Abstract
Natural Miscanthus grasses are useful for improving biomass production. We found a population of putative triploid interspecific hybrids between Miscanthus sacchariflorus and Miscanthus sinensis in southern Kyushu, Japan. This study aims to investigate its morphological variation, genetic structure, and origin. Miscanthus plants were collected from 114 points, mainly beside a river along a distance of 2.8 km in the Tashiro–Fumoto area. They resembled M. sacchariflorus but showed morphology intermediate between the two species. They had a nuclear DNA content corresponding to that of a hybrid between tetraploid M. sacchariflorus and diploid M. sinensis, and had species-specific alleles from both species revealed by DNA marker analysis. This indicates that the plants are triploid hybrids between M. sacchariflorus and M. sinensis. Genotyping using simple sequence repeat markers revealed only four genotypes among the hybrid population, of which two accounted for most plants. The genotypes showed mostly discrete geographical distributions. The two major genotypes showed contrasting phenotypes in pollen viability and in frequency of awns in florets. Some seeds collected from the population germinated and the seedlings showed a wide range of nuclear DNA content from diploid to tetraploid. In this area, many M. sinensis plants also grew, but we could not find M. sacchariflorus. The hybrid Miscanthus might be selected due to its improved adaptability introduced from M. sinensis. Furthermore, genetic and phenotypic characterization suggests the polyphyletic origin and clonal propagation of this population. Such partially fertile hybrids could be interesting for the improvement of Miscanthus as a biomass crop.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Miscanthus Andersson consists of approximately 12 species of C4 perennial grasses that originate in a region ranging from East Asia to the Pacific islands [1]. Miscanthus sacchariflorus (Maxim.) Bentham and Miscanthus sinensis Andersson are the predominant species in Japan. In comparison to M. sinensis, Miscanthus sacchariflorus prefers wetter soil conditions, such as river banks, but their ranges overlap, sometimes in sympatric populations [2]. In Japan, M. sinensis is generally diploid and M. sacchariflorus is tetraploid [3], but diploid M. sacchariflorus grows in China and Korea [4, 5].
Miscanthus grasses are a popular focus of biomass production in temperate regions as a source of renewable energy on account of their high productivity, low fertilizer requirement, and relatively wide adaptability [1]. In particular, Miscanthus ×giganteus Greef & Deuter ex Hodk. & Renvoize is a promising crop in Europe and the USA [1, 6, 7]. Cytogenetic and molecular analyses has shown that M. ×giganteus is an interspecific triploid hybrid between tetraploid M. sacchariflorus and diploid M. sinensis [8, 9]. It shows remarkably high productivity due to heterosis. Its sterility, due to its triploid nature [10, 11], is advantageous for limiting the risk of spread in non-native regions. One extensively studied genotype was exported from Yokohama, Japan in the 1930s by the Danish botanist Aksel Olsen [12, 13]. To expand the genetic variation, breeders have purposely crossed M. sacchariflorus and M. sinensis [1, 14].
Naturally growing interspecific hybrids are also useful for the acquisition of breeding materials. In sympatric population areas, natural interspecific hybrids may arise if the flowering periods overlap [2]. More than half a century ago, studies reported natural putative triploid hybrids between M. sacchariflorus and M. sinensis in central to southern Japan. A putative triploid hybrid, Miscanthus ×ogiformis, was collected in Kumamoto prefecture in Kyushu (southern Japan) and taxonomically named by Honda [15]. Adati [16] found two triploid Miscanthus plants in Hyogo prefecture (western Japan), and Hirayoshi et al. [3] identified triploid plants grown from caryopses collected from M. sinensis in Gifu prefecture (central Japan).
Since the importance of Miscanthus as a biomass crop has grown and molecular techniques such as DNA fingerprinting have become available, the identification of further triploid interspecific hybrids has been reported again in East Asia: Nishiwaki and colleagues [2, 9] used cytological and molecular techniques to identify three interspecific triploid plants grown from caryopses collected from M. sacchariflorus in a sympatric population in Miyazaki prefecture, Japan. Moon et al. [4] identified a triploid Miscanthus plant with a nuclear DNA content similar to that of M. ×giganteus in Chungcheongnam-do, Korea. Ibaragi et al. [17] reexamined plants intermediate in morphology between M. sacchariflorus and M. sinensis that were previously classified as M. sinensis Andersson var. sunanensis Y. N. Lee in Korea, and concluded that this taxon should be treated as M. ×ogiformis (not M. ×giganteus, which is distinguished from M. ×ogiformis by the absence of awns in florets). In China, 11 accessions of interspecific hybrids between M. sacchariflorus and M. sinensis were identified using genome-wide genotyping techniques [18]. In addition to triploid hybrids (M. ×giganteus or M. ×ogiformis), natural diploid hybrids, previously classified as Miscanthus oligostachyus ‘Purpurascens’, were confirmed using cytological and molecular methods in China [19, 20]. Dwiyanti et al. [9] proposed the existence of a tetraploid hybrid between M. sacchariflorus and M. sinensis in Japan.
