Abstract
The physicochemical pretreatment is an important step to reduce biomass recalcitrance and facilitate further processing of plant lignocellulose into bioproducts. This process results in soluble and insoluble biomass fractions, and both may contain by-products that inhibit enzymatic biocatalysts and microbial fermentation. These fermentation inhibitory compounds (ICs) are produced during the degradation of lignin and sugars, resulting in phenolic and furanic compounds, and carboxylic acids. Therefore, detoxification steps may be required to improve lignocellulose conversion by microoganisms. Several physical and chemical methods, such as neutralization, use of activated charcoal and organic solvents, have been developed and recommended for removal of ICs. However, biological processes, especially enzyme-based, have been shown to efficiently remove ICs with the advantage of minimizing environmental issues since they are biogenic catalysts and used in low quantities. This review focuses on describing several enzymatic approaches to promote detoxification of lignocellulosic hydrolysates and improve the performance of microbial fermentation for the generation of bioproducts. Novel strategies using classical carbohydrate active enzymes (CAZymes), such as laccases (AA1) and peroxidases (AA2), as well as more advanced strategies using prooxidant, antioxidant and detoxification enzymes (dubbed as PADs), i.e. superoxide dismutases, are discussed as perspectives in the field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Lignocellulosic biomass is made of polysaccharides comprising cellulose (C6 sugar monomers) and hemicellulose (C5 and C6 sugar monomers), and a phenolic heteropolymer named lignin. The renewable production of a wide range of biomolecules using monomeric sugars derived from plant biomass is a reality, such as sugarcane bagasse conversion to ethanol in the so-called second-generation ethanol biorefinery (Vieira et al. 2021). However, lignocellulosic materials are not readily accessible for bioconversion by enzymes and microorganisms, and thus require physical–chemical pretreatment steps to decrease the biomass recalcitrance (Lloyd et al. 2017; Suckling et al. 2017).
Several pretreatment methods have been studied and can be classified as mechanical, chemical, mechanical-chemical and biological. These classes include milling, pyrolysis, steam explosion, ammonia fiber explosion, liquid hot water, alkaline, acidic, organosolv, ionic liquids, enzymatic and microbial treatments (Cameron et al. 2015; Singh et al. 2016; Vaidya et al. 2016; Marques et al. 2020; Lorenci Woiciechowski et al. 2020). For instance, the pretreatment of biomass at elevated temperatures (ranging from 100 to 250 °C), and using acid as the catalyst, is widely employed nowadays at industrial scale to obtain a pentose (xylose and arabinose) and pseudo-lignin-rich hemicellulosic hydrolysate stream, and a solid fraction rich in cellulose, named cellulignin. The latter is composed mainly by cellulose and non-hydrolyzed lignin and can be subjected to enzymatic hydrolysis to obtain fermentable sugars (Suckling et al. 2017).
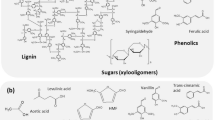
After lignocellulosic biomass pretreatment, different inhibitory compounds (ICs) are formed due to the chemical degradation of sugars and lignin. The amount and nature of the formed ICs is directly related to the pretreatment method and feedstock used (Jönsson and Martín 2015) (Fig. 1). The toxicity of these compounds to fermentative microorganisms is a limiting factor in the production of valuable products from plant biomass (Newman et al. 2013). ICs are mainly classified as weak aliphatic acids (acetic, formic and levulinic acid), furanaldehydes (for instance, furfural—FUR and hydroxymethylfurfural—HMF), phenolic and aromatic compounds (Jönsson and Martín 2015).
The cell-inhibitory effects of weak acid ICs are related to the reduction of the intracellular pH, accumulating anions in the cell and causing the reduction of ATP production (Jönsson et al. 2013). The weak acids can be removed from hydrolysates through layered double hydroxides (LDHs) as adsorbents, amongst other techniques (Jönsson and Martín 2015; Travália et al. 2019).
FUR and HMF can inhibit important enzymes such as alcohol dehydrogenase, and also promote membrane damage and cofactor depletion (Tramontina et al. 2017). These ICs can be removed through evaporation or even microbial metabolization (Nakagame et al. 2020).
A mixture of toxic phenolics has been reported as lignin degradation products, derived from 4-hydroxybenzyl (H), guaiacyl (G), and siringyl (S) units (Tramontina et al. 2020), including vanillin, coniferyl aldehyde, syringaldehyde, 4-hydroxybenzaldehyde, catechol, 4-hydroxybenzoic acid, dihydroconiferyl alcohol and syringic acid. Phenolic compounds can also originate from extractive components such as terpenes and tannins rather than lignin (Jönsson and Martín 2015).
Undissociated aromatic molecules are capable of diffusing passively across bacterial cell membranes (Nichols and Harwood 1997). Still, it is not currently established if higher molecular weight species (e.g., dimers) can cross cell membranes (Beckham et al. 2016). Therefore, there are several indications that lower molecular weight phenolics are more toxic to microorganisms (Klinke et al. 2004). These molecules cause biological membrane integrity loss, DNA damage via intracellular reactive oxygen species (ROS) generation, and inhibition of central metabolism enzymes (Jönsson et al. 2013).
