Abstract
Enzymes constitute a major monetary cost in the bioconversion of holocellulose to ethanol. Identifying enzyme inhibitors and moderating their effects is one approach that may help to overcome this issue. Most inhibitors that reduce the hydrolysis activity of holocellulases are released as the holocellulosic biomass is broken down in the pretreatment and hydrolysis steps. Recent reports in the literature have shown that the major inhibitors or deactivators of cellulases are phenols and xylooligosaccharides. The bioconversion of hemicelluloses by hemicellulases also has important practical applications in various agro-industrial processes in addition to the conversion of hemicellulosic biomass to fuels and chemicals. Hemicellulases, such as β-xylosidases, may also help alleviate the inhibitory effect of xylooligosaccharides to cellulases. However, compared to cellulases, less is known about the inhibition or deactivation of hemicellulases and pectinases, especially for inhibitors that are generated during pre-treatment and the hydrolysis of lignocellulosic substrates. Considering the importance of such enzymes for the complete degradation of lignocellulosic substrates, this review provides a broad view of the effect of inhibitors of holocellulases (cellulases, hemicellulases, and pectinases).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vast amounts of lignocellulosic materials are available for exploitation as sources of food, fuel, and chemical feedstock. The complexity of lignocellulose structures is reflected in the battery of enzyme systems required for their breakdown. Knowledge of these enzyme’s modes of action is crucial to overcoming the recalcitrance of lignocellulose structures to digestion and metabolism. After the removal of lignin from lignocelluloses, the total carbohydrate content obtained is the holocellulose, and it is composed of hemicelluloses, cellulose, and pectin [2].
The structure of holocellulose is dependent upon the species, tissue, and growth conditions of the plant. The hydrolysis of the holocellulosic components of the plant cell-wall structure involves the concerted action of endohydrolases and exohydrolases, which release a wide range of products, including short and long oligosaccharides and monosaccharide units. The term holocellulases refers to those enzymes that are specific to the breakdown of the holocellulosic structure and includes cellulases, hemicellulases, and pectinases. As holocelluloses comprise a variety of structures, the complete hydrolysis requires the synergistic actions of main-chain and side-chain cleaving enzymes [2].
The role of each enzyme in the breakdown of the holocellulosic structure is defined by their mechanisms of action, i.e., the hydrolysis of the terminal glycosidic linkage bonds or internal specific linkage-bond positions. Due to the heterogeneity of holocellulose, the presence of holocellulases with different specificities that release different hydrolysis products is essential. Moreover, considerable information on the enzymatic mechanisms of action can be obtained by characterizing the products of hydrolysis and their kinetic parameters with and without inhibitors. The role of inhibitors of holocellulases (cellulases, hemicellulases, and pectinases), especially those derived from the biomass, such as phenols and sugars, will be discussed in this review. We hope to stimulate continued efforts to understand the molecular mechanisms of enzymatic holocellulose breakdown.
An Overview of Holocellulose Hydrolysis
The hydrolysis of holocellulose is complex because of the heterogeneous nature of the substrate. Endo- and exoglucanases, β-glucosidases, β-xylanases, β-xylosidases, β-mannanases, and polygalacturonases are important enzymes that catalyze the release of short- and long-chain oligosaccharides. In addition, enzymes such as acetyl and ferulic acid esterases and arabinofuranosidases remove side chain groups from holocellulose, especially when the fragments of cleaved holocellulose have a high proportion of branched substituents [16, 45, 51]. The hydrolysis of holocellulose is carried out on the surface of the solid substrate (primary hydrolysis) and in the liquid phase (secondary hydrolysis). The primary hydrolysis is the rate-limiting step for the whole holocellulose hydrolysis process.
Specifically for cellulases, the primary hydrolysis is carried out by endo- and exoglucanases, while the secondary hydrolysis involves the hydrolysis of cellobiose to glucose by β-glucosidases [67, 72]. The synergistic action of holocellulases changes the holocellulose topography over time. The holocellulose structure affords the synergistic actions of a variety of main- and side-chain cleaving enzymes [16]. Homeosynergy is defined as cooperativity between two main-chain cleaving enzymes (for example, β-xylanase and β-xylosidase) or two side-chain cleaving enzymes (for example, α-arabinofuranosidase and acetylxylan esterase). Heterosynergy is the synergistic interaction between side-chain and a main-chain cleaving enzymes (for example, α-galactosidase and β-mannanase). The lack of β-mannosidase activity in the enzyme preparation significantly influenced glucose release from pretreated Douglas fir [21]. Qing and Wyman [50] reported that the supplementation of cellulase preparations with hemicellulases (β-xylanase and β-xylosidase) improved the enzymatic hydrolysis of cellulose and hemicellulose in solids after ammonia fiber expansion and dilute acid pretreatment of corn stover. Thus, the enzymatic removal of xylan from lignocellulosic residue seems to be an efficient method of enhancing cellulase effectiveness.