Hybridization and introgression in plants are thought to contribute to range expansion and adaptation in new environments by increasing genetic variability in hybridizing populations through new gene combinations, changes in polyploidy levels, or both [21, 22]. This hypothesis could be tested by phenotypic and genetic characterization of the natural hybrid population, in addition to the investigation of the habitats and growth environments [e.g., 23]. However, for Miscanthus hybrids, no natural spontaneous populations have been reported.
In this study, we found putative interspecific hybrids of triploid Miscanthus in the Tashiro–Fumoto area of southern Kyushu, Japan; this area lies at the southern limit of the range of M. sacchariflorus. This paper firstly aims to describe this putative hybrid population at the landscape scale using morphological, cytological, and molecular genetic methods. Secondly, we propose an explanation of the process of emergence and dispersal of this triploid Miscanthus hybrid population.
Materials and Methods
Investigation Site
We originally collected one Miscanthus plant in the Tashiro–Fumoto area (31.20°N, 130.85°E, 150–180 m a.s.l.), a small basin along the Fumoto River in Kinko-cho, Kimotsuki-gun, Kagoshima prefecture, Kyushu, Japan (Fig. 1a, b). The Fumoto River runs from east to west in the Tashiro–Fumoto area and is a tributary of Ogawa River, which further flows ca. 9 km into a sea estuary (Fig. 1b, c). The collected plant looked like M. sacchariflorus, but showed some intermediate morphology between M. sacchariflorus and M. sinensis. So we investigated the Miscanthus population in this area.
Geographical distribution of putative triploid hybrids between M. sacchariflorus and M. sinensis, in the Tashiro–Fumoto area, southern Kyushu, Japan. Symbols mark locations of plants sampled; ▲ (blue), type A; ● (red), type B1; ○, type B2; ■ (green), type C. Genotypes were characterized by using 24 simple sequence repeat markers. Dashed circle indicates samples used for seed-set rate and germination tests. Two representative plants nos. 707 and 730 used for chromosome counting are indicated by arrows
Plant Materials and Measurements
We sampled putative hybrid plants and surveyed the distribution of the population from 114 sites mainly along the Fumoto River over a range of 2.8 km on 31 October 2012 and 3–4 October 2013. Sampling was performed at at least one site in each canopy we found. Leaf samples from 114 sites were used for flow cytometry assay and DNA analysis. One or two panicles were sampled per site. Panicles sampled from 13 out of 114 sites in 2012 were used to evaluate seed fertility (seed-set and germination rates). Flowering panicles from 39 out of 114 sites sampled in 2013 were used to evaluate pollen viability. Panicles from 29 out of 114 sites sampled in 2013 were used to count awned florets. Awns were counted in 30 florets on each of three rachis branches from one panicle. Plant length (length of a straightened shoot from the ground level to the top) and panicle length (first branch on the main axis to the top) were measured for representative shoots at nine sites. Stems and rhizomes extracted from soil were observed for representative plants. Twenty-three Miscanthus sinensis plants from this area were also analyzed. Ten Miscanthus sacchariflorus accessions collected in southern Kyushu (‘Uchinoura,’ ‘Takayamagawa,’ ‘Kimotsukigawa,’ ‘JM0126,’ ‘Hirosegawa,’ ‘Gotandagawa,’ ‘Sendaigawa’ and ‘Kumagawa’) and other regions in Japan (‘Morioka’ and ‘NARCH-OGI-62’), and one M. ×giganteus clone ‘Illinois’, were also used for each analysis.
Estimation of Nuclear DNA Content and Counting of Chromosomes
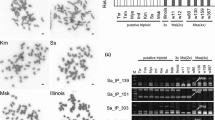
The relative nuclear DNA content was measured by flow cytometry assay. One tall fescue (Festuca arundinacea Schreb.) genotype was used as an internal standard. Nuclei prepared from chopped leaves were stained with 4′,6-diamidino-2-phenylindole (DAPI) fluorochrome by using a CyStain UV precise P reagent kit (Partec GmbH, Münster, Germany), and the filtered solution was analyzed with a Particle Analysing System (Partec). Each sample was assayed twice. Chromosome spreads were prepared by the enzymatic maceration/air-drying method [24, 25]. DAPI-stained chromosomes in root-tip squashes of two plants with two representative different genotypes revealed by DNA analysis (nos. 707 and 730, indicated in Fig. 1c) grown in pots in a glasshouse were counted under a fluorescence microscope (BX51; Olympus, Tokyo, Japan).