Therefore, inhibitor removal prior to fermentation is crucial for adequate microbial performance. Several methods for detoxification of lignocellulosic hydrolysates have been investigated including evaporation, ion exchange, adsorption with activated charcoal and enzyme treatments (Kumar et al. 2020). These methods seek to minimize the deleterious ICs by their removal, neutralization, absorption, or metabolization (Tramontina et al. 2020).
Enzymatic biological detoxification approaches have gained attention as a greener strategy, which can be substrate-specific, and offers the possibility to increase saccharification and fermentation rates, thus reducing the processing time with no carbohydrate consumption, and mild reaction conditions (Moreno et al. 2015). Therefore, this review presents the advances and a detailed description of widely used enzymatic approaches to improve microbial fermentation of lignocellulosic hydrolysates, focusing on aromatic ICs bioabatement. A dedicated section of this review brings successful cases using classical enzymes (laccases and peroxidases), along with novel strategies using carbohydrate-active enzymes (CAZymes), and prooxidant, antioxidant, and detoxication enzymes (PADs), i.e., superoxide dismutases, were discussed as perspectives in the field.
The use of enzymes for detoxification of plant biomass hydrolysates
Enzymatic processes can efficiently remove phenolic compounds and detoxify lignocellulosic hydrolysates while having a minimal effect on the environment, because they are biogenic catalysts and require mild conditions. The enzymes are generally added in low concentrations (1.00–0.01% w/w), before the fermentation step with no need for highly purified preparations (Parawira and Tekere 2011). However, biological processes present some disadvantages, such as the prolonged incubation time needed for detoxification as well as the protein production costs, which are higher than other compounds used for detoxification methods (Moreno et al. 2015; Plácido and Capareda 2015).
The first and most relevant studies with enzymatic detoxification started with laccases and peroxidases, representing the majority of findings in this field of study (Kurek and Monties 1994; Cho et al. 2009; Kapoor et al. 2015; Schroyen et al. 2017; García-Torreiro et al. 2018).
With the advance of omics studies, synthetic biology and cutting-edge chemical analyses, novel enzymes have been discovered which act on phenolic compounds present in lignocellulosic biomass, but their application in detoxification has been demonstrated in few studies (Tramontina et al. 2020; Granja-Travez et al. 2020). Accordingly, the following sections will discuss laccases and peroxidases applied for lignocellulosic hydrolysate bioabatement, as well as promising new enzymatic candidates of potential interest for detoxification processes. An overview on enzymatic detoxification strategies and applications is shown in Table 1.
Laccases (AA1)
A green alternative to detoxify sugar-rich streams containing phenolics and lignin-derived compounds is to employ lignolytic enzymes, in particular laccases. These enzymes belong to the family of multi-copper oxidases AA1 from the carbohydrate active enzymes (CAZy) database (EC 1.10.3.2). Using oxygen as an electron acceptor, laccases are able to oxidize phenolic compounds and generate water as a by-product. They are found in plants, bacteria, and insects and fungi (Yang et al. 2017). In plants, laccases are glycosylated proteins that exhibit low redox potential, and their physiological role is associated with lignin biosynthesis and polymerization (Arregui et al. 2019). In fungi, laccases have been studied in relation to their role in pigmentation and pathogenesis, as well as their application for plant biomass delignification and hydrolysate detoxification (Agrawal et al. 2018).
While laccases are potential options to increase the fermentability of lignocellulosic hydrolysates, their use should be carefully evaluated. Laccase-catalyzed oxidation generates radical species that can be transformed via different pathways (Christopher et al. 2014). Therefore, their activity may vary from polymerization or depolymerization, depending on the properties and characteristics of the enzyme, and the nature of the compounds in the hydrolysate, which could be monomeric phenolics or lignin-derived fragments. New molecules such as dimers or polymerized lignin-fragments can be formed via different oxidative phenol couplings, including homo-and cross-coupling of different phenols. These new compounds may stabilize, or undergo rearrangement and generate new products, which can also act as oxidizing agents. When combined with electron shuttles—so called mediators—laccases more effectively oxidize non-phenolic substrates via different mechanisms, such as electron transfer or radical hydrogen atom transfer (Christopher et al. 2014). Natural laccase redox mediators include vanillin or p-hydroxycinnamic acids, which are preferred to artificial mediators such as 2,2′-azinobis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) and 1-hydroxybenzotriazole, because they are less expensive and non-toxic (Kupski et al. 2019).
Detoxification methods using laccases or laccase-mediator-systems (LMS) can be performed using free or immobilized enzymes, with or without oxygen. For example, the purified fungal laccase from Trametes versicolor was successfully used to detoxify a lignocellulosic hydrolysate derived from willow wood after pretreatment using steam and sulfur dioxide, which lead to improved ethanolic fermentation by Saccharomyces cerevisiae (Jönsson et al. 1998). In this study, the mechanism of laccase detoxification involved removal of monoaromatic phenolic compounds and associated formation of high molecular weight phenolics.
In another work, the laccase from T. versicolor was able to remove nearly all kinds of phenolic monomers present in a hydrolysate derived from wheat straw pretreated with liquid hot water after 24 h (Kolb et al. 2012).