The substituents in the holocellulose, such as the side branches, act as spatial obstacles that prevent the formation of enzyme–substrate complexes during catalysis, thus limiting the action of the enzymes [2]. For instance, some holocellulases are not able to cleave oligomers containing branch points, indicating that the holocellulosic linkages are protected by the substituents. By contrast, other holocellulases cleave the backbone linkages only at or near branch points. Certain holocellulases have their highest activities against long-chain oligosaccharides or polysaccharides, while other types of holocellulases hydrolyze branch points in addition to cleaving main chain linkages [16]. Another group of holocellulases (for instance, β-glucosidases and β-xylosidases) are believed to relieve end product inhibition of other holocellulase enzymes. Substrate cross-specificity or relaxed specificity is a characteristic of many holocellulases, such as cellulases and hemicellulases [54]. Substrate specificity can range from absolute, such as for only one polysaccharide, to an equal affinity for multiple polysaccharides. In addition, holocellulases can be active against a variety of oligosaccharides sizes and affect the formation of oligosaccharides by transferase activity. Different oligomers can be formed by holocellulosic components, such as xylan and cellulose, interacting with different binding subsites of a particular enzyme, which are formed due to structural differences between these substrates. The catalytic cores of various holocellulases behave as independent entities, showing catalytic activity and defined specificities toward soluble model substrates. To organize the different enzymes, they are ordered into families by substrate specificities and modes of action (http://www.cazy.org/Glycoside-Hydrolases.html).
Inhibition of Holocellulases by Biomass-Derived Compounds
The primary cell wall structure is composed of approximately 10% protein and 90% holocellulose [40]. Lignin, a three-dimensional network of p-hydroxyphenylpropane units, has a key role in restricting the breakdown of holocellulose structure by hydrolases [54]. The matrix of holocellulose presents a great structural diversity in the plant cell walls with the cellulose/hemicellulose network embedded in a jelly-like matrix of pectin [38]. Each molecule may contain different sugar residues with the same or different bonds, which are branched to varying degrees and conformations. Within this context of macromolecule interactions, phenylpropanoid compounds, such as lignins, suberins, and phenolic acids, are important structural components of the plant cell wall [10].
In the plant cell wall environment, lignin is physically entangled with or chemically linked to holocellulose components [54, 58]. In this context, several factors are responsible for biomass resistance to enzymatic hydrolysis, including lignocelluloses particle size, cellulose crystallization, an accessible surface area, protection by lignin, cellulose sheathing by hemicelluloses, mass transfer limitation affecting the transport to and from insoluble substrates, and the presence of phenolic compounds, fat, and proteins [37, 46].
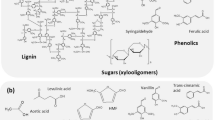
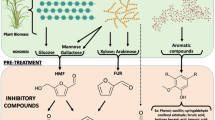
The pretreatment of lignocellulosic biomass is crucial before the enzymatic hydrolysis of the holocellulose structure of the plant cell wall. Chemical, physical, and morphological lignocellulose features are changed during pre-treatment and generally make the biomass more accessible to enzymatic saccharification [30, 33, 34, 42]. Various pretreatment options are available to fractionate, solubilize, hydrolyze, and separate cellulose, hemicellulose, and lignin components [52]. Figure 1 shows the lignocellulosic biomass treatment for enzyme saccharification and the generation of enzyme inhibitors. Lignocellulosic pretreatments, such as liquid hot water, steam explosion, and dilute acid, lead to the formation of soluble inhibitors that hamper the enzymatic hydrolysis of holocellulose [28, 29, 33, 70, 71]. These inhibitory compounds will vary according to the pretreatment and can include soluble sugars, furan derivatives, organic acids, and phenolic compounds. The amounts of these soluble inhibitors and their distribution depend on the type and severity of pretreatment, the concentration of lignocellulosic solids during pretreatment hydrolysis, and the type of biomass [33]. In the following sections, we will discuss the inhibitory effects of the biomass derived compounds on holocellulases, with a special emphasis on phenolic compounds and sugars.
Phenolic Compounds
As was already noted by Mandels and Reese [39], compounds with the ability to inhibit enzyme activity occur in a wide variety of plants. These authors also considered these inhibitors to be universal, varying only in amounts and degree, and they found that the naturally occurring enzyme inhibitors belonged chiefly to the phenolic, tannin, or leukoanthocyanin chemical families.
Phenols in higher plants range from simple low molecular weight phenolic glycosides to polymeric compounds [68, 69]. The molecular formula of major enzyme inhibitors are C10H10O4 (ferulic acid), C11H12O5 (sinapic acid), C9H10O5 (syringic acid), C76H52O46 (tannic acid), and C8H8O4 (vanillic acid). As a first line of defense, these phenylpropanoid compounds act as a barrier to many different microorganisms to which plants are exposed. During enzyme hydrolysis of pretreated lignocellulosic materials, modifications of lignin structures occur, leading to the generation of exposed phenolic moieties and the formation of simple oligomeric phenolics [22, 23, 55, 58]. As a consequence of this formation, lignin-derivable phenolic compounds have an inhibitory effect on the enzymatic conversion of the holocellulosic biomass to useful chemicals. Thus, a crucial point for the enzymatic hydrolysis of holocellulose is to reduce the inhibition and deactivation effects on enzymes to enhance hydrolysis and reduce enzyme loading. Table 1 shows examples of phenolic compound’s effects on cellulase and hemicellulase activities.
The physicochemical processes of lignocellulosic biomass pretreatment modify the lignin structure and promote phenol solubilization. Many simple phenolic compounds have been identified in the solution phase of the pretreated biomass, and they often inhibit fermentative fungi or bacteria that convert glucose, xylose, or other carbohydrates into ethanol. In addition to simple monomeric phenolics, oligomeric phenolics, such as tannin, may inhibit or deactivate lignocellulose-hydrolyzing enzymes [69]. The effective concentration range for tannic acid’s inhibition was studied on Avicel, filter paper, cellobiose, and p-nitrophenyl-β-d-glucopyranoside hydrolysis with a cellulase mix. Tannic acid became inhibitory at concentrations higher than 0.05 mM [58, 69].