Genomic DNA Marker Assay and Clustering Analysis
To reveal the degree of genetic uniformity within the putative hybrid population, we examined the extent of genomic DNA polymorphisms among plants by using SSR markers. Genomic DNA was extracted from leaves by the cetyltrimethylammonium bromide (CTAB) method [26]. Table S1 lists the primer sequences of 7 intron-flanking markers used to distinguish M. sacchariflorus and M. sinensis [27] and 24 simple sequence repeat (SSR) markers used to evaluate genetic polymorphisms for phylogenetic analysis [28, 29]. Six random genotypes were used to select SSR primers showing polymorphisms in putative hybrids. In SSR marker analysis, in addition to putative hybrids, six M. sinensis, six M. sacchariflorus, and one M. ×giganteus plants were genotyped. The polymerase chain reaction (PCR) was performed as described [27]. Products amplified with intron-flanking primers were loaded into 2 % or 4 % agarose gels in TAE (Tris, acetic acid, EDTA) buffer, and those amplified with SSR primers were loaded into 6 % acrylamide gels in TBE (Tris, borate, EDTA) buffer for electrophoresis. Following staining with ethidium bromide, the banding patterns were photographed under ultraviolet light. Reproducibility of polymorphisms among genotypes of putative Miscanthus hybrids was confirmed using one representative sample from each type. Genetic distance matrixes based on SSR polymorphisms were calculated using Nei’s index [30]. Clustering was performed by the unweighted pair group method, using the arithmetic mean procedure in R software v. 3.0.2 [31].
Chloroplast DNA Analysis
Four chloroplast DNA regions previously sequenced in some Miscanthus genotypes were analyzed; namely, psbC-trnS, trnS-trnT, trnL-trnF, and rpl20-rps12 [9, 32]. DNA was extracted from 14 putative Miscanthus hybrids, six M. sinensis plants from Tashiro–Fumoto, and ten M. sacchariflorus accessions from other regions of Japan and was used as a PCR template. PCR reaction mixtures (10 μl) contained ca. 100 ng of genomic DNA, 0.2 units of AmpliTaq 360 polymerase (Applied Biosystems), 1× PCR buffer (Applied Biosystems), 1.5 mM MgCl2, 0.2 mM each dNTP, and forward and reverse primers (0.5 mM each) as described by Dwiyanti et al. [9] and Shimono et al. [32]. The cycling regime for the PCR amplification consisted of an initial denaturation step of 2 min at 95 °C; 32 cycles of 30 s at 95 °C, 30 s at 56 °C and 1 min 20 s at 72 °C; and a final extension step of 7 min at 72 °C. PCR products were cleaned up using Exo-SAP IT reagent (Affymetrix, Santa Clara, CA, USA) and directly sequenced using an ABI Prism 3130 Genetic Analyzer (Applied Biosystems). All sequences were deposited in the DDBJ database (accession nos. LC060067 to LC060146). Multiple alignment was performed using DNASIS v. 3.0 software (Hitachi Software Engineering, Tokyo, Japan).
Evaluation of Pollen Viability and Seed Fertility
Pollen viability of 39 samples of putative hybrids was investigated. Two lots of pollen grains from five to ten florets each per individual were stained with 2 % acetocarmine solution [33]. Fully stained and unstained grains were counted under a microscope.
Seed-set and germination rates of 13 putative hybrids and 3 M. sinensis plants collected in the western part of the Tashiro–Fumoto area were evaluated (Fig. 1c). To estimate the seed-set rate, we used an X-ray inspection apparatus (C-60 TV-PbO-1; Softex, Ebina, Japan) to count filled and empty seeds in three lots of about 100 seeds each per individual. Before the germination test, seeds were maintained at 4 °C over a period of 4 months. Seeds (500–5,000; including empty ones) were placed on wet filter paper and incubated for 4 weeks at 25/15 °C under a daylength of 14 h. The germination ratio (%) was estimated as:
Statistical Analysis
Statistical analyses (ANOVA, Tukey’s and Wilcoxon’s tests) were performed in JMP software v. 9 (SAS Institute, Cary, NC, USA).
Results
Identification of a Putative Hybrid Miscanthus Population in the Tashiro–Fumoto Area of Japan
Putative hybrid Miscanthus plants were distributed mainly alongside the Fumoto River over a range of 2.8 km (Fig. 1c). As well as on river banks, some plants grew along the edges and footpaths of fields and on roadsides near the river. Some plants were found around tributaries, up to 1.4 km upstream of their confluence with the river (Fig. 1c). Miscanthus sinensis also grew naturally in this area. We could not find any putative hybrid plants in the Ogawa River basin.