The laccase redox potential is a crucial parameter to be considered for increasing the potential to oxidize a wide range of soluble phenolic compounds. In this sense, the bacterial laccase from Streptomyces ipomoeae showed lower efficiency than the commercial fungal laccase from T. villosa to detoxify the hydrolysate derived from a steam-exploded wheat straw slurry (De La Torre et al. 2017). The laccase from S. ipomoeae displays lower redox potential, which could explain its limited capability to oxidize syringaldehyde and ferulic acid, while the fungal laccase with higher redox potential can oxidize these compounds, as well as vanillin, p-coumaric acid and other phenolics.
Immobilized laccase from T. versicolor, in combination with anion exchange resin, were employed to reduce the amount of toxic phenolic compounds, furans and organic acids from organosolv-pretreated wheat straw hydrolysate, which improved fermentability of Pichia stipitis to produce ethanol (Ludwig et al. 2013). The laccase from T. versicolor was reported to detoxify furanic and phenolic aldehyde derivatives, and in the presence of a redox mediator, this enzyme broadened its activity to phenolic ketone derivatives (Saravanakumar et al. 2016). The butanol production by Clostridium acetobutylicum was 2.7-fold higher than the untreated wood hydrolysate (Allard-Massicotte et al. 2017). According to the previous study, a combination of hydrolysate flocculation and laccase treatment reduced phenolic concentration from 1.20 to 0.28 g/L.
Although many studies observed positive effects when employing different types of laccases, detrimental effects have also been reported. For instance, the laccase (Novozymes NS-22127) treatment provided little benefit to improve the fermentation of sugarcane bagasse hydrolysates using the ethanologenic Escherichia coli LY180. However, laccase treatment was more effective when combined with alkaline and vacuum treatment (Geddes et al. 2015). In another study, the laccase from Myceliophthora thermophila was combined with a cocktail containing redox enzymes to detoxify a sugarcane hemicellulosic hydrolysate, but this did not improve butanol production by the bacteria Clostridium saccharoperbutylacetonicum, or ethanol production by the yeast Scheffersomyces stipitis (Tramontina et al. 2020). According to this previous study, the absence of redox mediators together with the laccase reaction system could explain the absence of fermentation improvements.
Unquestionably, the application of laccases is a valuable strategy to detoxify hydrolysates and improve fermentation processes for biofuels production. However, it is important to consider the possible challenges and limitations of using laccases at an industrial scale and in biorefineries, including enzyme production cost, the need for redox mediators and enzyme recycling (immobilization systems). Laccase inactivation and removal of high molecular weight phenolics derived from lignin prior to fermentation are processes that need to be evaluated for their improvement on the overall fermentation performance (Cho et al. 2009; Tramontina et al. 2020). Genetic engineering of fermenting microorganisms to increase laccase production could also promote improvement by eliminating steps in the overall process (Larsson et al. 2001).
Peroxidases (AA2)
Fungal lignin peroxidases (LiP, EC 1.11.1.14), manganese peroxidases (MnP, EC 1.11.1.13) and versatile peroxidases (VP, EC 1.11.1.16) have shown potential for applications in detoxification of lignocellulosic hydrolysates. These enzymes are classified as family AA2 in the CAZy database and are included on the class II superfamily of plant and microbial peroxidases. Together with prokaryotic peroxidases from class I and plant peroxidases from class III, all these enzymes comprise the superfamily of heme peroxidases (Pandey et al. 2017).
The LiPs can catalyze the H2O2‐dependent oxidative depolymerization of lignin, resulting in side-chain cleavage, demethylation, intramolecular addition, and rearrangements. (Chandra et al. 2017). The general LiP-catalyzed mechanism is a two-step reaction involving the native enzyme of the ferric state, an unstable intermediate (compound I) and the impartial oxoferryl intermediate (compound II) (Kumar and Chandra 2020).
The MnPs are heme-containing glycoproteins produced by almost all wood‐colonizing basidiomycetes (Pandey et al. 2017). MnPs oxidize the one-electron donor Mn2+ to Mn3+, which in turn oxidizes phenolic substrates such as phenols and dyes, and have recently been employed in beech wood organosolv hemicellulosic fraction detoxification (Yee et al. 2018).
The VP is a broad heme-containing ligninolytic peroxidase with different oxidation-active catalytic sites of high redox potential. This enzyme can oxidate lignin with no need of any redox mediator (Kumar and Chandra 2020).
DyPs are a new class of heme peroxidases (Sugano 2009) with a broad substrate specificity, and a lack of homology to most peroxidases. These enzymes function well in lower pHs, catalyzing one-electron oxidative transfers, resulting in free radicals. The latter can mediate the oxidation of non-phenolic and phenolic lignin models, as well as synthetic dyes with high-redox potential, such as anthraquinone (Moreno et al. 2015).
The peroxidases have been applied in detoxification strategies to improve microbial fermentation, for example plant class III peroxidases, such as horseradish peroxidase (Tramontina et al. 2020); and from fungi such as Coprinus cinereus and T. versicolor DyP peroxidase (Cho et al. 2009) (Table 1). These enzymes can remove 50 to 90% of total phenolics from plant biomass hydrolysates (Kurek and Monties 1994; Jönsson et al. 1998; Cho et al. 2009; Guo et al. 2013). Supplementation with H2O2 is often applied in peroxidase treatments, improving phenolic compound removal from lignocellulosic hydrolysate (Yee et al. 2018). A possible strategy to avoid the peroxide supplementation is to combine enzymes that are known to generate H2O2 (See item below), thus expanding the application of redox active enzymes for detoxification of lignocelullosic hydrolysates (Tramontina et al. 2020). In a recent study, the Pleurotus ostreatus class II peroxidase (AA2) had its activity enhanced by a lytic polysaccharide monooxygenase (LMPO—AA9), and the main mechanism was based on reactive oxygen species (ROS) generation (Li et al. 2019).