The experiments developed by Jing et al. [29] showed that the action of the lignocellulose degradation products from corn stover on industrial cellulase (Spezyme CP) during cellulose hydrolysis strongly inhibited enzyme activity, and the order of the inhibitory strength by the lignocellulose degradation products on cellulase was lignin derivatives > furan derivatives > organic acids > ethanol. The inhibition ability of phenolic compounds on cellulases was also tested in the presence of birdsfoot trefoil polyphenols (condensed tannins) and sunflower polyphenols (chlorogenic, ferulic, caffeic, and sinapic acids) [4, 41, 53]. The degree of inhibition of endoglucanases from Neocallimastix frontalis RE1, Neocallimastix patriciarum 27, Piromyces communis 22, and Orpinomyces joyonii 19-2 was nearly complete at 300 μg condensed tannins/mL. With respect to sunflower polyphenols, the inhibitory effects were not cumulative because in the presence of phenolic compounds (chlorogenic, ferulic, caffeic, and sinapic acids), the avicelase retained 75–85% of its activity. The deactivation effects of polymeric and monomeric phenols were also determined on the cellulase activities from Trichoderma reesei and Aspergillus niger [69] fungal species. Within the groups of phenolic compounds tested, tannic acid showed the most damaging effect, causing deactivation and reversible loss on all of the enzymes activities tested. On the other hand, β-glucosidase from A. niger was the enzyme that was most resistant to inhibition and deactivation.
The combination of solka-floc, which is a lignin-free cellulose, with phenolic compounds released during liquid maple-pretreatment decreased the rate and extent of cellulose hydrolysis due to both deactivation of the cellulase activity and protein precipitation [33]. In another article, it was observed that oligomeric phenolics were more inhibitory on cellulase than simple phenolics and could inactivate cellulases by reversibly forming complexes with them, while simple and oligomeric phenolics could inhibit cellulase by adsorbing onto cellulose [58]. According to this article, polyethylene glycol is believed to prevent tannin adsorption onto cellulose or disrupt active cellulase–tannin complexes by “coating” the surface of insoluble lignocelluloses. Furthermore, polyethylene glycol may be involved in the formation of micelles that separate enzymes from their inhibitors. Gustavson [27] found that the formation of a precipitate between β-glucosidase and tannic acid was dependent on pH, ionic strength, and the concentrations of both proteins and tannins. By adding different amounts of tannic acid to the cellulose enzyme in a phosphate buffer (0.1 M, pH 7.0), it was found that 1 mg of β-glucosidase in 1 mL was completely precipitated by 0.75 mg of tannic acid, i.e., no enzyme activity was measurable in the supernatant. An independent assay on the amount of tannic acid in the supernatant showed that 0.460 mg of the acid was bound to the enzyme and that this amount remained constant when up to 5 mg of tannic acid was used [24].
The inhibition of xylanases and pectinases by phenolic compounds has also been reported in the literature. Significant inhibition of xylanase activity by vanillic acid, syringic acid, acetosyringone, and syringaldehyde has been observed [47]. El Modafar and El Boustani [19] tested the effects of cell wall-bound phenols, such as p-hydroxybenzoic, p-coumaric, ferulic, and sinapic acids, on the cell wall-degrading enzyme (CWDE) production by Fusarium oxysporum f. sp. Albedinis. In the presence of 2.5 μmol cm−3 of sinapic acid, the production of the different pectinolytic enzymes (pectin methylesterase, polygalacturonase, and polygalacturonase transeliminase) was completely inhibited, and the production of cellulase was strongly reduced (by approximately 90%). The production of cellulases was completely inhibited in the presence of 3 μmol cm−3 of sinapic acid. In the presence of 3 μmol cm−3 of ferulic acid, the production of pectinases was completely inhibited, and the production of the cellulases was reduced to 74%. However, p-coumaric acid and p-hydroxybenzoic acid did not induce a total inhibition of the CWDE at the concentrations tested. The effect of an artificially applied allele chemical, ferulic acid, on in vitro F. oxysporum revealed that pectinase activity was repressed by ferulic acid at a low concentration (200 mg/L), while it was stimulated at high concentrations (400–1,600 mg/L), as shown by an activity increase of 12.3%. The activity of pectinase was stimulated at high concentrations of ferulic acid (400–1,600 mg/L) in liquid culture, while it was depressed at a low concentration (200 mg/L). The activity was 0.091 ± 0.007 U/mL/min at the highest concentration of 1,600 mg/L [66]. Boukari et al. [11] reported the noncompetitive multi-site inhibition mechanism of phenolic compounds, including cinnamic acid, p-coumaric acid, caffeic acid, ferulic acid, and 3,4,5-trimethoxy-cinnamic acid, on the enzyme activity of a purified GH11 endoxylanase (Tx-Xyl) from Thermobacillus xylanilyticus. According to the authors, the inhibitory effects of phenolic compounds on Tx-Xyl activity do not involve a direct interaction with the enzyme active site. These interactions are likely to involve residues located at the surface of Tx-Xyl.
Ximenes et al. [69] suggested alternative strategies to improve enzyme activity and especially to alleviate the effects of phenolic compounds on such enzymes. They suggested carrying out enzyme hydrolysis over shorter periods of time to decrease the time-dependent deactivation or removing phenolics prior to enzyme hydrolysis by separation methods. Separation methods could include washing the solids or using microbial, enzymatic, or chemical methods for converting the phenolics to inactive forms.