The putative hybrids had elongated rhizomes, and some plants formed continuous stands up to 50 m long alongside the river or fields (Fig. 2a, b). Stems grew vertically (Fig. 2b), with buds at the nodes like M. sacchariflorus (Fig. 2c, d). The length of the plants on riverbanks ranged from 2.5 to 3.0 m at flowering stage, with panicles measuring 40 to 55 cm. Spikelets had long white callus hairs and either short or no awns on the lemmata (Fig. 2e, f).
Morphology of putative triploid hybrids between M. sacchariflorus and M. sinensis found in the Tashiro–Fumoto area. a Miscanthus plants growing along Fumoto River (hyb, putative triploid hybrid; Msi, M. sinensis). b Putative hybrid plants growing at the edge of a field. c Rhizomes (rh). d Buds at nodes. e Awnless spikelet of a putative hybrid. f Awned spikelet of a putative hybrid. g Spikelet of M. sinensis. h Spikelet of M. ×giganteus ‘Illinois’
The mean fluorescence level of DAPI-stained nuclear DNA of the putative hybrids from 114 sites was 0.396 ± 0.008 relative to that of F. arundinacea. It was 1.30 ± 0.03 times the mean fluorescence level of 20 genotypes of M. sinensis (0.305 ± 0.011) growing in this area, almost the same as that of M. ×giganteus ‘Illinois’ (1.32 times; Table 1). The fluorescence level of DAPI-stained nuclear DNA of a tetraploid M. sacchariflorus strain ‘Uchinoura’ was 1.57 times that of M. sinensis. The chromosome number of two putative hybrids sampled was 2n = 57 (Fig. 3a,b), three times the basic number in Miscanthus (x = 19). Lastly, primer pairs, which generate specific fragments to M. sacchariflorus or M. sinensis, amplified five fragments specific to M. sacchariflorus and three specific to M. sinensis in all 114 samples (Fig. 4).
Genotyping by the intron-flanking markers showing fragments specific to Miscanthus sacchariflorus (Msa) or Miscanthus sinensis (Msi) [28]. Asterisks indicate fragments detected in all M. sinensis genotypes and in some M. sacchariflorus genotypes in the study of Tamura et al. [28]. M, 100-bp ladder marker; 1–10, randomly selected putative Miscanthus plants; 11, M. ×giganteus ‘Illinois’; 12, M. sacchariflorus ‘Uchinoura’; 13, M. sacchariflorus ‘Miyakonojyo’; 14–16, M. sinensis genotypes collected in the Tashiro–Fumoto area
All these results indicate that all tested plants are triploid hybrids between M. sacchariflorus and M. sinensis.
Genetic characterization of a putative hybrid Miscanthus population in the Tashiro–Fumoto area
Genotyping of the 114 samples using 24 polymorphic SSR markers revealed 94 polymorphic fragments among all 135 fragments. Only four genotypes were evident: A (n = 63), B1 (n = 47), B2 (n = 2) and C (n = 2). Types B1 and B2 were distinguished by only one fragment specific to each type.
Clustering analysis of the putative hybrids and other Miscanthus accessions revealed three major clusters: M. sinensis, M. sacchariflorus and putative hybrid Miscanthus from Tashiro–Fumoto (Fig. 5). Miscanthus ×giganteus was clustered with M. sacchariflorus (Fig. 5). The putative hybrid cluster was genetically closer to M. sacchariflorus than to M. sinensis. Genetic distances among A, C and B1 + B2 were similar to those among M. sinensis from Tashiro–Fumoto and among M. sacchariflorus from southern Kyushu (Fig. 5).
Regarding the geographical distribution of the four types of genotypes, type A plants were distributed mainly in the center of the range along a 0.8-km stretch of the Fumoto River (Fig. 1c). A cluster of type B plants was distributed mainly in the eastern part of the range along a 1-km stretch of the river. The two type B2 plants grew 60 m apart. The two plants of type C grew 12 m apart within a continuous band of vegetation at the edge of the type A area. Types A and B1 plants also grew sympatrically in the western part of the range along the river banks or the edges of fields and roads.
Six randomly selected plants of type A and six of type B1 had the same haplotype of four chloroplast DNA regions within each type. There were no differences between types A and C, or between types B1 and B2 (Table 2). There was only one difference (insertion–deletion in the trnL-trnF region) between the A + C and B groups (Table 2). All mutations identified in the putative hybrids were also present in M. sacchariflorus plants collected in Japan but not in M. sinensis plants in Tashiro–Fumoto area (Table 2). Mutations at the six loci detected in putative hybrids (Table 2) were not reported by Shimono et al. [32], who investigated chloroplast DNA variations in 636 M. sinensis individuals in Japan. The haplotype of types A + C was the same as that of M. sacchariflorus ‘Morioka’, whereas the haplotype of type B was same as those of M. sacchariflorus ‘JM0126,’ ‘Hirosegawa,’ and ‘Kumagawa’ (Table 2). Namely, two chloroplast haplotypes found in the putative hybrids were common to some M. sacchariflorus genotypes but not found in any M. sinensis genotypes.
