Abstract
Although biomass gasification using concentrated solar energy is an attractive technology for the production of storable renewable energy and CO2 reduction, several challenges have stalled its deployment over the last decades: high temperature and/or large reactor volume required for complete fuel (char) conversion, the achievement of a steady syngas generation independent of solar radiation variation, and accomplishing effective heat supply at high temperature in large-scale reactors. An alternative approach overcoming these technical challenges is analyzed in this paper. It is based on conducting the gasification of biomass with steam in a fluidized bed using solid particles that are separately heated in a solid particle receiver using concentrating solar energy. The present study includes the thermodynamic and kinetic analysis of solar steam gasification in a completely allothermal single fluidized bed gasifier as well as in a partially allothermal dual fluidized bed gasification system. A theoretical analysis of the reactor performance and its integration with the solar receiver is examined and the state of the art of the most important aspects for developing the proposed technology is reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The use of solar energy as external heat source for steam reforming of fuels has been recognized as highly attractive method for increasing the share of renewable energy and reduction of CO2 emissions. The generation of energy vectors carrying the energy from the sun and the biomass is an encouraging concept towards full renewable energy production and energy storage. Solar steam gasification of biomass is one of the most attractive technologies considered for the achievement of such objectives [1].
Steam gasification of biomass proposed until now is either autothermal (the heat is supplied by partial burning of the fuel) or just laboratory studies where the heat is supplied by electrical heaters [2, 3]. Providing the gasifier with external heat would allow maximizing the chemical energy of the syngas (instead of burning part of the fuel to provide the heat), hence improving the syngas yield and composition and the overall energy efficiency of the process. However, it is yet to be determined how to supply the solar heat to industrial-scale reactors.
Due to the great interest of hybridizing steam biomass gasification with solar energy, a lot of prototypes of solar gasifiers have been developed during the last three decades [4, 5]. However, there are still several critical issues that have hindered the deployment of the technology. The main challenges are related to the high rate at which the external heat has to be transferred to the reactor, the high temperature required for the solar source, the large reactor volumes needed to convert the fuel (char) completely, and the difficulties of producing a steady syngas independent of solar radiation variation [6].
The objective of this paper is to examine the possibilities of hybridization of steam gasification of biomass fuels with concentrated solar energy and to present the potential of carrying out the process using solids particles in dual fluidized beds. The new system overcomes the mentioned critical issues by using solid particles as a thermal energy carrier and thermal energy storage, enabling continuous syngas generation with high thermal integration.
2 Background
In order to identify the hybridization possibilities of steam gasification and solar energy, the state of the art of solar and non-solar steam gasification, as well as key aspects of concentrating solar thermal energy for high-temperature applications based on solid particle receivers are reviewed in this section.
2.1 Steam gasification of biomass
The steam gasification of biomass with general formula CH1.44O0.66 to yield syngas (H2/CO) or (H2/CO2) can be represented by [7]:
The standard heat of reaction at 298 K of R1 and R2 is, respectively, 102.5 kJ/molbio (4.27 MJ/kgbio) and 61.3 kJ/molbio (2.55 MJ/kgbio). Therefore, gasification is an endothermal process, requiring significant heat to drive the reactions. In addition, because of equilibrium and kinetic reasons, the process must be conducted at high temperature, in the range of 750–1000 °C, depending on the fuel and reactor type.
Gasification technologies include allothermal steam gasification, using heat from an external source to drive the process, and autothermal gasification, in which part of the fuel is burnt to generate the necessary heat to maintain the process, using pure oxygen or air. Figure 1 shows schematically different gasification options that have been suggested for solid fuels: allothermal steam gasification, heated by an external source (Fig. 1a), and autothermal gasification, in which part of the fuel is burnt to generate the necessary heat (Fig. 1b), whether using pure oxygen (Fig. 1b.1) or air (Fig. 1b.2). In autothermal gasification using pure oxygen (Fig. 1b) for the production of nitrogen-free syngas, an air separation unit (ASU) is needed, being energy-intensive and costly. In order to produce a similar syngas quality using air, a dual fluidized bed (DFB) reactor system must be used (Fig. 1b.2), hence trading the cost and energy penalty of an ASU for a more complex gasification. Since in autothermal gasification part of the fuel is consumed to provide thermal heat, the cold gasification efficiency is lower than in allothermal gasification.
In allothermal gasification (Fig. 1a), a heat source at the gasification temperature or higher, in the range of 800–1000 °C depending on the fuel and reactor type, must be available to support the endothermic reactions. The external heat source would ideally make it possible to transfer all the energy available in the fuel into the produced syngas, instead of burning some to provide the heat, hence raising the yield significantly and improving the overall energy efficiency of the process. In turn, steam gasification transforms heat into chemical energy in the syngas with higher exergy and thus acts as a chemical heat pump. On the other hand, allothermal gasification can be seen as a method of water reduction (splitting) using the carbon in the fuel as reducing agent, generating additional hydrogen from the fuel during its reforming (so H2 comes from water and fuel).
Despite the fact that a great number of allothermal steam gasifiers have been developed at laboratory or bench scale [8], none has been scaled up or commercialized due to practical problems derived from the difficulty of producing a steady syngas in spite of solar radiation variation, as well as implementation of heat transfer at large scale. Therefore, steam (non-solar) gasification has been implemented at scale by the use of indirect air-steam dual fluidized bed gasifiers (DFBG) [2], i.e., by autothermal gasification using air. In this arrangement, the biomass is devolatilized in a bubbling FBG where volatiles are released, and char is partially gasified (≈ 5–30% depending on the fuel) with large excess of steam. The process is thermally driven by burning the char in the combustor and, if necessary, additional fuel. The heat from combustion is conveyed to the gasifier by sensible heat of the circulating solid material. The gasification unit is allothermal since the heat comes from the combustor unit (external to the gasifier), although the “gasification system” as a whole (gasifier + combustor) is autothermal (in the case of no addition of additional fuel). As a result, only about 70% of the energy (and 2/3 of the carbon) from the fuel is stored as chemical energy in the syngas. This is the usual way to operate the existing DFBGs as developed by TUV, ECN, or some universities/research centers and commercialized by companies like Repotec or Dahlman. Sometimes these DFBGs are operated burning also natural gas to elevate the combustion temperature [2, 3].
