Abstract
Background
Lipoblastomas (LPBs) are benign adipocytic neoplasms believed to recapitulate the development of embryonal fat.
Methods
We investigated the clinicopathologic and immunohistochemical features of 20 lipoblastomas arising in the head and neck in 18 patients.
Results
Patients included 6 males and 12 females (1:2 ratio) with age at diagnosis ranging from 4 months to 28 years. Tumors occurred more commonly in the neck (12, 66.7%) and less commonly in the forehead, scalp, and tongue (2, 11.1%). Tumor size ranged from 1.4 to 6.0 cm (median 5.0 cm). Two patients, a 4-month-old female and 3-year-old male, had local recurrence of neck tumors at 4 months and 3 years after excision, respectively. Microscopically, tumors had a lobulated growth pattern and consisted of adipocytes at varying stages of differentiation. In addition to the classical histologic features, lipoma-like and myxoid variants constituted 45% of cases. Metaplastic elements, including brown fat and cartilage, were identified in two cases.
Conclusions
LPBs arising in the head and neck region are not uncommon and occurred at a rate of 9% in our cohort. They should be kept in the differential diagnosis when a fatty tumor is encountered in an older child or occurring at an unusual location.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lipoblastomas (LPBs) are benign adipocytic neoplasms believed to recapitulate the development of embryonal fat [1,2,3]. They typically arise as circumscribed, slowly growing masses on the extremities and or trunk, but occasionally they manifest in the head and neck region [1, 4]. These tumors are more commonly noted in males and most often, but not exclusively, occur during the first decade [1, 4, 5]. They present as localized or diffuse tumors with a tendency for local recurrence [1].
Histologically, the tumors are lobulated with a varying proportion of mature and immature fat cells separated by fibrous septa. Immunostains show frequent positivity for S100 protein, desmin, CD34, and PLAG1. LPBs are characterized by abnormalities of chromosome 8q11-13, resulting in rearrangement of the PLAG1 gene with a plethora of partners [2, 6]. Rarely, genetic aberration of chromosome 12q14.3, resulting in rearrangement of the HMGA2, gene, is identified [6, 7].
We report a series of 18 patients with LPB occurring in the head and neck region to explore the clinical and pathologic feature of these tumors in this anatomic location.
Materials and Methods
This study was approved by the Institutional Review Board at Boston Children's Hospital. Twenty in-house LPB cases arising in the head and neck region between January 1987 and January 2020 were retrieved from the Boston Children's Hospital slide archives, Boston, MA. Consult cases were excluded. Hematoxylin and eosin-stained slides of all cases along with available immunohistochemistry were re-reviewed by the senior author.
Patients’ age and sex, anatomic location of tumor, and size of tumor (maximal dimension) were recorded. When available, clinical information and follow-up were obtained from the electronic medical record. Immunohistochemical stains for PLAG1 (vendor catalog Abnova, H00005324-M02, antibody clone 3B7), desmin (vendor catalog Leica Biosystems, PA0032, antibody clone DE-R-11), and S100 (vendor catalog Agilent Dako, IR504, polyclonal antibody) were performed on 7, 9, and 3 cases, respectively. Anchored multiplex RNA sequencing assay was performed on one case. Briefly, RNA was extracted from formalin-fixed paraffin-embedded (FFPE) tissue, followed by cDNA synthesis and library preparation using reagents provided by ArcherDX (Boulder, CO, USA). Gene targets varied by assay: a custom panel from Boston Children’s Hospital Anchored multiplex polymerase chain reaction amplicons were sequenced on Illumina sequencers (HiSeq or MiSeq). Analysis was performed using the Archer Analysis software.
Results
Clinical Features
Clinical and pathologic data are summarized in Table 1. The cohort consisted of 18 patients and included 12 (66.7%) females and 8 (33.3%) males. Patients’ mean age at time of diagnosis was 5.5 years, with a wide age range (4 months to 28 years). Overall, the neck was preferentially involved, followed by forehead and scalp. Resected tumor specimens ranged in maximum dimension from 1.4 to 6.0 cm (median 5 cm). Tumors presented as asymptomatic soft tissue masses in most patients with the exception of a rapidly growing posterior neck mass in a 16-month-old male and persistent cervical lymphadenopathy in 3-year-old female. All patients underwent surgical excision of the primary mass. Re-excision was documented in two cases in which tumors had locally recurred. One case recurred in the right supraclavicular area of a 4-month-old female after 4 months and the second case recurred in the left posterior neck of a 3-year-old male 3 years post excision.
Pathologic Features and Immunohistochemistry
Histologically, typical areas of the LPB were distinctly nodular, consisting of lobules of fat in various stages of maturation surrounded by thick fibrous bands (Fig. 1). Six tumors had predominantly mature adipose tissue resembling lipoma, while eleven cases showed classic morphology composed of lobules of primitive mesenchymal cells, lipoblasts, and small capillaries (Figs. 1, 2). Prominent myxoid stroma was noted in three cases. One LPB arising in the tongue of a 3-year-old female exhibited striking nodular areas of mature and immature cartilage without atypia (Fig. 3). One tumor in the neck of 3-year-old with marked myxoid stroma exhibited prominent delicate vasculature with numerous uni- and multi-vacuolated lipoblasts mimicking myxoid liposarcoma (Fig. 4). Additionally, one case arising in the neck of a 3-year-old male had small foci of brown fat adjacent to the normal soft tissue components (Fig. 4).