Phenotypic Characterization of a Putative Hybrid Miscanthus Population in the Tashiro–Fumoto Area
Most florets of type B plants (B1 & B2) had awns (≥95 %) (Table 3). In contrast, only 25 % of florets of type C plants had awns (Table 3). Despite a wide variance, type A were intermediate with 36.3 % of florets with awns (Table 3).
Acetocarmine staining showed that type A plants had relatively high pollen viability, although there was variation among samples (Fig. 6a). In contrast, most pollen grains of type B1 plants were not viable (Fig. 6b). The difference between types A (73.2 %, Fig. 6c) and B1 (7.1 %, Fig. 6d) was significant (P < 0.001, Wilcoxon’s test).
To examine the possibility of expansion of the putative hybrid Miscanthus population by seed propagation, we investigated the seed fertility of putative hybrids and M. sinensis plants growing sympatrically. All panicles collected from putative hybrids contained filled seeds. The seed-set rate of type A plants was significantly lower than those of type B1 and M. sinensis (Table 4). Seedlings were raised from five of the nine samples of type A and all four samples of type B. The germination rate of the putative hybrid seeds (0.4 to 2.1 %) was marginally lower than that of M. sinensis seeds (Table 4).
The nuclear DNA content of 26 progeny obtained from eight parents of types A and B1 varied widely, ranging from 0.29 to 0.51 relative to F. arundinacea, which corresponded to 0.94 to 1.68 relative to diploid M. sinensis (Fig. S2). The mean nuclear DNA contents were not significantly different between progeny of types A (n = 14) and B1 (n = 12).
Discussion
The Hybrids Identified in the Tashiro–Fumoto Area are Triploid With a Tetraploid M. Sacchariflorus as Maternal Parent
Since the 1930s, several studies have reported naturally growing hybrids between sympatric M. sacchariflorus and M. sinensis in East Asia [4, 9, 15, 16]. However, these studies focused on individual plants, not populations. In this study, we found a large population of plants morphologically intermediate between M sacchariflorus and M. sinensis. The results of our cytological and genetic analyses indicate that the intermediate type of Miscanthus in the Tashiro–Fumoto area is a triploid hybrid between M. sacchariflorus and M. sinensis.
The genomic DNA contents of the parents are 4.5 pg per nucleus in M. sacchariflorus and 5.5 pg in M. sinensis [8]. On this basis, the DNA content of a triploid hybrid containing two M. sacchariflorus and one M. sinensis genomes is 1.32× that of diploid M. sinensis, and that of a triploid hybrid containing one M. sacchariflorus and two M. sinensis genomes is 1.41×. The mean DNA content of putative hybrids from Tashiro–Fumoto, 1.30, suggests that these plants are hybrids between tetraploid M. sacchariflorus and diploid M. sinensis, similar to M. ×giganteus [8, 9]. Similar to other triploid Miscanthus hybrids (M. ×giganteus) [18, 34], putative hybrids from Tashiro–Fumoto had M. sacchariflorus-specific haplotypes in the chloroplast genome. This suggests that maternal parents of the hybrids from Tashiro–Fumoto were tetraploid M. sacchariflorus.
Process of Emergence and Dispersal of This Triploid Miscanthus Hybrid Population
Why and how has this large (kilometer scale) hybrid population formed? In the Tashiro–Fumoto area, we found M. sinensis but no M. sacchariflorus. The native distribution of M. sacchariflorus is limited to the northern range of M. sinensis, which extends much further south to the tropics [1, 35]. The Global Biodiversity Information Facility (www.gbif.org) records the most southerly occurrence of M. sacchariflorus as 29.57°N in China, further south than the Tashiro–Fumoto area (31.2°N). However, as far as we investigated, the southern limit of M. sacchariflorus in Japan is ca. 9 km north of the Tashiro–Fumoto area (31.3°N, data not shown). The Tashiro–Fumoto area lies about 150–180 m above sea level, and the temperature is a little cooler than in the surrounding lowlands. Therefore, we suppose that the parental M. sacchariflorus had grown in this area in the not-too-distant past before dying out owing to environmental changes such as warming, while the triploid hybrids survived owing to better adaptability introduced from M. sinensis. Clark et al. [36] reported that the genome of tetraploid M. sacchariflorus in Japan has introgressions from M. sinensis (on average 7 % of a whole genome), which are more frequent in southern Japan than in the northern region. They suggested that the ancestral M. sinensis introgression into M. sacchariflorus contributed to the adaptation to a warmer environment [36]. The existence of a large hybrid Miscanthus population at the southern limit of the range of M. sacchariflorus fits this hypothesis as an extreme example of the M. sinensis genome contributing to adaptation.