In Fig. 2, the possibilities of the classical air-steam DFBG are extended by considering that the system can be thermally balanced by some external heat or additional fuel. For obvious reasons, the most attractive case is when all external energy supplied to the gasifier is renewable (i.e., solar) and the fuel is biomass. In this case, solar energy is transferred to the bio-syngas and all carbon from the biomass is converted to fuel volatiles. In turn, it produces a syngas containing not only all the energy of the biomass (in the form of chemical and sensible energy) but also an additional share of solar energy embodied within the chemical energy of the syngas. Despite the great advantage of this operational mode, sustaining the gasifier with external heat only is difficult and is still to be developed. The approach proposed later in the present paper is to convert the existing conventional design of DFBG to admit external solar heat, maximizing the solar share in the syngas.
2.2 Solar gasifiers
Solar gasifiers can be classified according to the gas-solid contact (packed bed, fluidized bed, and entrained flow gasifiers) or by the way in which solar radiation contacts the reactants (directly irradiated, where the solid carbonaceous reactants are directly exposed to radiation, and indirectly irradiated, where the radiation strokes an intermediate material (like an opaque wall or thermal energy carrier). A variety of combinations of gas-solid and solar radiation-reactant contact reactors have been proposed, and some prototypes have been tested at a laboratory scale [1, 4,5,6, 9].
Regarding the gas-solid contact, packed bed gasifiers are simpler and robust and can accommodate a wide range of feedstock sizes, making them cost-effective, but they suffer from mass and heat transfer limitations, ash build-up, and energy losses. Entrained flow gasifiers exhibit more efficient transport, increasing the syngas throughput significantly, but imposing strict requirements on the feedstock size. Fluidized bed reactors achieve high mass and heat transfer rates, overcoming the transport limitations of packed bed and the particle size needs of entrained flow reactors. Both directly and indirectly irradiated have been developed for all gas-solid contacts [4]. Due to the high rate of heat transfer in fluidized bed designs, solar FBGs have been considered those with the highest scaling-up potential, although some issues remain to be resolved as discussed below and also in [6, 10].
Direct irradiation offers superior heat transfer characteristics and energy efficiency, but the reactor must have a transparent window, which can be fouled by operation. A particular effort has been made to develop directly heated solar FBG and many devices have been tested at lab-scale in transparent devices (silica glass or quartz tubes) for the gasification of carbonaceous fuels. Taylor et al. [11] gasified charcoal with CO2 under direct downward irradiation of vertical silica glass FB reactor of 5 cm diameter located at the focus of a 2-kW solar furnace. Murray and Fletcher [12] investigated the steam gasification of cellulose in a quartz tube FB reactor and a solar furnace. Kodama’s group studied the performance of CO2 gasification of bituminous coal in a conventional (bubbling) FB gasifier comprising a steel tube with a glass window irradiated with solar furnace whose concentrated visible light reached a maximum power of 3 kWth [6, 13]. In the earlier design, poor performance was detected and was attributed to the large temperature gradient between the irradiated bed surface and inside the bed reaction zone, as a result of the narrow internal diameter of the bed (63 mm ID). An improved FB concept based on internally circulating fluidized bed reactor was proposed, resulting in more uniform bed temperature distribution, higher fuel conversion, and reduction in fluidization agent compared with a conventional fluidized bed. In addition, two modes of operation were investigated: coal-only fluidized and mixtures of coal and quartz sand, the latter improving the conversion ratios indicating promising results for scale-up. Despite the improvement in reactor performance, achieving more uniform bed temperature, the performance was questionable and scaling-up remains unsolved. Some lab-scale prototypes have been recently proposed by hybridizing directly irradiated reactors with combustion process, providing some benefits related to carrying out both processes in the same device [14, 15].
Indirect irradiation, in which an intermediate medium is heated by solar radiation and transported to the gasifier, has less favorable heat transfer but avoids the difficulties with direct irradiation design [1]. Three approaches have been proposed to indirectly supply the concentrated solar radiation to the reactor: (i) irradiating the reactor external sidewalls [8]; (ii) using a two-cavity reactor [16]; and (iii) using solid particles as a heat transfer carrier [17, 18].
The superior heat transfer characteristics of FBs would allow the solar heat transfer fluid to be introduced into a jacket around the vessel, and also through tubes inside the bed. An intermediate storage also smooths out the intermittent supply of solar energy. Three configurations have been proposed [1]. Flamant et al. [8] proposed the use of dense suspension of upward particles circulating inside vertical tubes externally exposed to the concentrated solar energy. The suspension acts as a heat transfer fluid (HTF) with a heat capacity similar to a liquid HTF but only limited in temperature by the working temperature limit of the receiver tubes. Gordillo and Belghit [16] developed a FB model to simulate the gasification of biochar in a two-cavity reactor where the emitter plate heats the solids in the upper side of a FB. Large thermal gradients throughout the bed were predicted concluding that to improve the gas production, the source of solar radiation should have been directed to the bottom of the reactor to heat the steam at that point. In [17], a hypothetical hybridized DFB gasifier to theoretically assess the production of Fischer-Tropsch liquid from coal was tentatively considered. In [1], a lift pipe as part of a fluidized bed was proposed where the bed material and char are heated by externally irradiated heat pipes. The gasification proposed in [17] is not completely allothermal since the char and some auxiliary fuels are burnt, decreasing significantly the solar share in the syngas and the syngas yield per unit of fuel input. In addition, no experimental support for this concept has been published.
To sum up, none of the aforementioned directly irradiated gasifiers can be implemented at scale and, in the best cases, only lab-scale devices have been tested to understand the process limits of gasification subjected to solar radiation. The only existing FB solar gasifier reaching the lab-scale is directly irradiated, but the performance is poor and cannot be implemented in large-scale applications. The use of solid particles as an energy carrier for gasification has been theoretically proposed in two earlier works, but it has neither been experimentally tested nor theoretical analyzed from the reactor engineering perspective, but its performance has been assumed for the economic analysis of a biomass-to-liquid system.
2.3 Solid particle solar receivers
Solid particles were proposed as heat transfer and thermal storage medium in the early 1980s [19, 20] because of their ability to withstand high temperatures and the straightforward integration of solar energy collection and Thermal Energy Storage (TES), but the technology plummeted very quickly. Only in recent years, driven by the need of achieving higher temperatures together with efficient and cheap TES, solid particle technology has been the subject of new research interest [13, 21].