In addition to the inhibitory effect of phenolic compounds, nonspecific adsorption of holocellulases onto insoluble lignin decreases enzyme reactivity [2]. The use of additives, such as Tween, polyethylene glycol (PEG), and bovine serum albumin (BSA), has shown promise in enhancing lignocellulose hydrolysis [9, 35]. These additives adsorb to lignin surfaces, preventing non-productive binding of holocellulases onto lignin. The competitive adsorption of Tween 80 onto the hydrophobic lignin surface decreased the adsorption of cellulase onto CEL-SELP, a commercial cellulase onto steam-exploded (SELP) lodgepole pine cellulolytic enzyme lignin (CEL), by 60% [60]. Eriksson et al. [20] found that the non-ionic surfactant (Tween 20) was the most effective at improving the conversion of steam-pretreated spruce, as it was able to lower the enzyme load by 50% and, at the same time, retain cellulose conversion. They concluded that the addition of surfactant to prevent enzymes from binding to the residual lignin increased the possibility of recycling enzymes after complete cellulose hydrolysis. The surfactants Tween-80, dodecylbenzene sulfonic acid, and polyethylene glycol 4000 were also effective in increasing lignin removal during the pretreatment of corn stover and reduced the non-productive binding of enzymes on the biomass surface. In addition, surfactant pretreatment improved lignin solubility [48].
The addition of NaCl in the concentration range of 0–0.4 M showed that ionic strength also has a considerable effect on cellulase adsorption to CEL-SELP lignin by altering enzyme adsorption. According to Can and Guner [13], the salt ions could either compete against the protein for binding sites or change the configuration of the protein, thereby resulting in a decrease in hydrophobic interactions between the protein and the solid surface. In contrast, the hydrophobic adsorption of enzymes to lignin induces the denaturation of enzymes on the lignin surface. It has also been demonstrated that temperature has significant effects on enzyme adsorption onto different lignocellulosic substrates [60]. Borjesson et al. [9] showed that the addition of PEG during the enzyme conversion of lignocellulose at 50°C hinders the deactivation of enzymes by excluding them from lignin surfaces. The effect of PEG addition on the enzyme conversion of lignocellulose increased with higher temperatures due to the increased adsorption of PEG on lignin, thereby resulting in a higher surface density of PEG on the lignin surface. A higher level of cellulase adsorption onto isolated lignin occurred at 45°C rather than at 4°C or 25°C.
Proteins such as BSA were also reported to prevent the non-productive adsorption of cellulase onto lignin. The results obtained by Yang and Wyman [70] suggest that the addition of BSA to dilute acid pretreated corn stover prior to enzyme addition reduces the adsorption of cellulase, particularly β-glucosidase, onto lignin. These authors reported that BSA may act as a competitive lignin blocker and may irreversibly attach to the lignin structure and reduce unproductive cellulase binding onto lignin.
Inhibition of Holocellulases by Sugars
Studies on the inhibitory effect of sugars on holocellulases (especially xylan, xylooligomers, and xylose) are instrumental to obtaining a better understanding of the loss of cellulase accessibility and enzyme effectiveness during the conversion of cellulose [33, 49, 50, 62]. However, the mechanism of inhibition of cellulase and other holocellulose-degrading enzymes is still unclear and is matter of intense research. One mechanism of inhibition is through the release of sugars and oligomer from hemicellulose during the enzymatic hydrolysis of holocellulose. Table 2 shows the effect of sugars on cellulase and hemicellulase activities.
In experiments reported by Qing et al. [49], the effect of xylan, xylose, and xylooligomers derived from xylan upon the enzymatic hydrolysis of pure cellulose (Avicel) was tested at low enzyme loadings. It was found that xylooligomers were more inhibitory to cellulase (Spezyme CP) than was xylan or xylose in terms of a decreased initial hydrolysis rate compared to xylan or xylose added at similar concentrations. Xylooligomer addition also led to a lower final glucose yield. Higher concentrations of each of these xylose compounds resulted in reduced initial hydrolysis rates. In addition, mixed DP xylooligomers showed little, if any, effect on β-glucosidase activity. In another study [50], the inhibitory effect of xylan and xylooligomers on cellulase (Spezyme CP) was reduced after enzyme supplementation with xylanase and β-xylosidase activity. In this case, the addition of these enzymes increased glucan conversion by 27% and 8% on corn stover pretreated with ammonia fiber expansion and dilute acid, respectively. This finding might suggest that the enzymatic removal of xylan and xylooligomers before adding cellulase may enhance cellulase effectiveness.
Ximenes et al. [68] found that oat spelt xylan hydrolysates (xylooligosaccharides and xylose) were responsible for the major inhibition of the activity of commercial cellulase (Spezyme CP) and β-glucosidase (Novozyme 188). Pectin and starch also inhibit glucan hydrolysis. β-Glucosidase from T. reesei was more sensitive to inhibition by starch, pectin, and their hydrolysis products than β-glucosidase from A. niger. To demonstrate that the sugar produced from the hydrolysate inhibits hydrolysis of lignocellulosic substrates, Xiao et al. [67] removed all of the produced sugar from the hydrolysate through ultrafiltration. Significant increases in the hydrolysis rate were observed after the sugar removal at both 24 and 48 h of incubation. Takagi [56] demonstrated that glucose strongly inhibited the cellulase activity from an enzyme preparation of T. reesei QM 9414, in which concentrations of 2.5% glucose inhibited 50% of the reaction. Ladisch et al. [36] and Gong et al. [25] showed that Trichoderma β-glucosidase activity was inhibited by 50% in the presence of 0.2–0.4 g/L glucose (i.e., K i = 1.0–2 mM), which is a ratio of enzyme activity to initial substrate of approximately 40 CBU/g cellobiose.