Other AAs and potential detoxifying enzymes acting on lignin
Auxiliary active enzymes (AAs) class from the CAZy database also display several protein families related to lignin oxidation/detoxification (Levasseur et al. 2013). Besides the traditional laccases in AA1 and peroxidases in AA2, which act on low and high molecular weight lignin fragments, the families AA4 and AA6 as well as the subfamilies AA3_2, AA_3 and AA5_1 have enzyme members that act directly on mono or di-lignols (Mori et al. 2016; Gygli et al. 2018). Other AA families, such as AA7 and subfamilies from AA3 and AA5 are indirectly associated with lignin degradation/detoxification since these families have enzymes related to the generation of Fenton reaction components, a mechanism observed in brown-rot fungi for lignocellulose oxidation (Janusz et al. 2017).
The subfamilies AA3_2 and AA3_3 is composed by aryl-alcohols oxidases—AAOs (EC 1.1.3.7) and methanol oxidases—MOXs (EC 1.1.3.13)—respectively, while AA5_1 harbors the glyoxal oxidases—GLOXs (EC 1.2.3.15). Theses enzymes act on alcohol molecules derived mainly from lignin oxidation (Sützl et al. 2018). For example, extracellular AAO from the white-rot fungi Pleurotus eryngii can methoxylate benzylic metabolites secreted by itself or derived from lignin, generating H2O2 which is used to supply peroxidases during lignin degradation (Hernández-Ortega et al. 2012). In addition, it is reported that AAOs from Pleurotus ostreatus are able to oxidize HMF, suggesting their application for detoxifying liquors derived from lignocellulose pre-treatments (Feldman et al. 2015). Lastly, glyoxal oxidases contribute to lignin detoxification via dicarbonyl and hydroxycarbonyl oxidation, especially in glyoxal and methylglyoxal alcohols derived from lignocellulose degradation and pretreatment (Goswami et al. 2013). The AA4 family is composed by FAD-dependent vanillyl-alcohol oxidases (VAOs; EC 1.1.3.38) and VAOs that converts a wide range of para-substituted phenols, transforming them into several different phenolic compounds such as vanillin and coniferyl alcohol (Gygli et al. 2018). However, their use in lignocellulose detoxification for fermentation has not been reported.
The AA6 family harbors the 1,4-benzoquinone reductases (EC. 1.6.5.6), mainly found in yeasts. These enzymes are related to detoxification of aromatic compounds, protecting the cells from reactive quinones (Koch et al. 2017). As an example, the protein Pst2p from S. cerevisiae is a NADPH-dependent 1,4-benzoquinone oxidoreductase that enables yeast cells to cope with quinone-induced damage, suggesting a role of the enzyme in managing oxidative stress (Koch et al. 2017). Another example is PsBQR, a benzoquinone reductase from the lignin-degrading fungus Phanerochaete sordida YK-624, which when overexpressed in this fungi, enhanced the metabolism of low-molecular weight lignin fragments due to the effects of quinone redox cycling to produce hydroxyl radicals (Mori et al. 2016).
The potential of pro-oxidant, antioxidant and detoxification enzymes (PADs) on lignin detoxification and degradation
The PADs group was recently denominated to correlate the broad range of activities found in the oxidoreductase enzyme class with lignin detoxification and degradation (Franco Cairo et al. 2016). The term has been accepted by other researchers to complement the CAZy database as well (Bissaro et al. 2018). Examples of PADs include catalases (CAT), p450 monooxygenases, alcohol dehydrogenases (ADH), glutathione S-transferases (GST), superoxide dismutases (SOD), aldo–keto reductases (AKR) and many other oxidoreductases, which were previous displayed at the extinct Detoxiprot database for redox enzymes (Yang et al. 2011).
One of the first reports of PAD enzymes acting in lignin degradation were the β-etherases (which belong to the GSTs superfamily—EC. 2.5.1.18) from Sphingomonas paucimobilis SYK-6, encoded by the genes LigE and LigF (Masai et al. 1993). Afterwards, many other reports were published showing the ability of GSTs to cleave the β-O-4 aryl ether bond of low molecular weight lignin (DeAngelis et al. 2013; Masai et al. 2003; Ohta et al. 2015).
The PAD enzymes were first associated with synergistic lignin degradation/detoxification a decade ago (Tartar et al. 2009), in works describing the digestion physiology of termites (Blattodea: Isoptera). Omics reports concerning the role of PAD and AA enzymes were published for the major urban pest in South America, the lower termite Coptotermes gestroi (Franco Cairo et al. 2016). Both reports indicated that PAD enzymes such as SOD, AKR, CAT and ADH could play a role in termite lignocellulose digestion.
