torrefaction. Torrefaction is a thermal treatment of biomass at temperatures of 200–300 °C in an inert atmosphere. Compared with untreated biomass, torrefied biomass has lower moisture content, reduced O/C ratio, increased heating value, and enhanced grindability and hydrophobicity [14,15,16]. Due to its superior properties, torrefied biomass is a much better feedstock than raw biomass for any thermochemical conversion process including gasification [10].
Recent literature survey reveals that using torrefied biomass as feedstock for gasification has been receiving increased interests, both experimental [17,18,19] and simulation [20,21,22,23,24] approaches. However, there is currently a limited number of work which investigates torrefied biomass gasification in DFB reactor. Experimental studies for this new feedstock may be time consuming while obtained information is limited due to a small number of investigated points. Moreover, construction of such a reactor is also costly. On the contrary, a simulation work can provide better understanding of the whole process, giving approximate answers to the factors that may affect the gasification products and the process efficiency. Those motivate the need of the current study.
In this work, a biomass gasification with DFB technology is established using a commercial process simulator. The model is validated by available experimental data. Both raw and torrefied spruce are selected as feedstock in order to understand the effects of torrefaction on the performance of the gasification process through the composition and heating value of the product gas as well as the cold gas efficiency.
Materials and Methods
Materials
In this study, raw and torrefied spruce (10-mm cubes) from a literature [25] are selected as feedstock for the gasification simulation. Torrefaction of spruce was conducted at 225 and 275 °C for 1 h. In addition, several biomass species including hardwood chips, sewage pellets, and blend pellets from an experimental gasification study [26] are adopted for the model validation. The fuel properties of all feedstock are listed in Table 1, including proximate analysis, ultimate analysis, and LHV.
Process Simulation
Model Description
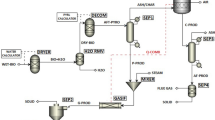
The flowsheet of the gasification model is constructed in Aspen Plus v8.4 and illustrated in Fig. 2. In this model, biomass is defined as a non-conventional component and assumed as totally dry. The stream BIOMASS containing biomass feedstock (either raw or torrefied biomass) is fed as a flowrate of 100 kg/h to the reactor DECOMP (RYield block). This reactor is used to simulate the decomposition of biomass and converts the material into conventional components [27, 28]. Thereafter, a portion of char is split by the block SEP-CHAR and burnt at the reactor COMB (RStoic block) to achieve the required temperatures for the gasifier. For this purpose, the splitting portions are varied with the gasification temperature. An excess air ratio of 1.2 is chosen for the inlet air to the combustor (AIR-COMB, RStoic block) to ensure a completed char combustion reaction [29]. The heat of combustion (Q-COMB) is used to supply the heat demand for the biomass decomposition (Q-DECOMP), the formation of NH3 and H2S (Q-NSRM), and the endothermic gasification reactions (Q-GASIF).
After splitting a part of char, the rest components are fed to the NS-RM reactor (RStoic block), which is assumed to convert minor elements (N and S) in the biomass into their gas forms (NH3 and H2S). Then, these gases are removed from the main stream by the block SEP-NS and mixed to the product gas after the gasification. Steam at 150 °C is introduced to the GASIFIER (user-defined block), where the main gasification reactions occur. The gasification reactions and kinetic information are adopted from [30, 31] and listed in Table 2. In addition, the steam feeding rates are varied depending on the desired steam-to-biomass ratios. After gasification, water is removed from the product gas (stream WETGAS) through the block SEP-H2O. Then, the dry gas (stream DRYGAS) is cooled to room temperature by the HX-PROD.
Assumptions
In order to simplify the complexity of gasification process, some common assumptions are made throughout the process:
Biomass (either raw or torrefied) is considered non-conventional component, and thus, the stream class used in the process is MIXCINC, which includes both conventional and non-conventional solids.
Because gasification is performed at high temperatures, the suitable thermodynamic property method for the process is Peng-Robinson with Boston-Mathias function (PR-BM) [27, 32, 33].
Char is assumed as 100% carbon.
Air consists of 79% nitrogen and 21% oxygen on molar basis.