Cellobiose that is produced during cellulolysis is inhibitory to both exo- and endocellulases, thereby retarding saccharification [18]. Cellobiose is considered to be a powerful inhibitor of cellulases and inhibits this enzyme 14 times more than glucose. This type of inhibition has been classified in many different ways. Misunderstandings regarding this inhibition result from the difficulty of conducting conclusive experiments that show the type of inhibition involved in a heterogeneous reaction environment (cellulose is an insoluble substrate) and the assumption that the cellulolytic system consists of a single enzyme [8]. Additionally, the conversion of cellobiose to ethanol can reduce the inhibition of cellulases by a factor of 16 [7]. Cellobiose inhibition of Cel 7A from T. reesei, which acts on low molecular weight chromogenic substrates, such as para-nitrophenyl cellobioside, has been well studied and reveals a strong competitive inhibition with an inhibition constant of approximately 20 μM [26]. Such a strong product inhibition is a drawback from a biological activity point of view, as it limits the level of soluble sugars that can be produced. However, it has been shown that the hydrolysis of natural, cellulosic substrates are more resistant to inhibition, and product inhibition is not responsible for the gradual decrease in the hydrolysis rate at the early stage of the process [26, 61].
Other Important Inhibitors of Holocellulases
Some other important inhibitors of holocellulases are listed in Table 3 and include inhibitors not derived from the biomass. Among these inhibitors, formic acid, acetone, 2,3-butanediol, and propionic acid have been shown to be strong inhibitors of holocellulase [47, 56]. The holocellulase activity is deeply affected by ions; however, the effects of these ions are most important on the environment characterization surrounding the enzyme [42, 43]. Metal ions can be involved in enzyme catalysis in a variety of ways: accepting or donating electrons, acting as electrophiles themselves, masking nucleophiles to prevent unwanted side reactions, bringing together enzyme and substrate by coordinate bonds, holding the reacting groups in the required 3D orientation, and simply stabilizing a catalytically active conformation of the enzyme [44]. Among the numerous studies in the literature involving metal ions, the properties mentioned above are highlighted particularly by salts of chloride, nitrate, and sulfate.
In addition, cellulases are inhibited by mercury, silver, copper, chromium, lead, and zinc salts at concentrations of approximately 10−3 M. Cellulases are also inhibited by large organic molecules, such as acid or basic dyes, quaternary ammonium salts, or other detergents. The cellulase enzymatic reaction being reviewed involves ionic binding, and it is strongly affected by the pH of the reaction mixture. Acid dyes and anionic detergents inhibit at low pH levels, while basic dyes and cationic detergents become inhibitory at high pHs. Halogens and compounds that release reactive halogens, such as hypochlorite, dichloramine-B, N-chlorosuccinimide, N-bromosuccinimide, chloromelamine, and tetraglycine potassium periodide, may be active at a concentration of 10−4 M [39].
Keskar et al. [31] reported the isolation of a high-xylanase-producing thermotolerant Streptomyces T7 and observed that the xylanase enzyme was completely inhibited by Hg2+ at a concentration of 2 × 10−6 M. Kim et al. [32] also studied the effects of various reagents on purified xylanase I from Trichoderma koningii (ATCC 26113) and verified that at a concentration of 10 mM, Hg2+ inhibited this enzyme’s activity. The authors also demonstrated that xylanase I, which is inactivated by Hg2+, could be completely reactivated by the addition of 10 mM cysteine. Teixeira et al. [57] verified that purified PXII-1 xylanase from Aspergillus awamori was inhibited 100% by Hg2+, probably due to its interaction with its sulfydryl groups. Vieira et al. [63] evaluated the influence of various ions on xylanase and mannanase activity of the partially purified enzyme complex from the thermophilic cellulolytic bacterium Clostridium thermocellum (ISO II). A significant negative effect on xylanase and mannanase activity was observed with Co2+. Calcium (Ca2+) did not affect the xylanase activity. Fe3+ and Ca2+ inhibited mannanase activity but not xylanase activity.
Recent studies carried out by Thakur et al. [59] with a purified and characterized polygalacturonase from the saprophytic fungus, Mucor circinelloides (ITCC 6025), identified inhibition ranging from 5% to 15% of the polylacturonase enzyme activity in the presence of Mn2+, Co2+, and Mg2+, while Fe3+ and Zn2+ caused inhibition ranging from 27% to 31%. In contrast, the activity of the purified extracellular pectinase produced by the thermotolerant fungus Acrophialophora nainiana was completely inactivated by Cu2+, Ca2+, Fe2+, Al3+, Mn2+, and Zn2+ [14].
Within the group of holocellulases, pectinases are the first cell wall-degrading enzymes that are secreted by microorganisms; therefore, they are exposed to inhibitors, which are mostly proteins [38]. Several plants produce proteins with inhibitory activity against extracellular holocellulases of fungal pathogens [1, 6, 15, 17]. The finding of a powerful glycoprotein inhibitor of pectin methylesterase (PME) in ripe kiwi was reported by Balestrieri et al. [5]. The glycoprotein inhibitor was described as having a molecular mass of 28 kDa and specificity for PME from several sources, including orange-, tomato-, potato-, apple-, and banana-producing plants. Furthermore, it was hypothesized to be involved in the pathogen defense mechanism of plants and in the control mechanism of the plant’s growth and/or maturation. In addition, a protein from sugar beet roots inhibited the pectin lyase (PNL) activity of Rhizoctonia solani [12]. This inhibitor was equally effective against the PNL from Phoma betae and was less effective against the PNL from Aspergillus japonicus.