Tramontina et al. (2017) depicted the role of AKR in the digestive physiology of the lower termite Coptotermes gestroi, showing multiple roles for this enzyme while working in synergy with the termite cellulase for cellulose cleavage. The AKR increased lignocellulose hydrolysis through the generation of H2O2 and was also able to reduce FUR content in sugarcane hydrolysates, thus increasing fermentation yields.
The role of SODs in lignin degradation was also reported recently. Rashid et al. described a MnSOD as a major enzyme in the secretome of Sphingobacterium sp. T2 when grown on lignin (Rashid et al. 2015). Posteriorly, the same authors showed that MnSOD from Sphingobacterium performs the oxidative demethylation of lignin via generation of hydroxyl radicals, producing lower molecular weight fragments (Rashid et al. 2018).
A mixture of redox enzymes for the detoxification of a hemicellulosic hydrolysate derived from sugarcane bagasse pre-treatment was evaluated (Tramontina et al. 2020). The cocktail contained the PAD enzymes AKR and the Cu/Zn SOD from lower termite C. gestroi, horseradish peroxidase (HRP) and a laccase. It was shown that the synergism of HRP and SOD performed the degradation and reduction of hydroxyphenyl- and feruloyl-derived units, and also polymerized the lignin fragments from the hemicellulosic hydrolysate. This detoxification process allowed for the increase in butanol fermentation by the bacteria Clostridium saccharoperbutylacetonicum by 24-fold.
The roles of the PAD enzymes and other AA families for lignin detoxification certainly deserve to be better explored and further studies are now required to understand the mechanisms applied by PAD enzymes for lignin modification in biomass hydrolysates, as well as the techno-economic analysis to use these enzyme mixtures as detoxifying cocktails.
Perspectives
The physical–chemical pretreatments are often mandatory for the efficient conversion of lignocellulose into biofuels and other bioproducts. The enzymatic detoxification can be adopted before the saccharification or the fermentation step to mitigate the harmful effects of ICs. Combining other detoxification treatments with enzymatic detoxification is recommended for overall process improvements (Geddes et al. 2015). For instance, to include the application of adsorbents, redox mediators, media alkalinization, and others, for the removal of all types of ICs (Moreno et al. 2015; Geddes et al. 2015; Travália et al. 2019).
The cost associated with enzyme production is the major obstacle for an economically feasible biocatalytic detoxification process (Ferreira et al. 2020). Thus, by developing 'tailor-made detoxification cocktails', through genetic editing of fungal hosts to produce AA and PAD enzymes at large scale can enhance the detoxification potential and minimize overall costs of this process. Finally, the development of enzymatic strategies for biomass to bioproducts applications is important to sustain the transition from a fossil fuel based-economy to a more sustainable bioeconomy, which is important not only because of environmental aspects related to climate change, but also because this action can stimulate job growth and economic opportunities.
References
Agrawal K, Chaturvedi V, Verma P (2018) Fungal laccase discovered but yet undiscovered. Bioresour Bioprocess 5:4. https://doi.org/10.1186/s40643-018-0190-z
Allard-Massicotte R, Chadjaa H, Marinova M (2017) Phenols removal from hemicelluloses pre-hydrolysate by laccase to improve butanol production. Fermentation 3:31. https://doi.org/10.3390/fermentation3030031
Arregui L, Ayala M, Gómez-Gil X et al (2019) Laccases: structure, function, and potential application in water bioremediation. Microb Cell Fact 18:200. https://doi.org/10.1186/s12934-019-1248-0
Beckham GT, Johnson CW, Karp EM et al (2016) Opportunities and challenges in biological lignin valorization. Curr Opin Biotechnol 42:40–53. https://doi.org/10.1016/j.copbio.2016.02.030
Bissaro B, Várnai A, Røhr ÅK, Eijsink VGH (2018) Oxidoreductases and reactive oxygen species in conversion of lignocellulosic biomass. Microbiol Mol Biol Rev 82:1–51. https://doi.org/10.1128/mmbr.00029-18
Cameron H, Campion SH, Singh T, Vaidya AA (2015) Improved saccharification of steam-exploded Pinus radiata on supplementing crude extract of Penicilliumsp. 3 Biotech. https://doi.org/10.1007/s13205-014-0212-2
Chandra R, Kumar V, Yadav S (2017) Extremophilic ligninolytic enzymes. In: Extremophilic enzymatic processing of lignocellulosic feedstocks to bioenergy. Springer, Cham, pp 115–154. https://doi.org/10.1007/978-3-319-54684-1_8
Cho DH, Lee YJ, Um Y et al (2009) Detoxification of model phenolic compounds in lignocellulosic hydrolysates with peroxidase for butanol production from Clostridium beijerinckii. Appl Microbiol Biotechnol. https://doi.org/10.1007/s00253-009-1925-8