Previous designs of particle receiver include free-falling, centrifugal, flow in tubes, with or without fluidization, multi-pass recirculation, and face-down configurations. The solid particle receiver may be either directly or indirectly irradiated. Directly heated particle receivers have the main advantage of higher heating efficiency, but particle loss may be a problem in open cavities with significant wind effects. Obstructed or impeded flow receivers, rotary kiln, and fluidized bed receivers have been proposed to increase the residence time of the particles. Indirect particle receivers have the advantage of particle containment and no particle losses, but additional heat transfer resistance between the irradiated surface and the particles is a challenge. Indirect designs include flow-in-tube receivers. Fluidizing the particles within tubes has been shown to enhance the heat transfer with respect to fixed bed or free-falling designs. Some technical challenges, mainly associated with the flow and heat transfer characteristics of solid particles in the receiver, must be solved for commercial viability of solid particle technologies [21, 22]. For large-scale applications, significant particle mass flow rates are required and gravity-driven flow (free-falling or with obstructions) appears to be the most promising [21]. Most current studies on particle receivers are focused on the solar tower system for high-efficiency power plants. The key problems using solid particles are the heat absorption and storage medium requirements in solar tower, the heat transfer characteristics of solid particles as the heat transfer medium in heat exchangers, wear of solid particles in the circulation process and metal wall, and other issues such as the regulation and operation optimization of the receiver [22].
The integration of solar energy conveying by solid particles coming from the hot storage (storing hot particles from the central receiver) is usually conducted by a heat exchanger to produce steam or to heat an additional HTF. New solid particle receivers are under development towards more efficient thermodynamic cycles based on gas turbines (Brayton cycle). No thermochemical applications have been considered so far under this operational mode, except for the theoretical study mentioned [17].
2.4 Main conclusions from literature review
From the critical review the following conclusions can be made:
-
Allothermal steam gasification of biomass has been widely investigated at lab-scale with heat from an external electrical oven. Scale-up has only been achieved using DFBG with partial combustion of the fuel (char). Dual FBG can be operated with air, still producing high-quality syngas without N2 and high content of H2. Although the process is not allothermal (about 20–40% of the fuel is burned), it is the most flexible technological development and it is the only one that has reached commercial status using biomass.
-
Allothermal gasification with external heat from concentrated solar radiation is the most interesting choice for steam gasification of biomass since the syngas produced contains all the energy from the biomass and a significant solar share. In addition, it maximizes the carbon of the biomass into the syngas as combustible species. In spite of this, solar steam gasification has been tested only with indoor devices using a high-flux solar simulator up to 1–10 kWth, which provides an artificial source of concentrated solar spectral distribution of energy mimicking the solar radiation. For a pilot-scale demonstration, the solar furnaces are used with solar input of 10–1000 kW at the reactor aperture but this step has not been achieved for solar gasification.
-
The only practical development on steam gasification (no-solar) is the air-steam indirect DFBG. In this technology, the gasifier is heated by burning part of the fuel (char) and providing this gasifier with external heat has not been considered. As a result, there is not a solar version of the technology.
-
Most of the solar gasifiers developed up to date are directly irradiated. They are more thermally efficient than indirectly irradiated gasifiers but scaling-up remains a challenge. As a result, only laboratory devices have been tested. Indirect irradiation to the reactor could overcome many problems allowing application at large scale in the short/medium term.
-
New technology of solid particle receivers (without reaction) reaching temperatures between 700 and 900 °C has been developed in the last 5 years. Moreover, research under development promises good future perspectives to come up with scalable prototypes reaching 1000 °C. The directly irradiated solar particle, tower-mounted, falling particle cavity receiver is the best choice developed to date and can achieve the thermal requirements of the biomass gasification (800–950 °C).
-
The solids receiver and the gasifier can be uncoupled using solid particles as an energy carrier and storage material. However, it may result in excessive storage volume and complications in conveying great amount of solid particles.
3 Theoretical analysis
3.1 Process layout
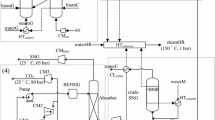
The proposed conceptual integration of the solar gasification system and particle receiver is presented in Fig. 3a. The solid particles act as a thermal energy carrier, circulating cyclically between the solid particle receiver and the gasifier. Two tanks are used to store the particles heated by the receiver and the particle cooled in the gasifier allowing for temporary thermal storage of solar energy. The use of solid particles as an energy carrier is attractive since they are an excellent thermal energy storage medium, operating at high temperature and low cost. A more detailed scheme of the DFBG using solar-heated solid particles (SDFBG) is presented in Fig. 3b. In this system, there are two types of solids circulations: internal solids circulation (between gasifier and combustor) and external circulation (between the DFBG and the solar receiver passing through the thermal energy storage). In a SDFBG, there are different possibilities for introducing/extracting the solids in the system as shown in Fig. 4, and the internal circulation from the gasifier to the combustor can be different from that from the combustor to the gasifier.
To sum up, the use of DFBG with thermal storage offers some practical advantages in that the gasifier and downstream processes can operate as a conventional (no-solar) DFBG, with sensible heat provided to the process from hot bed material that is heated either in a solar receiver or a fluidized bed combustor. This gasifier provides the system with the required flexibility, improving significantly the rigidity and disadvantages of intermittent time-variation typical of directly irradiated process. Although the analysis in [17] is interesting because it proposes uncoupling the gasification process from solar radiation, it is based on coal that needs higher temperatures (1000–1200 °C) and the process is not completely allothermal since a significant part of the char and some auxiliary fuel are burnt, decreasing significantly the solar share and thus the gasification efficiency. In addition, in [17], it is neither analyzed the potential idea of increasing the allothermal character of the process nor the way in which the DFBG has to be adapted to solar-mode operation. Our aim in the following sections is to analyze the DFBG processing biomass from the chemical reactor engineering perspective, coming up with optimal reactor design and coupling with the solar field. This contrasts with [17] where a global analysis of coal-to-liquid is made without analyzing the DFBG performance.
3.2 Modeling
3.2.1 Model approach
The steam gasification of 1 mol of biomass can be represented by the stoichiometry of reactions R1 and R2, establishing the amount of reactants involved (steam biomass ratio, SBR) and products (H2 and CO/CO2) generated provided the reactions are complete. This is just an ideal reference case and not the actual situation when using FBG, in which thermodynamic and kinetic limitations result in a more complex product distribution [23] as indicated in R3, [23].
where besides CO and H2, other components are present in the product gas, such as hydrocarbons (mainly CH4, and other light compounds, lumped into C2H4) and tars (lumped into naphthalene C10H8) as well as solid carbon (char, i.e., C(s)). In addition, there is always unconverted steam even when feeding the stoichiometric steam according to R1.