Following its establishment, how did the hybrid population expand to the landscape scale? On the one hand, we found that the genetic distances among types A, C and B were comparable to those among M. sinensis from Tashiro–Fumoto and among M. sacchariflorus in southern Kyushu. Mutations in chloroplast genomes were also found among the types of putative hybrids. These results demonstrate that the hybrids in the Tashiro–Fumoto area have a polyphyletic origin; namely, they originated from progeny of multiple pairs of M. sacchariflorus and M. sinensis. On the other hand, plants with the same genotype typically formed clusters. The simple genomic and geographic structures (Fig. 1c) imply that the population propagated clonally from a few (probably F1) genotypes. In M. sacchariflorus, vegetative rhizomes are the main reproductive source for population recruitment [37–39]. Rhizome systems can be fragmented and dispersed by natural or human disturbance such as flooding or roadside maintenance [37, 39] and are recognized as a potential invasive risk in M. ×giganteus, especially in riparian ecosystems [40, 41]. According to a chronicle of the history of the area that includes Tashiro–Fumoto, the Fumoto River used to meander and often flooded following heavy rains, but 15–30 years ago it was channelized. The hybrid population area corresponds to the site of these works. Therefore, rhizomes of a few hybrid genotypes (mainly A and B1) might have been fragmented and spread in soil disturbed by floods, river improvement works, or both. Moreover, such habitat disturbances might have not only dispersed the propagules, but also led to the new conditions to which plants may become adapted following hybridization, as observed in other species [e.g., 23]. It is unlikely that the hybrid plants expanded through seed propagation, because they are triploid, and only four types were found by SSR marker analysis. Expansion through seed dispersal would result in a more heterogeneous genetic background and ploidy level, as confirmed in the plants raised from seeds of hybrid plants in this study.
Suggestions Regarding the Classification of Miscanthus Hybrids
The triploid hybrid M. ×giganteus has no awns, but the putative triploid hybrid M. ×ogiformis [11, 15, 16] has awns [15, 17]. On this basis, the Tashiro–Fumoto triploid hybrids should be classified as M. ×ogiformis rather than M. ×giganteus, although Linde-Laursen [11] insisted that M. ×giganteus should be taxonomically reclassified as M. ×ogiformis. However, the presence or absence of awns in the hybrids seems to be genetically variable, as seen in types A and B of the putative hybrids, and to be influenced by environment, as seen in the large variance among type A plants. Therefore, we think that the presence or absence of awns is not a reliable trait for classification.
Conclusion and Prospects
A landscape-scale population of triploid Miscanthus hybrids was discovered in the southern limit area of the range of M. sacchariflorus. We have proposed the hypothesis that under the environment where the growth of M. sacchariflorus was limited, hybridization between M. sacchariflorus and M. sinensis had an advantage due to its improved adaptability. Furthermore, genetic and phenotypic characterization suggest the polyphyletic origin and clonal propagation of this population. Further detailed studies, including genomic comparison with Miscanthus plants growing around this area and evaluation of the environmental adaptability of the hybrids, could test this hypothesis.
Natural Miscanthus hybrids adapted to different environments could be used as materials for breeding biomass crops. In sympatric areas where flowering times of M. sacchariflorus and M. sinensis overlap, such as in central to southern Japan, Miscanthus hybrid populations could occur; in fact, we have confirmed some other hybrid populations in Kyushu (unpublished data). As triploid hybrids can resemble M. sacchariflorus in natural habitats, previously collected M. sacchariflorus germplasms may include natural hybrids or plants with introgression from M. sinensis, which requires further inspections in the future.
Such natural hybrids, i.e., hybrids we found in a natural environment and that occurred spontaneously, set some seeds, some of which germinated. Although further testing is required to evaluate fertility accurately, these results show that the putative hybrids have partial paternal or maternal fertility. Type A plants in particular could be used as a pollen source to genetically improve hybrid Miscanthus as a biomass crop.