Ash and bed material are inert and do not react with any other components. In addition, no catalytic or fluidizing effects are considered for the bed material.
Tar formation is significantly reduced during steam gasification of torrefied biomass feedstock [17]; thus, it is neglected throughout this simulation.
The system operates at the atmospheric pressure, and all pressure drops are neglected.
The heat loss was assumed to be 5% of the energy flow to the blocks.
Results and Discussion
Model Validation
Gasification of hardwood chips, straw pellets, and sewage pellets was experimentally conducted [26], and the obtained data were adopted to validate the model in this work. The fuel properties and the gasification parameters for each feedstock were input into the model to extract simulation data for comparison. The compositions of product gas from both the experiments and simulations are demonstrated in Fig. 3. Differences in the product gas composition are 0.6–8.6 vol% for H2 content, 3.1–7.2 vol% for CO content, 1.1–3.1 vol% for CO2 content, and 0.5–3.0 vol% for CH4 content. Figure 4 presents the cold gas heating values from the experiments and simulations, showing differences within 0.27–0.82 MJ/Nm3. The validation shows good agreements between the simulation data in this work and experimental results in the previous work, which indicates that the current model is appropriate for the gasification of a variation of biomass feedstock.
Gasification of Raw Spruce
In steam gasification, gasification temperature and steam-to-biomass ratio (SBR) are the most important factors showing significant influences on the process performance [6], which include the product gas composition, H2O/CO ratio, lower heating values (LHV) of cold gas, and cold gas efficiency (CGE). The SBR is defined as the mass flow rate of the injected steam divided by that of the dry fed biomass. Calculations of the LHV and CGE are presented in Eqs. 1–3 [34]:
where YC, YH, YO, and \( {Y}_{{\mathrm{H}}_2\mathrm{O}} \) are the mass fractions of carbon, hydrogen, oxygen, and moisture in the biomass; \( {y}_{{\mathrm{H}}_2} \), yCO, and \( {y}_{{\mathrm{CH}}_4} \) are the mole fractions of hydrogen, carbon monoxide, and methane in the product gas; Vgas is the volumetric flow rate of the product gas while mfeed is the mass flow rate of the feedstock.
In this part, these two factors are alternately varied to examine their effects on the gasification performance. In order to investigate the effects of temperature in the range of 700–900 °C, SBR is kept constant at 1. On the other hand, a temperature of 800 °C is applied for testing various SBRs (0.8–1.2).
Effects of Gasification Temperature
The results for raw spruce gasification at different temperatures are tabulated in Table 3. When the temperature rises from 700 to 900 °C, both the H2 and CO2 contents decrease (from 48.67 to 47.72 vol% for H2 and from 27.48 to 24.27 vol% for CO2) while the CO content increases (from 11.35 to 15.61 vol%). On the other hand, the CH4 content shows only a marginal change with increasing the gasification temperature. Reduction in the H2 content and increase in the CO content result in an increasing trend of the H2/CO ratio with increasing the gasification temperature. Due to relatively low sulfur and nitrogen contents in the biomass, the portions of H2S and NH3 in the product gas are inconsiderable.
Because gasification is a complex process and can be affected by many factors including types of feedstock, process parameters, gasification technologies, gasifying agents, catalytic effects, additional units (e.g., reformer), and simulation settings, disagreements in the trends of the product gas composition with changes in the gasification temperature are commonly found in the literature. For an example, in the case of trend for H2 content, some works reported that it increases [35, 36] while some others revealed that it decreases [37, 38] with increasing gasification temperature. Within a study, some researchers however found that, when increasing the temperature, H2 content first increases in a low temperature range and then decreases in a higher temperature window [39,40,41,42,43]. Similar contractions may be also observed for other gas components such as CO, CO2, and CH4. Therefore, to compare any trend during a gasification process in different works, it is worth noting that all the aforementioned factors must be similar between the studies; otherwise, the comparison may be voided.