Calculating the distribution of the syngas and char for different operating conditions in a FBG is complex and several models can be applied. Three approaches can be applied to model steam gasification in an FBG [24]: (i) the assumption of equilibrium (EM); (ii) the application of kinetic models (KM), taking into account chemical and fluid-dynamic rate considerations; (iii) the combined approach, sometimes called pseudo-equilibrium (PEM). EM is the most universal way to close the calculations but fails in predicting real gas composition and fuel utilization (char conversion). KM gives better representation of the process for a specified system (geometry, type of biomass, etc.) but a great deal of inputs are required, and the conclusions are system-dependent. PEM is based on equilibrium relations together with semiempirical inputs to take into account kinetic and flow rate limitations. It is a reasonable compromise between EM and KM using some comprehensive models supported by empirical closures.
3.2.2 Model of the SDFBG
The model of the DFBG considers the gasification and combustion units and the solids stream coming from the solid particle receiver. In the gasification unit devolatilization, secondary reforming of gas and tar and steam gasification of char takes place, summarized by R3. The PEM developed in [23] is adopted here to model the gasification unit. The process is simplified by decoupling primary and secondary conversion. The primary yields of fuel devolatilization are methane, ethylene, tar, and char. Then, the char is gasified, the light hydrocarbons reformed with steam, and the tar is converted by reforming/cracking. The composition of the outlet gas is obtained by applying the equilibrium of the water-gas-shift reaction to the compounds released after devolatilization, and considering the overall atomic mass and heat balance over the entire gasifier while taking into account the unconverted fraction of hydrocarbons, tar, and char. Other assumptions for modeling the gasification reactor are as follows: (i) conversion of gaseous species assuming perfect mixing of gases both, in the bed and freeboard, and first-order kinetics, with an average gas residence time of 1 s (the actual geometry of the reactor is not considered); (ii) char is removed from the gasifier with the solids circulating to the combustor (neither elutriation nor mechanical removal of bottom ash is considered); and (iii) the char is converted by steam gasification considering that the particles are perfectly mixed in the reactor and following the uniform conversion model (see Section 3.2.3).
The unconverted char from the gasifier is conveyed to the combustion unit together with inert material and catalyst (if any) and is considered to be completely burnt with air according to reaction R4. Finally, the heat balance between the gasifier and combustor is made to close the overall heat balance of the system. More details of the model are presented in [25].
Given the gasification and combustion temperature, the steam to biomass in the gasifier, the excess of oxygen in the combustor, and the temperature at which gasification agent and air are fed to the system, the model gives the yields and composition of the syngas and flue gas for any specified external heat. The solids circulation rate (per unit of kgbio,daf) is calculated to balance the heat between the two units since the overall system is autothermal. For the autothermal (no-solar) case, the external heat is zero and the amount of char burnt in the combustor is the maximum one. For the allothermal case, all the char is converted in the gasifier and the combustor is idle, being maximum the external heat to the system.
3.2.3 Model of char conversion in an FBG
The conversion of the char is the rate-controlling process in FBG gasifiers and must be carefully modeled to analyze the performance of both stand-alone and DFBG. In a conventional DFBG, only a limited fraction of the char generated after fuel devolatilization is converted with steam, while the rest is driven to the combustor. In a solar DFBG, however, high char conversion could be achieved if the proper rate of external heat transfer is provided to the gasifier.
In general, it is difficult to fully convert the solid carbon in a single FBG, both in a stand-alone FBG and in the gasification unit of a DFBG. Even in units operating with high temperature and reactor volumes, or when an external catalyst is used, the backmixing of solid particles makes complete char conversion difficult in one single unit. The extent of char conversion in the gasifier depends on the carbon-steam gasification rate of the char particles (CO2-carbon rate is much slower) and their residence time in it. On the one hand, the rate of reaction depends on the temperature, the species concentration (mainly steam, but hydrogen can be also important as it inhibits the carbon-steam reaction rate), the intrinsic reactivity of the char (fuel type and form of char generation), and the quality/extent of gas-solid contact. On the other hand, the residence time of the char particles depends on the rate of solids removal that can be unintentionally happen by gas-solid elutriation from the bed and/or, in the case of DFBG, by removing the solids to carry them to the combustor. Theoretically, in a FBG without any kind of char removal, i.e., in an ideal stand-alone FBG processing an ash-free biomass without elutriation, the residence time of char could be infinite, and consequently, the char conversion could be complete, provided the reactor volume is high enough. In practice, due to kinetic limitations and ash-related issues, there is a throughput of char flow rate (i.e., kg m−2 s−1) that can be converted in a gasifier with a given solid inventory.
The model of char conversion developed in [23] is applied here to analyze the char residence time required to achieve the desired conversion in the gasification unit of a DFBG (conventional and solar). In this model, the average conversion in the gasifier is calculated by considering a population balance of char particles, each one following a uniform conversion model with kinetics control, and perfect mixing of gas and char in the fluidized bed gasifier.
As discussed above, the required char conversion in a DFBG depends on the heat balance of the system: in a conventional autothermal DFBG, the char conversion in the gasifier is very limited because a significant fraction of the char has to be burnt in the combustor to thermally maintain the system. In a solar DFBG, the higher the external solar heat provided, the higher the char conversion in the gasifier and the lower in the combustor. As we discuss below, the char conversion and residence time in the gasifier is strongly related to the required gasifier mass inventory and solids circulation flowrate between the gasifier and combustor and must be carefully analyzed to properly design a SDFBG.
3.2.4 Equilibrium model of a completely allothermal gasifier
As a useful reference, the results from a model considering equilibrium in a completely allothermal gasifier, where all the biomass is converted with steam (in absence of air) giving the maximum amount of external heat to the gasifier will be used. This is the limiting case of an SDFBG without a combustion unit and with an infinite residence time of the char. This ideal case will be shown to be very useful as it results in the syngas with the maximum solar share for a specified gasification temperature.
3.3 Performance indicators
To quantify the contribution of external solar energy to the system, the following parameters are defined:
-
Specific external heat (SEH) defined as the solar heat supplied to the system per unit of dry-and-ash-free biomass (MJ/kgbio,daf).