Christopher LP, Yao B, Ji Y (2014) Lignin biodegradation with laccase-mediator systems. Front Energy Res. https://doi.org/10.3389/fenrg.2014.00012
De La Torre M, Martín-Sampedro R, Fillat Ú et al (2017) Comparison of the efficiency of bacterial and fungal laccases in delignification and detoxification of steam-pretreated lignocellulosic biomass for bioethanol production. J Ind Microbiol Biotechnol. https://doi.org/10.1007/s10295-017-1977-1
DeAngelis KM, Sharma D, Varney R et al (2013) Evidence supporting dissimilatory and assimilatory lignin degradation in Enterobacter lignolyticus SCF1. Front Microbiol. https://doi.org/10.3389/fmicb.2013.00280
Feldman D, Kowbel DJ, Glass NL et al (2015) Detoxification of 5-hydroxymethylfurfural by the Pleurotus ostreatus lignolytic enzymes aryl alcohol oxidase and dehydrogenase. Biotechnol Biofuels. https://doi.org/10.1186/s13068-015-0244-9
Ferreira RG, Azzoni AR, Freitas S (2020) On the production cost of lignocellulose-degrading enzymes. Biofuels Bioprod Biorefining. https://doi.org/10.1002/bbb.2142
Franco Cairo JPL, Carazzolle MF, Leonardo FC et al (2016) Expanding the knowledge on lignocellulolytic and redox enzymes of worker and soldier castes from the lower termite Coptotermes gestroi. Front Microbiol 7:1518. https://doi.org/10.3389/fmicb.2016.01518
García-Torreiro M, Martínez-Patiño JC, Gullón B et al (2018) Simultaneous valorization and detoxification of the hemicellulose rich liquor from the organosolv fractionation. Int Biodeterior Biodegrad. https://doi.org/10.1016/j.ibiod.2017.10.010
Geddes R, Shanmugam KT, Ingram LO (2015) Combining treatments to improve the fermentation of sugarcane bagasse hydrolysates by ethanologenic Escherichia coli LY180. Bioresour Technol 189:15–22. https://doi.org/10.1016/j.biortech.2015.03.141
Giacobbe S, Piscitelli A, Raganati F et al (2019) Butanol production from laccase-pretreated brewer’s spent grain. Biotechnol Biofuels 12:1–8. https://doi.org/10.1186/s13068-019-1383-1
Goswami P, Chinnadayyala SSR, Chakraborty M et al (2013) An overview on alcohol oxidases and their potential applications. Appl Microbiol Biotechnol 97:4259–4275. https://doi.org/10.1007/s00253-013-4842-9
Granja-Travez RS, Persinoti GF, Squina FM, Bugg TDH (2020) Functional genomic analysis of bacterial lignin degraders: diversity in mechanisms of lignin oxidation and metabolism. Appl Microbiol Biotechnol 104:3305–3320. https://doi.org/10.1007/s00253-019-10318-y
Guo X, Cavka A, Jönsson LJ, Hong F (2013) Comparison of methods for detoxification of spruce hydrolysate for bacterial cellulose production. Microb Cell Fact 12:1–14. https://doi.org/10.1186/1475-2859-12-93
Gygli G, de Vries RP, van Berkel WJH (2018) On the origin of vanillyl alcohol oxidases. Fungal Genet Biol. https://doi.org/10.1016/j.fgb.2018.04.003
Hernández-Ortega A, Ferreira P, Martínez AT (2012) Fungal aryl-alcohol oxidase: a peroxide-producing flavoenzyme involved in lignin degradation. Appl Microbiol Biotechnol 23:257–262
Janusz G, Pawlik A, Sulej J et al (2017) Lignin degradation: microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol Rev 41:941–962. https://doi.org/10.1093/femsre/fux049
Jönsson LJ, Martín C (2015) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol 199:103–112. https://doi.org/10.1016/j.biortech.2015.10.009
Jönsson LJ, Palmqvist E, Nilvebrant N-O, Hahn-Hägerdal B (1998) Detoxification of wood hydrolysates with laccase and peroxidase from the white-rot fungus Trametes versicolor. Appl Microbiol Biotechnol 49:691–697. https://doi.org/10.1007/s002530051233
Jönsson LJ, Alriksson B, Nilvebrant N-O (2013) Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuels 6:16. https://doi.org/10.1186/1754-6834-6-16
Kapoor RK, Rajan K, Carrier DJ (2015) Applications of Trametes versicolor crude culture filtrates in detoxification of biomass pretreatment hydrolyzates. Bioresour Technol 189:99–106. https://doi.org/10.1016/j.biortech.2015.03.100
Klinke HB, Thomsen AB, Ahring BK (2004) Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol 66:10–26. https://doi.org/10.1007/s00253-004-1642-2
Koch K, Hromic A, Sorokina M et al (2017) Structure, biochemical and kinetic properties of recombinant Pst2p from Saccharomyces cerevisiae, a FMN-dependent NAD(P)H:quinone oxidoreductase. Biochim Biophys Acta 1865:1046–1056. https://doi.org/10.1016/j.bbapap.2017.05.005
Kolb M, Sieber V, Amann M et al (2012) Removal of monomer delignification products by laccase from Trametes versicolor. Bioresour Technol. https://doi.org/10.1016/j.biortech.2011.11.080
Kumar A, Chandra R (2020) Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 6:e03170. https://doi.org/10.1016/j.heliyon.2020.e03170