While increasing the gasification temperature, the LHV of the cold gas increases (from 11.12 to 11.51 MJ/Nm3) but the CGE decreases (from 67.64 to 59.48%). It is clear that the LHV of the product gas is strongly depended on the fractions of H2, CO, and CH4 as stated in Eq. 2. Therefore, increased LHV of the cold gas can be explained by increases in both CO and H2 contents the product gas with increasing temperature (Table 3). On the other hand, although CH4 has the highest contribution to the LHV, it shows only a marginal change and thus, its effect on the cold gas LHV is less than those of CO and H2. Moreover, the decreased CGE may be due to that more char is split (at the block SEP-CHAR) to supply for more heat demand (for the GASIFIER) at higher temperatures. Consequently, less char enters the GASIFIER and thus, less product gas is produced.
Effects of Steam-to-Biomass Ratio
Table 4 shows the compositions of the product gas, H2O/CO ratio, cold gas LHV and efficiency at different SBRs. Compared with the gasification temperature, which may affect the gasification process differently depending on other factors as mentioned above, influences of the SBR can be summarized in Eq. 4 [44], from which more additional steam favors the production of more hydrogen and carbon dioxide during steam gasification of biomass (CxHyOz).
As a result, while the SBR increases from 0.8 to 1.2, the H2 and CO2 contents are respectively increased from 45.61 to 50.20 vol% and from 24.74 to 26.47 vol%. Consequently, the CO and CH4 contents are respectively reduced from 15.82 to 12.07 vol% and 13.71 to 11.15 vol% when the SBR increases. Due to changes in the gas composition, high SBR reduces the LHV of cold gas from 11.83 to 10.93 MJ/Nm3; however, the CGE increases from 62.20 to 64.24%. Compared with other works in the literature [28, 33, 45], the trends in the product gas composition, the cold gas LHV, and the CGE are in good agreements with previous studies. In addition, increase in the H2 content and decrease in the CO content result in a decreasing trend of the H2/CO ratio with increasing the SBR.
Gasification of Torrefied Spruce
Effects on Product Gas Composition
Figures. 5 and 6 show the effects of torrefaction on the product gas composition at different gasification temperatures and SBR. Due to relatively low contributions, NH3 and H2S contents are not included in this comparison. At the first sight, one can see from the figures that the trends for the product gas composition when changing the gasification parameters (e.g., temperature and SBR) of the torrefied spruce are similar to those of the raw spruce. However, torrefaction affects the values of the gas contents. At the same gasification condition, the H2 and CO2 contents reduce while the CO and CH4 contents increase with increasing the torrefaction severity. In details, gasification of spruce torrefied at 275 °C shows reductions of 2.40–2.77 vol% and 0.56–1.11 vol% in the H2 and CO2 contents. On the other hand, increases of 2.08–2.70 vol% and 1.06–1.21 vol% in the CO and CH4 contents are observed. In addition, differences in the CO2 and CH4 contents between the raw spruce and the spruce torrefied at 225 °C are marginal, while a torrefaction at 275 °C shows more significant effects. In addition, it can be observed that torrefaction also reduces the H2/CO ratio in the product gas which is resulted from the decreasing trend of H2 and increasing trend of CO when the torrefaction condition is severer.
The trends for the product gas composition (decreases for H2 and CO2 contents and increases for CO and CH4 contents) from gasification of torrefied biomass, compared with that of untreated biomass, are in good agreement with the results from other studies [21, 23, 24, 46]. These observations are due to the changes in the elemental composition (i.e., C, H and O) of the fuel after torrefaction, which may affect the equilibriums of gasification reactions. In addition, the torrefied spruce has higher fixed carbon content than the raw spruce, which can also influence the product gas composition. The lower oxygen content and higher carbon content (i.e., lower O/C ratio) as well as higher fixed carbon content in the torrefied spruce (compared with untreated spruce) may favor the incomplete char oxidation (2C + O2 → 2CO) and Boudouard reaction (C + CO2 → 2CO). These two reactions are attributed to the reduction of CO2 and increase of CO in the product gas from torrefied spruce gasification. On the other hand, the lower hydrogen content and the higher carbon content (i.e., low H/C ratio) in the torrefied spruce may support the methanation reaction (C + 2H2 → CH4), and result to lower hydrogen but higher methane contents in the gasification product of torrefied spruce.