Ethanol inhibition is much less potent than cellobiose and glucose inhibition [56, 65], but the inhibitory effect of ethanol on cellulase enzymes is of a particular concern in performing the simultaneous saccharification and fermentation of biomass because the cellulase enzyme and ethanol coexist in the same reactor [7, 64]. Ethanol has been found to inhibit the crude exoglucanase enzyme at a much higher rate than it inhibits purified Cel 7A, which in fact appears to be almost not inhibited by ethanol.
Conclusion
The reaction between an enzyme and a complex polymeric substrate is not simple, nor is it, in this case, fully understood as yet [39, 71]. This problem is even more complicated when the polymer substrate is insoluble, thereby requiring the initial enzyme attack on crystalline portions of the cellulosic fiber [64]. The initial stage of the enzymatic saccharification of the holocellulose (amorphogenesis) is closely associated with the enzymatic access to the insoluble cellulose microfibrils which is limited by lignin and hemicelluloses, encasing the microfibrils [3]. Economical processing of lignocellulose at high-solid concentrations is challenging, not only due to mass transfer limitations, low water activities, and insufficient mixing but also because of the presence of soluble inhibitors. Soluble inhibitors, such as phenolic and xylooligosaccharide compounds, are major inhibitors that lead to decrease holocellulase activity. In addition, inhibitors not derived from the biomass, including ions and ethanol, can deeply affect the holocellulase activity. The isolation or development of holocellulases with increased resistance to inhibition of sugars, phenols, and inhibitors not derived from the biomass would enhance lignocelluloses hydrolysis activity.
References
Abu-Goukh AA, Greve LC, Labavitch JM (1983) Purification and partial characterization of ‘Bartlett’ pear fruit polygalacturonase inhibitors. Physiol Plant Pathol 23:111–122
Andreaus J, Filho EXF, Bon EPS (2008) A review on biotechnology of holocellulose-degrading enzymes. In: Hou CT, Shaw JF (eds) Biocatalysis and bioenergy. Wiley, New Jersey, pp 1–41
Arantes V, Jack N, Saddler JN (2010) Access to cellulose limits the efficiency of enzymatic hydrolysis: the role of amorphogenesis. Biotechnol Biofuels 3:4
Bae HD, McAllister TA, Yanke J, Cheng KJ, Muir AD (1993) Effects of condensed tannins on endoglucanase activity and filter paper digestion by Fibrobacter succinogenes S85. Appl Environ Microbiol 59:2132–2138
Balestrieri C, Castaldo D, Giovane A, Quagliuolo L, Servillo L (1990) A glycoprotein inhibitor of pectin methylesterase in kiwi fruit (Actinidia chinensis). Eur J Biochem 193:183–187
Barmore CR, Nguyen TK (1985) Polygalacturonase inhibition in rind of Valencia orange infected with Diplodia natalensis. Phytopathol 75:446–449
Bezerra RMF, Dias AA (2005) Enzymatic kinetic of cellulose hydrolysis inhibition by ethanol and cellobiose. Appl Biochem Biotech 126:49–59
Bezerra RMF, Dias AA, Fraga I, Pereira AN (2006) Simultaneous ethanol and cellobiose inhibition of cellulose hydrolysis studied with integrated equations assuming constant or variable substrate concentration. Appl Biochem Biotech 134:27–38
Borjesson J, Engqvist M, Sipos B, Tjerneld F (2007) Effect of poly(ethylene glycol) on enzymatic hydrolysis and adsorption of cellulase enzymes to pretreated lignocelluloses. Enz Microb Technol 41:186–195
Borneman WS, Ljungdahl LG, Hartley RD, Akin DE (1993) Feruloyl and p-coumaroyl esterases from the anaerobic fungus Neocallimastix strain MC-2: properties and functions in plant cell wall degradation. In: Coughlan MP Hazlewood GP (eds) Hemicellulose and hemicellulases. Portland, London, pp 85–102
Boukari I, O’Donohue M, Rémond C, Chabbert B (2011) Probing a family GH11 endo-β-1,4-xylanase inhibition mechanism by phenolic compounds: role of functional phenolic groups. J Mol Cat B: Enzymatic 72:130–138
Bugbee WM (1993) A pectin lyase inhibitor protein from cell walls of sugar beet. Phytopathol 83:63–68
Can HK, Guner A (2006) Investigation of adsorption-desorption dynamism of bovine serum albumin on crosslinked N, N-diethylaminoethyl dextran microbeads: solution phase. J Appl Polym Sci 99:2288–2299
Celestino SMC, de Freitas SM, Medrano FJ, de Souza MV, Filho EXF (2006) Purification and characterization of a novel pectinase from Acrophialophora nainiana with emphasis on its physicochemical properties. J Biotechnol 123:33–42
Cervone F, De Lorenzo G, Degra L, Salvi G, Bergami M (1987) Purification and characterization of a polygalacturonase-inhibiting protein from Phaseolus vulgaris L. Plant Physiol 85:631–637
Coughlan MP, Thuohy MG, Filho EXF, Puls J, Claeyssens M, Vrsanská M et al. (1993) Enzymological aspects of microbial hemicellulases with emphasis on fungal systems. In: Coughlan MP Hazlewood GP (eds) Hemicellulose and hemicellulases. Portland, London, pp 53–84