References
Clifton-Brown J, Chang YC, Hodkinson TR (2008) Miscanthus: Genetic resources and breeding potential to enhance bioenergy production. In: Vermerris W (ed) Genetic improvement of bioenergy crops. Springer, New York, pp 273–294
Nishiwaki A, Mizuguti A, Kuwabara S, Toma Y, Ishigaki G, Miyashita T, Yamada T, Matuura H, Yamaguchi S, Rayburn AL, Akashi R, Stewart JR (2011) Discovery of natural Miscanthus (Poaceae) triploid plants in sympatric populations of Miscanthus sacchariflorus and Miscanthus sinensis in southern Japan. Am J Bot 98:154–159
Hirayoshi I, Nishikawa K, Kubono M, Murase T (1957) Cytogenetical studies on forage plants (VI). On the chromosome number of ogi (Miscanthus sacchariflorus). Res Bull Fac Agric Gifu Univ 8:8–13, [In Japanese with English summary]
Moon YH, Cha YL, Choi YH, Yoon YM, Koo BC, Ahn JW, An GH, Kim JK, Park KG (2013) Diversity in ploidy levels and nuclear DNA amounts in Korean Miscanthus species. Euphytica 193:317–326
Feng XP, Lourgant K, Castric V, Saumitou-Laprade P, Zheng BS, Jiang D, Brancourt-Hulmel M (2014) The discovery of natural Miscanthus accessions related to Miscanthus × giganteus using chloroplast DNA. Crop Sci 54:1645–1655
Lewandowski I, Clifton-Brown JC, Scurlock JMO, Huisman W (2000) Miscanthus: european experience with a novel energy crop. Biomass Bioenerg 19:209–227
Heaton EA, Dohleman FG, Miguez AF, Juvik JA, Lozovaya V, Widholm J, Zabotina OA, McIsaac GF, David MB, Voigt TB, Boersma NN, Long SP (2010) Miscanthus: a promising biomass crop. In: Kader JC, Delseny M (eds) Advances in botanical research, Vol 56. Elsevier, Amsterdam, pp 75–137
Rayburn AL, Crawford J, Rayburn CM, Juvik JA (2009) Genome size of three Miscanthus species. Plant Mol Biol Rep 27:184–188
Dwiyanti MS, Rudolph A, Swaminathan K, Nishiwaki A, Shimono Y, Kuwabara S, Matuura H, Nadir M, Moose S, Stewart JR, Yamada T (2013) Genetic analysis of putative triploid Miscanthus hybrids and tetraploid M. sacchariflorus collected from sympatric populations of Kushima, Japan. Bioenerg Res 6:486–493
Adati S, Mitsuishi S (1955) Wild growing plants of the Far east, especially Japan, suitable for breeding purposes. Part I. Karyological study in miscanthus (1). Bull Fac Agric Mie Univ 12:1–10
Linde-Laursen I (1993) Cytogenetic analysis of Miscanthus giganteus, an interspecific hybrid. Hereditas 119:297–300
Nielsen PN (1990) Elefantengrassanbau in Dänemark — Praktikerbericht. Pflug und Spaten 3:1–4 [In German]
Greef JM, Deuter M (1993) Syntaxonomy of Miscanthus × giganteus Greef-Et-Deu. Angew Bot 67:87–90
Głowacka K, Clark LV, Adhikari S, Peng J, Stewart JR, Nishiwaki A, Yamada T, Jørgensen U, Hodkinson TR, Gifford J, Juvik JA Sacks EJ (2015) Genetic variation in Miscanthus ×giganteus and the importance of estimating genetic distance thresholds for differentiating clones. Glob Change Biol Bioenergy 7:386–404
Honda M (1939) New report of plants in Japan XXXVIII. Bot Mag 53:144 [In Japanese]
Adati S (1958) Studies on the Miscanthus genus with special reference to the Japanese species suitable for breeding purposes as fodder crops. Bull Fac Agric Mie Univ 17:1–112 [In Japanese with English summary]
Ibaragi Y, Lim SH, Yook MJ, Chang CS, Kim DS (2013) Taxonomic notes on Korean miscanthus × ogiformis Honda (Poaceae)––a new record from Korea. J Jpn Bot 88:184–187
Clark LV, Brummer JE, Głowacka K, Hall MC, Heo K, Peng J, Yamada T, Yoo JH, Yu CY, Zhao H, Long SP, Sacks EJ (2014) A footprint of past climate change on the diversity and population structure of Miscanthus sinensis. Ann Bot 114:97–107
Chae WB, Hong SJ, Gifford JM, Lane Rayburn A, Sacks EJ, Juvik JA (2014) Plant morphology, genome size, and SSR markers differentiate five distinct taxonomic groups among accessions in the genus Miscanthus. Glob Change Biol Bioenergy 6:646–660
Jiang JX, Zhu MD, Ai X, Xiao L, Deng GT, Yi ZL (2013) Molecular evidence for a natural diploid hybrid between Miscanthus sinensis (Poaceae) and M. sacchariflorus. Plant Syst Evol 299:1367–1377
Anderson E, Stebbins GL (1954) Hybridization as an evolutionary stimulus. Evolution 8:378–388
Arnold ML, Cornman RS, Martin NH (2008) Hybridization, hybrid fitness and the evolution of adaptations. Plant Biosyst 142:166–171