Effects on Heating Value of Cold Gas and Cold Gas Efficiency
Effect of torrefaction on the cold gas LHV is presented in Fig. 7, and that on the cold gas efficiency is demonstrated in Fig. 8. Again, the SBR is kept at 1 to investigate the effects of temperature, and the temperature is 800 °C during the test of SBR. The figures show that both the cold gas LHV and efficiency are enhanced with increasing the torrefaction severity. Although the spruce torrefied at 225 °C offers only small improvements (compared with the raw spruce) in the two factors, they increase significantly in the case of spruce torrefied at 275 °C, which offers improvement up to 0.46 MJ/Nm3 for the LHV and 5.96% for the CGE. Therefore, it can conclude that torrefaction has positive effects on both the cold gas LHV and efficiency, which are in good agreement with other experimental study on gasification of torrefied biomass [10]. However, torrefaction requires more energy than conventional drying process; thus, it recommends further studies on the overall energy efficiency for more comprehensive understanding of the biomass torrefaction-gasification integrated process. Ideally, waste heat from high temperature gasification process can be utilized to supply for the heat demand of torrefaction process.
Conclusions
A model for DFB gasification of spruce has been successfully constructed and validated by experimental data. The simulation results show that the gasification temperature and SBR have opposite effects on the product gas composition, cold gas LHV, and CGE. Increasing the temperature reduces the H2 and CO2 contents, but increasing the SBR enhances these contents. On the other hand, the CO content is increased with increment of the temperature and reduction of the SBR. Moreover, the cold gas LHV increases when the temperature increases and the SBR decreases. However, the CGE shows opposite trends, i.e., the CGE decreases with increasing the gasification temperature and with decreasing the SBR.
Torrefaction does not influence the trends of the composition, heating value, and efficiency of the product gas while varying the gasification temperature and SBR, but it has strong effects on the values. Remarkably, at the same gasification condition, the torrefied spruce offers lower H2 but higher CO contents in the product gas than the raw spruce. Moreover, the cold gas LHV and CGE from gasification of the torrefied biomass are both increased, up to 0.46 MJ/Nm3 for the LHV and 5.96% for the CGE, compared with those of the raw biomass. In addition, further studies to estimate the overall energy efficiency of integrated torrefaction and gasification process are recommended for deeper understanding of this combined process.
Abbreviations
- CGE:
-
Cold gas efficiency
- DFB:
-
Dual fluidized bed
- LHV:
-
Lower heating value
- SBR:
-
Steam-to-biomass ratio
- TS-225:
-
Spruce torrefied at 225 °C
- TS-275:
-
Spruce torrefied at 275 °C
References
Bhattacharya A, Manna D, Paul B, Datta A (2011) Biomass integrated gasification combined cycle power generation with supplementary biomass firing: energy and exergy based performance analysis. Energy 36:2599–2610
Kumar A, Demirel Y, Jones DD, Hanna MA (2010) Optimization and economic evaluation of industrial gas production and combined heat and power generation from gasification of corn stover and distillers grains. Bioresour Technol 101:3696–3701
Tijmensen MJA, Faaij APC, Hamelinck CN, van Hardeveld MRM (2002) Exploration of the possibilities for production of Fischer Tropsch liquids and power via biomass gasification. Biomass Bioenergy 23:129–152
Swanson RM, Platon A, Satrio JA, Brown RC (2010) Techno-economic analysis of biomass-to-liquids production based on gasification. Fuel 89:S11–S19