D’hallewin G, Schirra M, Powell ALT, Greve C, Labavitch JM (2004) Properties of a polygalacturonase-inhibiting protein isolated from “Oroblanco” grapefruit. Physiol Plantarum 120:395–404
Dekker RFH (1986) Kinetic, inhibition, and stability properties of a commercial β-D-glucosidase (cellobiase) preparation from Aspergillus niger and its suitability in the hydrolysis of lignocelluloses. Biotechnol Bioeng 28:1438–1442
El Modafar C, El Boustani E (2001) Cell wall-bound phenolic acid and lignin contents in date palm as related to its resistance to Fusarium oxysporum. Biol Plantarum 44:125–130
Eriksson T, Borjesson J, Tjerneld F (2002) Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzyme MicrobTechnol 31:353–364
Fang X, Shen Y, Zhao J, Bao X, Qu Y (2010) Status and prospect of lignocellulosic bioethanol from China. Biores Technol 101:4814–4819
Fenske JJ, Griffin DA, Penner MH (1998) Comparison of aromatic monomers in lignocellulosic biomass prehydrolysates. J Ind Microbiol Biotechnol 20:364–368
Gamble GR, Snook ME, Henriksson G, Akin DE (2000) Phenolic constituents in the bast tissue and inhibition of cellulose and pectinase. Biotechnol 22:741–746
Goldstein JL, Swain T (1956) The inhibition of enzymes by tannins. Phytochem 4:185–192
Gong CS, Ladisch MR, Tsao GT (1977) Cellobiase from Trichoderma viride: purification, properties, kinetics, and mechanism. Biotechnol Bioeng 19:959–981
Gruno M, Väljamä P, Petterson G, Johansson G (2004) Inhibition of the Trichoderma reesei cellulases by cellobiose is strongly dependent on the nature of the substrate. Biotechnol Bioeng 86:503–511
Gustavson KH (1956) The chemistry of tannins processes. Academic, New York
Hodge DB, Karim MN, Schell DJ, McMillan JD (2008) Soluble and insoluble solids contributions to high-solids enzymatic hydrolysis of lignocellulose. Biores Technol 99:8940–8948
Jing X, Zhang X, Bao J (2009) Inhibition performance of lignocellulose degradation products on industrial cellulase enzymes during cellulose hydrolysis. Appl Biochem Biotechnol 159:696–707
Kawakubo T, Karita S, Araki Y, Watanabe S, Oyadomari M et al. (2010) Analysis of exposed cellulose surfaces in pretreated wood biomass using carbohydrate-binding module (CBM)–cyan fluorescent protein (CFP). Biotechnol Bioeng 105:49–508
Keskar SS, Srinivasan MC, Deshpande VV (1989) Chemical modification of a xylanase from a thermotolerant Streptomyces. Evidence for essential tryptophan and cysteine residues at the active site. Biochem J 261:49–55
Kim HJ, Kang SO, Yung CH (1993) Purification and characterization of xylanase I from Trichoderma koningii ATCC 26113. Korean J Microbiol 31:63–71
Kim Y, Ximenes EA, Mosier NS, Ladisch MR (2011) Soluble inhibitors/deactivators of cellulase enzymes from lignocellulosic biomass. Enz Microbial Technol 48:408–415
Kristensen JB (2009) Enzymatic hydrolysis of lignocellulose substrate interactions and high solids loadings. In: Faculty of Life Sciences, Vol. Doctor of Philosophy (PhD), University of Copenhagen, Frederiksberg, p 130
Kumar R, Wyman CE (2009) Effect of additives on the digestibility of corn stover solids following pretreatment by leading technologies. Biotechnol Bioeng 102:1544–1557
Ladisch MR, Gong C-S, Tsao GT (1977) Corn crop residues as a potential source of single cell protein: kinetics of Trichoderma viride cellobiase action. Dev Ind Microbiol Ser 18:157–168
Ladisch M, MosierN KY, Ximenes EA, Hogsett D (2010) Conversion of cellulose to biofuels. Chem Eng Progress 106:56–63
Lagaert S, Beliën T, Volckaert G (2009) Plant cell walls: protecting the barrier from degradation by microbial enzymes. Sem Cell Develop Biol 20:1064–1073
Mandels M, Reese ET (1965) Inhibition of cellulases. Annu Rev Phytopathol 3:85–102
McNeil M, Darvill AG, Fry SC, Albersheim P (1984) Structure and function of the primary cell walls of plants. Annu Rev Biochem 53:625–663
McAllister TA, Bae HD, Yanke LJ, Cheng KJ (1994) Effect of condensed tannins from birdsfoot trefoil on endoglucanase activity and the digestion of cellulose filter paper by ruminal fungi. Can J Microbiol 40:298–305
McMillan JD (1994) Pretreatment of lignocellulosic biomass. In: Himmel ME, Baker JO, Overend RP (eds) Enzymatic conversion of biomass for fuels production. ACS symposium series, vol. 566. ACS, Washington, DC, pp 292–324
Mohamed SA, Farid NM, Hossiny EN, Bassuiny RI (2006) Biochemical characterization of an extracellular polygalacturonase from Trichoderma harzianum. J Biotechnol 127:54–64
Mohana S, Shah A, Divecha J, Madamwar D (2008) Xylanase production by Burkholderia sp. DMAX strain under solid state fermentation using distillery spent wash. Biores Technol 99:7553–7564
Moreira LRS, Filho EXF (2008) An overview of mannan structure and mannan-degrading enzyme systems. Appl Microbiol Biotechnol 79:165–178