-
Solar share (SS), defined as the ratio between the solar heat supplied to the system and the lower heating value of the syngas (expressed in percentage).
4 Results and discussion
4.1 Analysis of the allothermal gasification
Figure 5a shows the equilibrium molar yields (mol of species i per mol of dry ash-free biomass) of the main gases and solid carbon at 1 bar of pressure as a function of temperature for a steam equivalence ratio, ERH2O = 1 (ERH2O, steam fed/stoichiometric steam to produce syngas according to R1). According to equilibrium predictions, at low temperatures, solid carbon (C(s)) and CH4 are present in the product gas, but both disappear by reforming as the temperature is increased. The carbon boundary point (disappearance of solid carbon) is about 1200 K for ERH2O = 1, decreasing with ERH2O (for instance, although not shown in the figure, it is about 900 K for ERH2O = 4.33). CH4 virtually disappears at 1200 K and the CO and H2 increase with temperature as they are the main products of carbon and methane reforming. The increase is very significant as long as there is solid carbon in the system (lower temperatures) whereas the increase slows down beyond intermediate temperature, once the solid carbon is reformed. For ERH2O = 1 and T > 1200 K, the syngas is practically H2 and CO as given by the stoichiometry of reaction R1.
The effect of temperature in allothermal steam gasification on a the molar gas yields of the main species for steam gasification of biomass according to R1 (tars and other light hydrocarbons are not depicted since their concentrations are very low compared with the rest of species included in the figure); b specific heat of steam gasification at temperature T according to R1 (per kg of dry-and-ash-free fuel) for different fuels: carbon, char, and biomass (the hatched region in the figure corresponds to a typical biomass). Simulation corresponds to equilibrium predictions with ERH2O = 1
The corresponding heat required for gasification (the heat of reaction R3 assuming the equilibrium is achieved) as a function of temperature (Tgas) is presented in Fig. 5b for various fuels (biomass, coal, char, and pure carbon). It is shown that the heat for gasification increases with temperature up to 1200 K (923 °C) for practically all the fuels, i.e., up to the point where the fuel is practically converted into CO and H2 as shown in Fig. 5a. For biomass with LHV of approximately 18–18.5 MJ/kgbio,daf at around 1000–1200 K (727–927 °C), the heat of gasification ranges between 3.5 and 5.5 MJ/kgbio,daf (80–130 kJ/molbio,daf). The region of biomass in the figure (region marked in green in Fig. 5b) is well below that for char from wood (blue) and pure carbon (represented as C in black). Obviously, the higher the carbon to hydrogen ratio, the larger the heat necessary and the more water is split. However, on the basis of the heating value of the fuel, the ratio to be supplied to the gasifier (MJ/kgfuel,daf) is of the same order for all fuels, in the order of 25–35% of the LHV of the fuel. For instance, for pure carbon, the maximum heat of gasification is around 11.5 MJ/kgfuel,daf while LHV is roughly 33 MJ/kgfuel,daf, so the ratio is approximately 35%.
To understand the effect of kinetic limitations, usually prevailing in a real FBG, the effect of steam equivalence ratio over syngas composition is presented in Fig. 6 over a wide range of ERH2O for 850 °C, calculated with the pseudo-equilibrium model (graph a), while in graph b, the equilibrium predictions are plotted for comparison. In the PEM, the char conversion model is calculated using the reactivity of a typical wood char as input and the average residence time of the char is fixed at 20 min. In fact, the residence time of the char has to be calculated case-by-case by considering entrainment and elutriation and then the geometrical factor has to be defined [23]. That is the reason why a typical value of 20 min is taken as a reference for the simulations of Fig.6a. This value is justified below after analysis of Fig. 9.
Effect on steam equivalence ratio in allothermal steam gasification on syngas composition (water and solid carbon are referred to wet gas, the rest to dry syngas) at gasification temperature 850 °C. Graph a for PEM, i.e., taking into account kinetic factors, and b for EM, i.e., thermodynamic predictions
It is seen in Fig.6 that due to kinetic limitations, at 850 °C and ERH2O = 1, the syngas is 80% CO and H2 with H2/CO molar ratio of about 1, whereas all the syngas is made of CO and H2 according to equilibrium predictions. This is explained by the reforming of CH4 and hydrocarbons as well as char gasification, which are complete according to equilibrium but only partially achieved in a FBG due to kinetic limitations. The kinetic limitations are seen to occur at 850 °C at any ERH2O since the quantitative predictions of kinetics and equilibrium differ significantly, although the variation trends of gas species concentration with ERH2O are similar for both models.
An important issue for the present work is that the heat to be supplied to the gasifier is much higher if an equilibrium is attained, as the reforming of hydrocarbons and the gasification of char (highly endothermic reactions) are almost completed, releasing more H2 species. This effect is quantified in Fig. 7a where the heat required in the gasifier (normalized by the low heating value of the biomass, which is 18.6 MJ/kgbio,daf) is plotted as a function of the gasification temperature. Although the trends are similar (the heat supplied is higher at higher temperature and steam to biomass ratio), the EM predicts 2–3 times more heat than PEM. This is because as the gasifier is limited by kinetics factors, a great deal of carbon, methane, and hydrocarbons are not reformed and the heat required is much lower. In effect, at 850 °C, for instance, around 0.14 LHV of the energy is necessary in the gasifier according to PEM, whereas about 0.25 LHV is predicted by EM. The figure also shows that the hydrogen benefits from increasing the gasification temperature, especially if the process is kinetically limited (EM predicts a maximum H2 yield and gasification efficiency at high ERH2O and intermediate temperature of around 750 °C). The conclusion is that the more complete the ideal reaction (R1) to syngas (CO and H2), the higher the heat storage in the product gas. This is an important information for the design of the solar gasifier discussed below.