Beheshti SM, Ghassemi H, Shahsavan-Markadeh R (2015) Process simulation of biomass gasification in a bubbling fluidized bed reactor. Energy Convers Manag 94:345–352
Parthasarathy P, Narayanan KS (2014) Hydrogen production from steam gasification of biomass: influence of process parameters on hydrogen yield – a review. Renew Energy 66:570–579
Kern S, Pfeifer C, Hofbauer H (2013) Gasification of wood in a dual fluidized bed gasifier: influence of fuel feeding on process performance. Chem Eng Sci 90:284–298
Corella J, Toledo JM, Molina G (2007) A review on dual fluidized-bed biomass gasifiers. Ind Eng Chem Res 46:6831–6839
Li YH, Chen Z, Watkinson P, Bi X, Grace J, Lim CJ, Ellis N (2018) A novel dual-bed for steam gasification of biomass. Biomass Convers Biorefinery 8:357–367
Prins MJ, Ptasinski KJ, Janssen FJJG (2006) More efficient biomass gasification via torrefaction. Energy 31:3458–3470
Vélez JF, Chejne F, Valdés CF, Emery EJ, Londoño CA (2009) Co-gasification of Colombian coal and biomass in fluidized bed: an experimental study. Fuel 88:424–430
Aigner I, Pfeifer C, Hofbauer H (2011) Co-gasification of coal and wood in a dual fluidized bed gasifier. Fuel 90:2404–2412
Saw WL, Pang S (2013) Co-gasification of blended lignite and wood pellets in a 100 kW dual fluidised bed steam gasifier: the influence of lignite ratio on producer gas composition and tar content. Fuel 112:117–124
Ciolkosz D, Wallace R (2011) A review of torrefaction for bioenergy feedstock production, Biofuels. Bioproducts Biorefining 5:317–329
Chen W-H, Lin B-J, Huang M-Y, Chang J-S (2015) Thermochemical conversion of microalgal biomass into biofuels: a review. Bioresour Technol 184:314–327
van der Stelt MJC, Gerhauser H, Kiel JHA, Ptasinski KJ (2011) Biomass upgrading by torrefaction for the production of biofuels: a review. Biomass Bioenergy 35:3748–3762
Di Marcello M, Tsalidis GA, Spinelli G, de Jong W, Kiel JHA (2017) Pilot scale steam-oxygen CFB gasification of commercial torrefied wood pellets. The effect of torrefaction on the gasification performance. Biomass Bioenergy 105:411–420
Tsalidis GA, Di Marcello M, Spinelli G, de Jong W, Kiel JHA (2017) The effect of torrefaction on the process performance of oxygen-steam blown CFB gasification of hardwood and softwood. Biomass Bioenergy 106:155–165
Bach Q-V, Chen W-H, Sheen H-K, Chang J-S (2017) Gasification kinetics of raw and wet-torrefied microalgae Chlorella vulgaris ESP-31 in carbon dioxide. Bioresour Technol 244:1393–1399
Chen W-H, Chen C-J, Hung C-I, Shen C-H, Hsu H-W (2013) A comparison of gasification phenomena among raw biomass, torrefied biomass and coal in an entrained-flow reactor. Appl Energy 112:421–430
Kuo P-C, Wu W, Chen W-H (2014) Gasification performances of raw and torrefied biomass in a downdraft fixed bed gasifier using thermodynamic analysis. Fuel 117:1231–1241
Tapasvi D, Kempegowda RS, Tran K-Q, Skreiberg Ø, Grønli M (2015) A simulation study on the torrefied biomass gasification. Energy Convers Manag 90:446–457
Ku X, Lin J, Yuan F (2016) Influence of torrefaction on biomass gasification performance in a high-temperature entrained-flow reactor. Energy Fuel 30:4053–4064
Ku X, Jin H, Lin J (2017) Comparison of gasification performances between raw and torrefied biomasses in an air-blown fluidized-bed gasifier. Chem Eng Sci 168:235–249
Tapasvi D, Khalil R, Skreiberg Ø, Tran K-Q, Grønli M (2012) Torrefaction of Norwegian birch and spruce: an experimental study using macro-TGA. Energy Fuel 26:5232–5240
Schmid JC, Wolfesberger U, Koppatz S, Pfeifer C, Hofbauer H (2012) Variation of feedstock in a dual fluidized bed steam gasifier—influence on product gas, tar content, and composition. Environ Prog Sustain Energy 31:205–215
Fernandez-Lopez M, Pedroche J, Valverde JL, Sanchez-Silva L (2017) Simulation of the gasification of animal wastes in a dual gasifier using Aspen Plus®. Energy Convers Manag 140:211–217