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M et al. (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Biores Technol 96:673–686
Panagiotou G, Olsson L (2007) Effect of compounds released during pretreatment of wheat straw on microbial growth and enzymatic hydrolysis rates. Biotechnol Bioeng 96:250–258
Qing Q, Yang B, Wyman CE (2010) Impact of surfactants on pretreatment of corn stover. Biores Technol 101:5941–5951
Qing Q, Yang B, Wyman CE (2010) Xylooligomers are strong inhibitors of cellulose hydrolysis by enzymes. Biores Technol 101:9624–9630
Qing Q, Wyman CE (2011) Supplementation with xylanase and β-xylosidase to reduce xylooligomer and xylan inhibition of enzymatic hydrolysis of cellulose and pretreated corn stover. Biotechnol Biofuels 4:18
Ryan SE, Nolan K, Thompson R, Gubitz GM, Savage AV, Tuohy MG (2003) Purification and characterization of a new low molecular weight endoxylanase from Penicillium capsulatum. Enz Microb Technol 33:775–785
Saha B (2003) Hemicellulose bioconversion. J Ind Microbiol Biotechnol 30:279–291
Sineiro J, Dominguez H, Núñez MJ, Lema JM (1997) Inhibition of cellulase activity by sunflower polyphenols. Biotechnol Lett 19:521–524
Siqueira FG, Filho EXF (2010) Plant cell wall as substrate for the production of enzymes with industrial applications. Mini-Rev Org Chem 7:54–60
Szengyel Z, Zacchi G, Varga A, Réczey K (2000) Cellulase production of Trichoderma reesei Rut C30 using steam-pretreated spruce. Appl Biochem Biotechnol 84–86:679–691
Takagi M (1984) Inhibition of cellulase by fermentation products. Biotechnol Bioeng 26:1506–1507
Teixeira RSS, Siqueira FG, Souza MV, Filho EXF, Bon EPS (2010) Purification and characterization studies of a thermostable β-xylanase from Aspergillus awamori. J Ind Microbiol Biotechnol 37:1041–1051
Tejirian A, Xu F (2011) Inhibition of enzymatic cellulolysis by phenolic compounds. Enz Microb Technol 48:239–247
Thakur A, Pahwa R, Singh S, Gupta R (2010) Production, purification and characterization of polygalacturonase from Mucor circinelloides ITCC 6025. Enz Res 2010:1–7
Tu M, Pan X, Saddler JN (2009) Adsorption of cellulase on cellulolytic enzyme lignin from lodgepole pine. J Agric Food Chem 57:7771–7778
Väljamäe P, Sild V, Petterson G, Johansson G (1998) The initial kinetics of hydrolysis by cellobiohydrolases I and II is consistent with a cellulose surface—erosion model. Eur J Biochem 253:469–475
Várnai A, Siika-aho M, Viikari L (2010) Restriction of the enzymatic hydrolysis of steam-pretreated spruce by lignin and hemicelluloses. Enz Microb Technol 46:185–193
Vieira WB, Moreira LRS, Monteiro Neto A, Filho EXF (2007) Production and characterization of an enzyme complex from a new strain of Clostridium thermocellum with emphasis on its xylanase activity. Braz J Microbiol 38:237–242
Walton JD (1994) Deconstructing the cell wall. Plant Physiol 104:1113–1118
Wu Z, Lee YY (1997) Inhibition of the enzymatic hydrolysis of cellulose by ethanol. Biotechnol Lett 19:977–979
Wu HS, Luo J, Raza W, Liu YX, Gu M, Chen G et al. (2009) Effect of exogenously added ferulic acid on in vitro Fusarium oxysporum f. sp. Niveum. Sci Horticul 124:448–453
Xiao Z, Zhang X, Gregg D, Saddler J (2004) Effects of sugar inhibition on cellulases and β-glucosidase during enzymatic hydrolysis of softwood substrates. Appl Biochem Biotechnol 115:1115–1126
Ximenes EA, Kim Y, Mosier N, Dien B, Ladisch M (2010) Inhibition of cellulases by phenols. Enz Microb Technol 46:170–176
Ximenes EA, Kim Y, Mosier N, Dien B, Ladisch M (2011) Deactivation of cellulases by phenol. Enz Microb Technol 48:54–60
Yang B, Wyman CE (2006) BSA treatment to enhance enzymatic hydrolysis of cellulose in lignin containing substrates. Biotechnol Bioeng 94:614–617
Yang B, Willies DM, Wyman CE (2006) Changes in the enzymatic hydrolysis rate of Avicel cellulose with conversion. Biotechnol Bioeng 94:1122–1128
Zhang YHP, Himmel ME, Mielenz JR (2006) Outlook for cellulase improvement: screening and selection strategies. Biotechnol Adv 24:452–481
Acknowledgments
E.X.F. acknowledges the receipt of a research fellowship from the Brazilian Research Council (CNPq). P.M.D.J. and L.R.S.M acknowledge the receipt of a postgraduate maintenance scholarship from CNPq and Coordination for the Improvement of Higher Education, respectively. This work is funded by CNPq (research grants 563260/2010-6 and 563823/2010-0), Foundation for Research Support of Federal District (Brazil, Pronex Program) and National Institute of Science and Technology of Bioethanol. The authors would like to thank Dr. Eduardo de Aquino Ximenes for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duarte, G.C., Moreira, L.R.S., Jaramillo, P.M.D. et al. Biomass-Derived Inhibitors of Holocellulases. Bioenerg. Res. 5, 768–777 (2012). https://doi.org/10.1007/s12155-012-9182-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-012-9182-6