Effect of gasification temperature on the heat to be supplied to the gasifier (normalized by the heating value of the biomass, 18.6 MJ/kgbio,daf) (a) and H2 molar yield (b) in an allothermal FBG, as a function of gasification temperature for two steam equivalence ratios ERH2O = 1 (black lines) and ERH2O = 3 (blue lines), using EM (solid lines) and PEM (broken lines)
Figure 8 shows the heat balance over a system with heat recovery from the syngas according to equilibrium predictions for ERH2O = 1. It is concluded that if equilibrium conversion is attained, about 105 kJ/molbio,daf (4.25 MJ/kgbio,daf) is necessary to be supplied to the gasifier even if the system is energetically integrated (i.e., steam is produced from water and superheated at 700 °C by heat exchange with the syngas). This means that 20.68 kJ/molbio,daf is employed in water vaporization and steam superheating for the gasification process (typically 5 times less than the heat required in the gasifier, i.e., 105 kJ/molbio,daf). This is an important consideration because the heat in the gasifier has to be provided at high temperature (at a significantly higher temperature than 823 °C, so typically in the range of 900–950 °C) whereas steam generation and superheating can be obtained with a lower temperature heat source (in the figure by the sensible heat of the syngas). For higher steam to biomass ratio, for instance for operation at ERH2O = 4.33 (SBR = 0.95 kgH2O/kgbio,daf), the heat required to vaporize 1.3 mol of water and superheat the steam up to 700 ºC is about 90 kJ/molbio,daf which is of the order of the 113 kJ/molbio,daf that is needed in the gasifier at 823 °C for that ERH2O = 4.33). Nevertheless, operation with such high steam to biomass ratio is not practical and it is only applied in lab-scale studies. Therefore, the conclusion is that for practical ERH2O (1–2), most of the heat for gasification (60–80%) has to be supplied from a high-temperature external heat source (> 900 °C).
As discussed above, simulation of char conversion in the gasifier is a key step to predict the performance of steam allothermal stand-alone gasification (Fig. 1a) since the main effort must be concentrated to fully convert the carbon with steam, for instance by operating at high temperature, high solid residence times or by catalytic addition. In DFBG, in contrast, the char generated after fuel devolatilization is barely converted, but it is directed to the char combustor. Even in this case, it is necessary to calculate the char available for combustion since the gasifier heat needs must be satisfied by the combustion heat, and the amount of char converted in the gasifier establishes the additional fuel needed to be supplied in the combustor to energetically balance the system.
The results from the char conversion model for a generic FBG (so useful to examine the performance of a stand-alone FBG or a gasification unit of a DFBG) are shown in Fig. 9. Graph a shows the residence time of the char particle needed to attain 50% char conversion as a function of gasifier temperature using steam-char reactivities for two different biomass species, assuming average steam partial pressures of 0.20 and 0.60 bar. It is seen that at 800 °C, the char particle needs to stay in the reactor between 10 and 30 min to achieve half conversion (so that the yield of this biomass would be 0.06 kgchar/kgbio,daf at the gasifier exit, assuming an initial char yield of about 0.12 kgchar/kgbio,daf), whereas at 900 °C, the residence time required to achieve the same 50% conversion is reduced about an order of magnitude (to 1–3 min). Graph b shows how the char conversion is increased with temperature and residence time for a fixed char (from wood) and steam concentration in the gasifier. For instance, to increase the char conversion from 0.5 to 0.8 at 850 °C, it is necessary to increase the residence time from 10 to 40 min. For a given gasifier design and inventory, the residence time does not change much within reasonable fluid-dynamic conditions, so the conversion is more affected by temperature and gas composition effects and these effects are well captured by the model. Overall, this information is of great relevance to know for instance how much char will circulate with the sand from the gasifier to the combustor with different biomass species and operating conditions (temperature and residence time) in a DFBG.
a Residence time of char for reaching 50% average conversion in a FBG as a function of gasification temperature for various biomass chars and steam partial pressures; b Residence time of char particles in the gasifier as a function of average char conversion for various gasification temperatures for wood char and steam partial pressure of 0.2 bar
4.2 Analysis of the conventional (non-solar) DFBG
In Fig. 10a, the solids circulation rate (per unit of kgbio,daf) required to maintain the system autothermal (providing neither ancillary fuel nor external heat) is plotted as a function of combustion temperature for three gasification temperatures. For a fixed gasification temperature, the solids circulation rate decreases rapidly with combustion temperature (i.e., higher difference of temperature between the two reactors, ΔT). It is concluded that for keeping solids circulation rates under reasonable values, ΔT should be in the order of 50 °C and above. In Fig. 10b, the corresponding conversion as a function of the residence time of the char in the gasifier is presented for the same three gasification temperatures of Fig. 10a. As shown, the residence time of the char in the gasifier decreases (so it does the char conversion) with gasification temperature, since more char yield has to be produced in the gasifier to be burned in the combustor. Note that although the char reactivity is higher as gasification temperature is increased, the char conversion in the gasifier decreases because of the significant reduction in the char residence time. It is observed in Fig. 10b that, for a given gasification temperature, the higher the combustion temperature, the lower the degree of char conversion, especially at lower gasification temperatures. The reason is that more sensible energy escaped with the flue gas when the combustor is operated at a higher temperature, so a slightly more char is necessary to be burnt in the combustor for equal gasification temperature. Although not shown in the figure, the corresponding cold gasification efficiency (chemical energy in the product gas/chemical energy in the biomass) for the simulations made in Fig. 10 decreases slightly with temperature, from to 71 (800 °C) to 68 (950 °C), and the lower heating value of the dry syngas ranges between 12.8 and 13 MJ/Nm3.
a Solids circulation ratio (flow rate of solids/flow rate of biomass) as a function of combustion temperature for three gasification temperatures. b Corresponding char residence time as a function of char conversion (the numbers indicated below the lines are the two extreme combustion temperatures simulated for each gasification temperature). Char after devolatilization 0.12 kg/kgbio,daf, ERH2O = 2 (SBR = 0.44 kg/kgbio,daf)
Figure 11 presents the performance of a standard (non-solar) DFBG at different gasification temperatures for fixed combustion temperature, steam equivalence ratio ERH2O, and inlet steam temperature. It is shown that the solids circulation increases as the gasifier operates at a higher temperature. Consequently, lower char residence time is required and lower char conversion is attained in the gasifier (Fig. 11a). The yields of syngas and H2 (Fig. 11b) decrease with gasification temperature as a result of the lower char conversion in the gasifier.