Doherty W, Reynolds A, Kennedy D (2009) The effect of air preheating in a biomass CFB gasifier using ASPEN Plus simulation. Biomass Bioenergy 33:1158–1167
Blok K, Nieuwlaar E (2016) Introduction to Energy Analysis, 2nd edn. Routledge, London
Xie J, Zhong W, Jin B, Shao Y, Huang Y (2013) Eulerian–Lagrangian method for three-dimensional simulation of fluidized bed coal gasification. Adv Powder Technol 24:382–392
Umeki K, Yamamoto K, Namioka T, Yoshikawa K (2010) High temperature steam-only gasification of woody biomass. Appl Energy 87:791–798
Formica M, Frigo S, Gabbrielli R (2016) Development of a new steady state zero-dimensional simulation model for woody biomass gasification in a full scale plant. Energy Convers Manag 120:358–369
Pala LPR, Wang Q, Kolb G, Hessel V (2017) Steam gasification of biomass with subsequent syngas adjustment using shift reaction for syngas production: an Aspen Plus model. Renew Energy 101:484–492
Cheng Y, Thow Z, Wang C-H (2016) Biomass gasification with CO2 in a fluidized bed. Powder Technol 296:87–101
Gao N, Li A, Quan C, Gao F (2008) Hydrogen-rich gas production from biomass steam gasification in an updraft fixed-bed gasifier combined with a porous ceramic reformer. Int J Hydrog Energy 33:5430–5438
Doherty W, Reynolds A, Kennedy D (2015) Process simulation of biomass gasification integrated with a solid oxide fuel cell stack. J Power Sources 277:292–303
Pfeifer C, Rauch R, Hofbauer H (2004) In-bed catalytic tar reduction in a dual fluidized bed biomass steam gasifier. Ind Eng Chem Res 43:1634–1640
Koppatz S, Pfeifer C, Rauch R, Hofbauer H, Marquard-Moellenstedt T, Specht M (2009) H2 rich product gas by steam gasification of biomass with in situ CO2 absorption in a dual fluidized bed system of 8 MW fuel input. Fuel Process Technol 90:914–921
Prestipino M, Chiodo V, Maisano S, Zafarana G, Urbani F, Galvagno A (2017) Hydrogen rich syngas production by air-steam gasification of citrus peel residues from citrus juice manufacturing: experimental and simulation activities. Int J Hydrog Energy 42:26816–26827
Dong J, Nzihou A, Chi Y, Weiss-Hortala E, Ni M, Lyczko N, Tang Y, Ducousso M (2017) Hydrogen-rich gas production from steam gasification of bio-char in the presence of CaO. Waste Biomass Valoriz 8:2735–2746
Gao N, Li A, Quan C (2009) A novel reforming method for hydrogen production from biomass steam gasification. Bioresour Technol 100:4271–4277
Xiao Y, Xu S, Song Y, Shan Y, Wang C, Wang G (2017) Biomass steam gasification for hydrogen-rich gas production in a decoupled dual loop gasification system. Fuel Process Technol 165:54–61
Rupesh S, Muraleedharan C, Arun P (2016) ASPEN plus modelling of air–steam gasification of biomass with sorbent enabled CO2 capture. Resour-Effic Technol 2:94–103
Prasad BVRK, Kuester JL (1988) Process analysis of a dual fluidized bed biomass gasification system. Ind Eng Chem Res 27:304–310
Franco C, Pinto F, Gulyurtlu I, Cabrita I (2003) The study of reactions influencing the biomass steam gasification process. Fuel 82:835–842
Li Y-H, Chen H-H (2018) Analysis of syngas production rate in empty fruit bunch steam gasification with varying control factors. Int J Hydrog Energy 43:667–675
Funding
This research was supported by the Chung-Ang University Research Grants in 2018.
This work was supported by the Human Resources Development (No.20184030202070) of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government Ministry of Trade, Industry and Energy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bach, QV., Nguyen, D.D. & Lee, CJ. Effect of Torrefaction on Steam Gasification of Biomass in Dual Fluidized Bed Reactor—a Process Simulation Study. Bioenerg. Res. 12, 1042–1051 (2019). https://doi.org/10.1007/s12155-019-10011-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-019-10011-y