Kumar V, Yadav SK, Kumar J, Ahluwalia V (2020) A critical review on current strategies and trends employed for removal of inhibitors and toxic materials generated during biomass pretreatment. Bioresour Technol 299:122633. https://doi.org/10.1016/j.biortech.2019.122633
Kupski L, Salcedo GM, Caldas SS et al (2019) Optimization of a laccase-mediator system with natural redox-mediating compounds for pesticide removal. Environ Sci Pollut Res 26:5131–5139. https://doi.org/10.1007/s11356-018-4010-y
Kurek B, Monties B (1994) Oxidation of spruce lignin by fungal lignin peroxidase and horseradish peroxidase: comparison of their actions on molecular structure of the polymer in colloidal solution. Enzyme Microb Technol 16:125–130. https://doi.org/10.1016/0141-0229(94)90075-2
Larsson S, Cassland P, Jönsson LJ (2001) Development of a Saccharomyces cerevisiae strain with enhanced resistance to phenolic fermentation inhibitors in lignocellulose hydrolysates by heterologous expression of laccase. Appl Environ Microbiol. https://doi.org/10.1128/AEM.67.3.1163-1170.2001
Levasseur A, Drula E, Lombard V et al (2013) Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnol Biofuels 6:41. https://doi.org/10.1186/1754-6834-6-41
Li F, Ma F, Zhao H et al (2019) A lytic polysaccharide monooxygenase from a white-rot fungus drives the degradation of lignin by a versatile peroxidase. Appl Environ Microbiol. https://doi.org/10.1128/AEM.02803-18
Lloyd JA, Murton KD, Newman RH et al (2017) Careful selection of steaming and attrition conditions during thermo-mechanical pretreatment can increase enzymatic conversion of softwood. J Chem Technol Biotechnol. https://doi.org/10.1002/jctb.4975
Lorenci Woiciechowski A, Dalmas Neto CJ, de Souza P, Vandenberghe L et al (2020) Lignocellulosic biomass: acid and alkaline pretreatments and their effects on biomass recalcitrance—conventional processing and recent advances. Bioresour Technol 304:122848. https://doi.org/10.1016/j.biortech.2020.122848
Ludwig D, Amann M, Hirth T et al (2013) Development and optimization of single and combined detoxification processes to improve the fermentability of lignocellulose hydrolyzates. Bioresour Technol. https://doi.org/10.1016/j.biortech.2013.01.053
Marques FP, Silva LMA, Lomonaco D et al (2020) Steam explosion pretreatment to obtain eco-friendly building blocks from oil palm mesocarp fiber. Ind Crops Prod 143:111907. https://doi.org/10.1016/j.indcrop.2019.111907
Masai E, Katayama Y, Kubota S et al (1993) A bacterial enzyme degrading the model lignin compound β-etherase is a member of the glutathione-S-transferase superfamily. FEBS Lett. https://doi.org/10.1016/0014-5793(93)81465-C
Masai E, Ichimura A, Sato Y et al (2003) Roles of the enantioselective glutathione S-transferases in cleavage of β-aryl ether. J Bacteriol. https://doi.org/10.1128/JB.185.6.1768-1775.2003
Moreno AD, Ibarra D, Alvira P et al (2015) A review of biological delignification and detoxification methods for lignocellulosic bioethanol production. Crit Rev Biotechnol 35:342–354. https://doi.org/10.3109/07388551.2013.878896
Mori T, Koyama G, Kawagishi H, Hirai H (2016) Effects of homologous expression of 1,4-benzoquinone reductase and homogentisate 1,2-dioxygenase genes on wood decay in hyper-lignin-degrading fungus Phanerochaete sordida YK-624. Curr Microbiol. https://doi.org/10.1007/s00284-016-1089-6
Nakagame S, Shimizu Y, Saddler JN (2020) The production of lipids using 5-hydorxymethy furfural tolerant rhodotorula graminis grown on the hydrolyzates of steam pretreated softwoods. Sustainability 12:755. https://doi.org/10.3390/su12030755
Newman RH, Vaidya AA, Campion SH (2013) A mathematical model for the inhibitory effects of lignin in enzymatic hydrolysis of lignocellulosics. Bioresour Technol 130:757–762. https://doi.org/10.1016/j.biortech.2012.12.122
Nichols NN, Harwood CS (1997) PcaK, a high-affinity permease for the aromatic compounds 4-hydroxybenzoate and protocatechuate from Pseudomonas putida. J Bacteriol 179:5056–5061. https://doi.org/10.1128/JB.179.16.5056-5061.1997
Ohta Y, Nishi S, Hasegawa R, Hatada Y (2015) Combination of six enzymes of a marine Novosphingobium converts the stereoisomers of β-O-4 lignin model dimers into the respective monomers. Sci Rep. https://doi.org/10.1038/srep15105
Pandey VP, Awasthi M, Singh S et al (2017) A Comprehensive review on function and application of plant peroxidases. Biochem Anal Biochem. https://doi.org/10.4172/2161-1009.1000308
Parawira W, Tekere M (2011) Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: review. Crit Rev Biotechnol 31:20–31. https://doi.org/10.3109/07388551003757816
Plácido J, Capareda S (2015) Ligninolytic enzymes: a biotechnological alternative for bioethanol production. Bioresour Bioprocess 2:23. https://doi.org/10.1186/s40643-015-0049-5