4.3 Analysis of solar DFBG
Figure 12 shows the performance of an SDFBG operating at fixed gasification temperature with solid addition/removal to/from the gasifier (Conf1, according to Fig. 4), as a function of the specific external heat supplied to the system, SEH. The internal solids circulation decreases significantly with SEH, whereas the biomass space time, defined as the ratio between the mass inventory of the gasification unit and the mass flowrate of biomass, increases as seen in Fig. 12a (note that larger biomass space time means larger mass inventory or reactor size for a given biomass flowrate). Logically, no circulation is necessary when all the heat required to the gasifier is externally supplied (SEH = 3 MJ/kgbio,daf). Significant char conversion is reached for reasonable residence time (80% of char conversion is attained in the gasifier with a residence time of 28 min) but higher char conversion requires excessive long residence time and thus reactor volumes (see biomass space time in Fig. 12a). The syngas produced is improved considerably (Fig. 12c) as a result of higher steam-char conversion. Figure 12d shows that, for SEH = 3 MJ/kgbio,daf, 15% of the chemical energy in the syngas comes from the sun; besides, under this operation, the syngas embodies all the energy of the biomass (in the form of chemical and sensible energy).
a–d Performance of an SDFBG with various levels of specific external heat (SEH) for configuration 1 at gasification temperature of 850 °C and temperature of hot solids from the solar loop of 950 °C (other operating conditions as in Fig.11)
Figure 13 shows the external solids circulation (that circulating through the solar loop) as a function of the SEH for the four configurations presented in Fig. 4. Configurations with the solid removal in the same unit (Confs 2 and 4 and Confs 1 and 3) present the same value of external solids circulation, as this results from the driving potential of temperature between the hot particles and the removal point. Therefore, since the driving potential is lower, the external solids circulation is higher in Confs 2 and 4.
Figure 14 compares the internal circulation for the four configurations in Fig. 4. Conf 1 requires the lowest internal solids circulation for equal SEH, whereas Conf 4 demands the highest one. Confs 2 and 3 present different internal circulations depending on the direction of the solids flow. Moreover, Confs 1 and 3 present the highest external solar heat absorption capacity (highest SEH), reaching a value of 3 MJ/kgbio,daf, corresponding to a solar share of 15%, as shown in Fig. 12d. In Conf 1, the internal solids circulation in the two directions is the same and decreases with SEH, while in Conf 3, the solids circulation to the gasifier is higher than that to the combustor, and the difference of the two solid flows increases with SEH. The internal solids circulation in Conf 1 becomes zero when the system reaches the maximum solar share, i.e., when it becomes completely allothermal. In this case, all the external solids pass through the gasifier only, full char conversion is attained in it, and the combustor is out of service.
An operational point is identified in Conf 2 where the system cannot absorb further external solar heat, even if more external hot solids were introduced in the gasifier. At that point (SEH ≈ 1 MJ/kgbio,daf) part of the char is burnt and char conversion in the gasifier is limited to 0.46 reaching a maximum solar share of about 6%. The maximum SEH attainable in Conf 4 is around 2.3 MJ/kgbio,daf, lower than the maximum SEH in Confs 1 and 3 (3 MJ/kgbio,daf). This results from the difference between the driving potentials of temperatures (hot particles, combustor 950–905 = 45 °C, and gasifier, combustor 905–850 = 55 °C). Since the latter temperature driving potential is higher than the former, some char is burnt in the combustor to heat up the solids from the gasifier to combustor and the char conversion is lower than unity in the gasifier, resulting in SEH lower than the maximum. For the same driving potentials, for instance, by taking combustor temperature at 900 °C (the two driving potentials would be 50 °C), full char conversion is attained in the gasifier and SEH would reach 3 MJ/kgbio,daf.
It is concluded that Conf 1 is the most attractive option from a gasification perspective. However, additional aspects from the solar receiver side need to be considered. Solid removal from the gasifier (Confs 1 and 3) will result in reacting particles in the solar loop, which in principle poses new problems (the solid particle receivers currently under development are mainly based on “open” designs [12] leading to the loss of active particles). On the other hand, the removal of solids from the combustor (Confs 2 and 4) will make it more difficult the operation of the solar receiver (solids reaching the receiver at higher temperature require higher circulation for a given SEH), although it has the advantage of allowing for the use of open solid particles receivers.
5 Conclusions and future work
In order to understand the possibilities of hybridization of steam gasification of biomass using solar energy, the thermodynamic and kinetic analysis of allothermal steam gasification of biomass carried out in fluidized bed conditions is analyzed. For a typical biomass with a heating value of 18 MJ/kgbio,daf, the equilibrium predictions establish that the maximum solar energy than can be transferred to the syngas ranges from 3.5 to 5.5 MJ/kgbio,daf (80–130 kJ/molbio,daf) for temperatures between 727 and 927 °C. The higher the temperature, the more complete the biomass is converted into pure syngas (CO and H2) and then the higher the solar share in the syngas. For practical steam equivalence ratios ERH2O (1–2) and gasification temperature in FBG (800–900 °C), most of the heat for gasification (60–80%) must be supplied from a high-temperature external heat source (> 900 °C). To assess the kinetic limitations for typical operation of FBG, a comparison between thermodynamic and kinetic predictions shows that kinetic factors limit the amount of solar energy that can be transferred to the syngas. For instance, at 850 °C, the actual external energy supplied to a stand-alone FBG (calculated taken into account kinetic factors) was estimated in the order of 2.4 MJ/kgbio,daf, which is significantly lower than the maximum one calculated by equilibrium, which is 4.5 MJ/kgbio,daf.
An analysis of the performance of an alternative form of integration of steam gasification of biomass in a dual fluidized bed (DFBG) using solid particles to carry the solar energy from the solid particle receiver to the reactor is presented. Instead of directly exposing the reaction chamber or an intermediate emitter material to the solar radiation, as it has been most often applied up to now, in this process, the solid particles act as an energy carrier and energy storage material, enabling continuous syngas generation with high thermal integration. A theoretical analysis of the DFBG system (using a kinetic model of the gasification unit) shows that, for a standard configuration (introducing and extracting the solids to/from the gasifier), the process can be conducted efficiently in a DFBG with char conversions of 80% in the gasifier corresponding to an average char residence time of 28 min, which is a reasonable figure for a bubbling gasification unit of a conventional DFBG. This mode of operation requires 2.4 MJ/kgbio,daf of solar energy, resulting in a syngas with 12% of solar share, i.e., embodying approximately 12% of solar energy in the form of chemical energy in the syngas (other modes of operation yield different figures but similar conclusions are obtained). The analysis of different configurations integrating the solar receiver and the DFBG showed that the addition and removal of solids from the gasifier unit is the most attractive option but limits the operation to closed solid particle receiver or enforces carrying out solids separation before sending them to the receiver. Nevertheless, additional aspects have to be considered like the operation of the system under daily and seasonal weather variations throughout the year coupled to a solar field using hourly data for different locations [25], and the design and optimization of the new gasifier considering operational flexibility, i.e., different modes of daily and seasonal performance. Overall, the analysis demonstrates the potential of adding solar thermal energy to a DFBG to increase the chemical energy of the syngas, providing first estimates for further analysis of the system.