Tsukaya H, Fukuda T, Yokoyama J (2003) Hybridization and introgression between Callicarpa japonica and C. mollis (Verbenaceae) in central Japan, as inferred from nuclear and chloroplast DNA sequences. Mol Ecol 12:3003–3011
Fukui K (1996) Plant chromosomes at mitosis. In: Fukui K, Nakayama S (eds) Plant chromosomes: laboratory methods. CRC Press, Boca Raton, FL, pp 1–17
Akiyama Y, Conner JA, Goel S, Morishige DT, Mullet JE, Hanna WW, Ozias-Akins P (2004) High-resolution physical mapping in Pennisetum squamulatum reveals extensive chromosomal heteromorphism of the genomic region associated with apomixis. Plant Physiol 134:1733–1741
Murray MG, Thompson WF (1980) Rapid isolation of high molecular-weight plant DNA. Nucleic Acids Res 8:4321–4325
Tamura K, Sanada Y, Shoji A, Okumura K, Uwatoko N, Anzoua KG, Sacks EJ, Yamada T (2015) DNA markers for identifying interspecific hybrids between Miscanthus sacchariflorus and Miscanthus sinensis. Grassl Sci 61:160–166
Zhou HF, Li SS, Ge S (2011) Development of microsatellite markers for Miscanthus sinensis (Poaceae) and cross-amplification in other related species. Am J Bot 98:E195–E197
Kim C, Zhang D, Auckland SA, Rainville LK, Jakob K, Kronmiller B, Sacks EJ, Deuter M, Paterson AH (2012) SSR-based genetic maps of Miscanthus sinensis and M. sacchariflorus, and their comparison to sorghum. Theor Appl Genet 124:1325–1338
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A 76:5269–5273
R Core Team (2013) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, available from URL: http://www.R-project.org/ [cited 1 May 2014]
Shimono Y, Kurokawa S, Nishida T, Ikeda H, Futagami N (2013) Phylogeography based on intraspecific sequence variation in chloroplast DNA of Miscanthus sinensis (Poaceae), a native pioneer grass in Japan. Botany 91:449–456
Singh RJ (2003) Plant cytogenetics, 2nd edn. CRC Press, Boca Raton, FL
Hodkinson TR, Chase MW, Takahashi C, Leitch IJ, Bennett MD, Renvoize SA (2002) The use of DNA sequencing (ITS and trnL-F), AFLP, and fluorescent in situ hybridization to study allopolyploid Miscanthus (Poaceae). Am J Bot 89:279–286
Hager HA, Sinasac SE, Gedalof Z, Newman JA (2014) Predicting potential global distributions of two Miscanthus grasses: implications for horticulture, biofuel production, and biological invasions. PLoS One 9:e100032
Clark LV, Stewart JR, Nishiwaki A, Toma Y, Kjeldsen JB, Jorgensen U, Zhao H, Peng J, Yoo JH, Heo K, Yu CY, Yamada T, Sacks EJ (2015) Genetic structure of Miscanthus sinensis and Miscanthus sacchariflorus in Japan indicates a gradient of bidirectional but asymmetric introgression. J Exp Bot 66:4213–4225
Deng ZM, Chen XS, Xie YH, Li X, Pan Y, Li F (2013) Effects of size and vertical distribution of buds on sprouting and plant growth of the clonal emergent macrophyte Miscanthus sacchariflorus (Poaceae). Aquat Bot 104:121–126
Chen XS, Cao CS, Deng ZM, Xie YH, Li F, Hou ZY, Li X (2015) Assessment of regeneration potential in the clonal macrophyte Miscanthus sacchariflorus (Poaceae) after burial disturbance based on bud bank size and sprouting capacity. PLoS One 10: e0120846
Hager HA, Rupert R, Quinn LD, Newman JA (2015) Escaped Miscanthus sacchariflorus reduces the richness and diversity of vegetation and the soil seed bank. Biol Invasions 17:1833–1847
Mann JJ, Kyser GB, Barney JN, DiTomaso JM (2013) Assessment of aboveground and belowground vegetative fragments as propagules in the bioenergy crops Arundo donax and Miscanthus × giganteus. Bioenergy Res 6:688–698
Matlaga DP, Davis AS (2013) Minimizing invasive potential of Miscanthus × giganteus grown for bioenergy: identifying demographic thresholds for population growth and spread. J Appl Ecol 50:479–487
Acknowledgments
We are grateful to Ms. Satomi Shimada, NARO Hokkaido Agricultural Research Center, for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no potential conflict of interest.
Rights and permissions
About this article
Cite this article
Tamura, Ki., Uwatoko, N., Yamashita, H. et al. Discovery of Natural Interspecific Hybrids Between Miscanthus Sacchariflorus and Miscanthus Sinensis in Southern Japan: Morphological Characterization, Genetic Structure, and Origin. Bioenerg. Res. 9, 315–325 (2016). https://doi.org/10.1007/s12155-015-9683-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-015-9683-1