Rashid GMM, Taylor CR, Liu Y et al (2015) Identification of manganese superoxide dismutase from Sphingobacterium sp. T2 as a novel bacterial enzyme for lignin oxidation. ACS Chem Biol 10:2286–2294. https://doi.org/10.1021/acschembio.5b00298
Rashid GMM, Zhang X, Wilkinson RC et al (2018) Sphingobacterium sp. T2 manganese superoxide dismutase catalyzes the oxidative demethylation of polymeric lignin via generation of hydroxyl radical. ACS Chem Biol 13:2920–2929. https://doi.org/10.1021/acschembio.8b00557
Saravanakumar T, Park HS, Mo AY et al (2016) Detoxification of furanic and phenolic lignocellulose derived inhibitors of yeast using laccase immobilized on bacterial cellulosic nanofibers. J Mol Catal B. https://doi.org/10.1016/j.molcatb.2016.11.006
Schneider WDH, Fontana RC, Baudel HM et al (2020) Lignin degradation and detoxification of eucalyptus wastes by on-site manufacturing fungal enzymes to enhance second-generation ethanol yield. Appl Energy 262:114493. https://doi.org/10.1016/j.apenergy.2020.114493
Schroyen M, Van Hulle SWH, Holemans S et al (2017) Laccase enzyme detoxifies hydrolysates and improves biogas production from hemp straw and miscanthus. Bioresour Technol. https://doi.org/10.1016/j.biortech.2017.07.137
Singh T, Vaidya AA, Donaldson LA, Singh AP (2016) Improvement in the enzymatic hydrolysis of biofuel substrate by a combined thermochemical and fungal pretreatment. Wood Sci Technol. https://doi.org/10.1007/s00226-016-0838-9
Suckling ID, Jack MW, Lloyd JA et al (2017) A mild thermomechanical process for the enzymatic conversion of radiata pine into fermentable sugars and lignin. Biotechnol Biofuels 10:61. https://doi.org/10.1186/s13068-017-0748-6
Sugano Y (2009) DyP-type peroxidases comprise a novel heme peroxidase family. Cell Mol Life Sci 66:1387–1403. https://doi.org/10.1007/s00018-008-8651-8
Sützl L, Laurent CVFP, Abrera AT et al (2018) Multiplicity of enzymatic functions in the CAZy AA3 family. Appl Microbiol Biotechnol 102:2477–2492. https://doi.org/10.1007/s00253-018-8784-0
Tartar A, Wheeler MM, Zhou X et al (2009) Parallel metatranscriptome analyses of host and symbiont gene expression in the gut of the termite Reticulitermes flavipes. Biotechnol Biofuels. https://doi.org/10.1186/1754-6834-2-25
Tramontina R, Franco Cairo JPL, Liberato MV et al (2017) The Coptotermes gestroi aldo–keto reductase: a multipurpose enzyme for biorefinery applications. Biotechnol Biofuels 10:4. https://doi.org/10.1186/s13068-016-0688-6
Tramontina R, Brenelli LB, Sousa A et al (2020) Designing a cocktail containing redox enzymes to improve hemicellulosic hydrolysate fermentability by microorganisms. Enzyme Microb Technol 135:109490. https://doi.org/10.1016/j.enzmictec.2019.109490
Travália BM, Santos NTDG, Vieira MGA, Forte MBS (2019) Adsorption of fermentation inhibitors by layered double hydroxides in synthetic hemicellulose hydrolysate: a batch multicomponent analysis. Ind Eng Chem Res. https://doi.org/10.1021/acs.iecr.9b03184
Vaidya AA, Donaldson LA, Newman RH et al (2016) Micromorphological changes and mechanism associated with wet ball milling of Pinus radiata substrate and consequences for saccharification at low enzyme loading. Bioresour Technol 214:132–137. https://doi.org/10.1016/j.biortech.2016.04.084
Vieira CFDS, Codogno MC, Maugeri Filho F et al (2021) Sugarcane bagasse hydrolysates as feedstock to produce the isopropanol-butanol-ethanol fuel mixture: effect of lactic acid derived from microbial contamination on Clostridium beijerinckii DSM 6423. Bioresour Technol 319:124140. https://doi.org/10.1016/j.biortech.2020.124140
Yang Z, Yu Y, Yao L et al (2011) DetoxiProt: an integrated database for detoxification proteins. BMC Genomics 12:S2. https://doi.org/10.1186/1471-2164-12-S3-S2
Yang J, Li W, Bun Ng T et al (2017) Laccases: production, expression regulation, and applications in pharmaceutical biodegradation. Front Microbiol. https://doi.org/10.3389/fmicb.2017.00832
Yee KL, Jansen LE, Lajoie CA et al (2018) Furfural and 5-hydroxymethyl-furfural degradation using recombinant manganese peroxidase. Enzyme Microb Technol. https://doi.org/10.1016/j.enzmictec.2017.08.009
Funding
This work was supported by the São Paulo Research Foundation (FAPESP) research Grants (2015/50590-4 to FMS) and fellowships (2017/15477-8 to LBB; 2016/07926-4 to RT, 2018/18101-1 to VS and 20/03051-9 to VYE), and National Council for Scientific and Technological Development (CNPq) Grants (305748/2017-3 and 428527/2018-3 to FMS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tramontina, R., Brenelli, L.B., Sodré, V. et al. Enzymatic removal of inhibitory compounds from lignocellulosic hydrolysates for biomass to bioproducts applications. World J Microbiol Biotechnol 36, 166 (2020). https://doi.org/10.1007/s11274-020-02942-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-020-02942-y