Abbreviations
- Conf:
-
configuration
- DFBG:
-
dual fluidized bed gasifier
- EM:
-
equilibrium model
- FB:
-
fluidized bed
- FBG:
-
fluidized bed gasifier/gasification
- HTF:
-
heat transfer fluid
- KM:
-
kinetic model
- LHV:
-
low heating value
- PEM:
-
pseudo-equilibrium model
- SDFBG:
-
solar dual fluidized bed gasifier
- TES:
-
thermal energy storage
- bio:
-
biomass
- daf:
-
dry ash free
- gas:
-
gasification, gasifier
- ERH2O :
-
Steam equivalence ratio (−)
- n i :
-
Molar yield of i (mol/mol)
- p :
-
pressure (bar)
- p i :
-
partial pressure of component i (bar)
- SEH:
-
Specific external heat (MJ/kgbio,daf)
- SBR:
-
Steam biomass ratio (kgH2O/kgbio,daf)
- SS:
-
Solar share (−)
- T :
-
Temperature (K)
References
Nzihou A, Flamant G, Stanmore B (2012) Synthetic fuels from biomass using concentrated solar energy – a review. Energy 42(1):121–131
Karl J, Pröll T (2018) Steam gasification of biomass in dual fluidized bed gasifiers: a review. Renew Sust Energ Rev 98:64–78
Corella J, Toledo J, Molina G (2007) A Review on dual fluidized-bed biomass gasifiers. Ind Eng Chem Res 46:6831–6839
Puig-Arnavat M, Tora E, Bruno J, Coronas A (2013) State of the art on reactor designs for solar gasification of carbonaceous feedstock. Sol Energy 97:67–84
Kodama T (2003) High-temperature solar chemistry for converting solar heat to chemical fuels. Prog Energy Combust Sci 29:567–597
Nathan G, Dally B, Alwahabi Z, van Eyk P, Jafarian M, Ashman P Research challenges in combustion and gasification arising from emerging technologies employing directly irradiated concentrating solar thermal radiation. Proc Combust Inst 36(2):17
Florin N, Harris A (2008) Enhanced hydrogen production from biomass with in situ carbon dioxide capture using calcium oxide sorbents. Chem Eng Sci 63:287–316
Flamant G, Gauthier D, Benoit H, Sans J, Garcia R, Boissière B, Ansart R, Hemati M (2013) Dense suspension of solid particles as a new heat transfer fluid for concentrated solar thermal plants: on-sun proof of concept. Chem Eng Sci 102:567–576
Alonso E, Romero M (2015) Review of experimental investigation on directly irradiated particles solar reactors. Renew Sust Energ Rev 41:53–67
Deepak Y, Rangan B (2016) A review of solar thermochemical processes. Renew Sust Energ Rev 54:497–532
Taylor R, Berjoan R, Coutures J (1983) Solar gasification of carbonaceous material. Sol Energy 30(6):513–525
Murray JP, Fletcher EA (1994) Reaction of steam with cellulose in a fluidized bed using concentrated sunlight. Energy 10(10):1083–1098
Kodama T, Bellan S, Gokon N, Cho H (2017) Particle reactors for solar thermochemical processes. Sol Energy 156(1):113–132
Boujjat H, Rodat S, Chuayboon S, Abanades S (2019) Experimental and numerical study of a directly irradiated hybrid solar/combustion spouted bed reactor for continuous steam gasification of biomass. Energy 189(15):116118
Moroyama A, Guscetti I, Schieber G, Haussener S, Loutzenhiser P (2018) Design and demonstration of a prototype 1.5 kWth hybrid solar/autothermal steam gasifier. Fuel 211(1):331–340
Gordillo E, Belghit A (2011) A bubbling fluidized bed solar reactor model of biomass char high temperature steam-only gasification. Fuel Process Technol 92(3):314–321
Guo P, van Eyk P, Saw W, Ashman P, Nathan G, Stechel E (2015) Performance assessment of fischer-trops liquid fuels production by solar hybridized dual fluidized bed gasification of lignite. Energy Fuel 29:2738–2751
Guo P, Saw W, van Eyk P, Stechel E, Ashman P, Nathan G (2017) System optimization for Fischer–Tropsch liquid fuels production via solar hybridized dual fluidized bed gasification of solid fuels. Energy Fuel 31(2):2033–2043
Falcone PK (1984) Technical review of the solid particle receiver program, January 25–26, 1984, Livermore. https://doi.org/10.2172/6719789
Falcone PK, Noring JE, Hruby JM (1985) Assessment of a solid particle receiver for a high temperature solar central receiver system, Livermore. https://doi.org/10.2172/6023191
Ho C (2016) A review of high-temperature particle receivers for concentrating solar power. Appl Therm Eng 109:958–969
Jiang K, Du X, Kong Y, Xu C, Ju X (2019) A comprehensive review on solid particle receivers of concentrated solar power. Renew Sust Energ Rev 116:109463
Gómez-Barea A, Leckner B (2013) Estimation of gas composition and char conversion in a fluidized bed. Fuel 107:419–431
Gómez Barea A, Leckner B (2010) Modeling of biomass gasification in fluidized bed. Prog Energy Combust Sci 36(4):444–509
Suarez Almeida M, Gómez-Barea A, Ghoniem A, Pfeifer C (2020) Solar gasification of biomass in a dual fluidized bed. Chem Eng J. https://doi.org/10.1016/j.cej.2020.126665
Funding
The authors acknowledge the financial support by MINECO of the Spanish government (project CTM2016-78089-R), and the grants PRX18/00629 and BES-2017-080653, as well as the Foundation Seed Fund MIT - Spain “la Caixa” (project SOLGASBI).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gómez-Barea, A., Suárez-Almeida, M. & Ghoniem, A. Analysis of fluidized bed gasification of biomass assisted by solar-heated particles. Biomass Conv. Bioref. 11, 143–158 (2021). https://doi.org/10.1007/s13399-020-00865-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00865-0