Common histological features of lipoblastoma. A Grossly, LPB is a circumscribed mass with a yellow vaguely nodular cut surface. B Most tumors exhibited classic features of LPB with an encapsulated lobulated growth pattern and intervening thin fibrous bands. C Focal areas exhibited myxoid stroma. D and E Immature lobules consist of primitive mesenchymal cells admixed with mature adipocytes
Lipoma-like lipoblastoma. A Grossly, a lobular brown mass with focal myxoid cut surface (left). B A multilobulated architecture of mature adipocytes with dense intervening fibrous bands. C Immunohistochemical stain for PLAG1 reveals strong nuclear positivity correlating with the presence of gene fusion
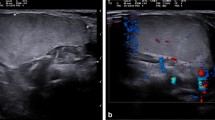
A 3-year-old female with tongue lipoblastoma. A Grossly, a round mass with a bright yellow surface macroscopically. B Preoperative sagittal MRI image of the head and neck reveals a well-delineated lingual mass (white arrows). C LBP showing lobules of fat in various stages of maturation, surrounded by thick fibrous bands. D Lobules consist of primitive mesenchymal cells admixed with mature adipocytes and a nodule of immature cartilage. E–G Multiple foci of cartilage in between the adipocytic lobules consist of primitive mesenchymal cells admixed with mature adipocytes. H Immunohistochemical stain for desmin shows strong positivity within lesional cells. I Immunohistochemical stain for PLAG1 reveals strong nuclear positivity. Note that positive staining includes both primitive cells and metaplastic cartilage
A and B HAS2::PLAG1 fused lipoblastoma in the neck of a 3-year-old female showing marked myxoid stroma with a delicate vascular pattern and numerous lipoblasts. C–F Lipoblastoma in the neck of a 3-year-old male showing a classic lobulated pattern with myxoid stroma and variable adipocytic maturation (C, D) in addition to foci of “hibernoma-like” brown adipose tissue with multi-vacuolated adipocytes (E, F)
As expected, all tumors were strongly immunopositive for PLAG1 (7/7), while desmin was positive in nine cases (9/11) and equivocal to negative in the remaining two cases. Three cases were positive for S100 protein (3/3).
Molecular Analysis
One case was confirmed to have HAS2::PLAG1 fusion by RNA-based targeted fusion assay.
Discussion
LPBs are benign mesenchymal tumors arising from embryonal white fat which recapitulates the process of embryonic adipocytic differentiation [1,2,3]. LPBs occur at various sites but most frequently affect the trunk and extremities [4]. Previous studies of LPBs arising in the head and neck reported the incidence rate as 10–15% [8,9,10]. These series mostly highlight radiologic and clinical features, while reports of pathologic features are limited to case reports, small series, or as part of large series of LPB involving all sites. Herein, we are reporting the largest series of LPB involving the head and neck region and in doing so we are highlighting the distinct pathologic features of these tumors. In our cohort, we report a rate of 9% of total LPB resected in our institution (20 out of 243 total LPB during the study period). As in prior reports, most LPBs in our series occurred in the neck (66.7%). To date, cases of LPBs in other head and neck sites have been documented in the literature in the parotid gland [11, 12], cheek [13, 14], tongue [15, 16], and orbit [17, 18]. Our cohort included forehead and scalp. A recent systematic review reported that the incidence of LPBs was more common in males (55%) with a median age of 3.30 years (range 2 months to 20 years) [8]. In our cohort, LPBs were more frequent in females (66.7%) and in patients older than 3 years of age with a wider overall age range (range 2 months to 28 years).
The most common rearrangement of LPBs involves the 8q11-13 region affecting the PLAG1 gene. Since its discovery, various fusion partners of PLAG1 have been reported in LPB including HAS2, COL1A2, RAD51B, COL3A1, RAB2A, BOC, CHCHD7, HNRNPC, SRSF3, PCMTD1, YWHAZ, CTDSP2, PPP2R2A, DDX6, KLF10, KANSL1L, PI15, and ZEB2 [2, 6, 19,20,21,22,23,24,25,26]. Recently, novel HMGA2 fusions have been documented in LPB, some of which are associated with EP400 and FGD6 as partners [6, 7]. This gene translocation event results in the upregulation of PLAG1 or HMGA2 by a promoter swapping mechanism [2]. Other chromosomal abnormalities include polysomy and ring chromosome involving chromosome 8 [2, 27,28,29]. The HAS2::PLAG1 fusion gene was identified in the single case tested in our cohort.
As expected histologically, the majority of our cases demonstrated lobular architecture. These lobules were composed of fat cells in various stages of differentiation separated by fibrous septa, recapitulating developing fat. Among histologic subtypes, the myxoid variant and lipoma-like variant were equally noted in addition to the classical variant. Although metaplastic elements such as cartilage and bone are documented in benign and malignant adipocytic tumors [30, 31], their presence in LPB is not common [32]. Here, we found metaplastic mature and immature cartilage in a tongue location. Immunohistochemical studies showed that tumors, including the metaplastic cartilage and primitive stroma cells, were positive for desmin and PLAG1, confirming the diagnosis of LPB (Fig. 3). In our cohort, only seven cases had PLAG1 immunostain performed. None of these cases had classic LPB histologic features, 5 were maturing lipoma-like, one with metaplastic cartilage, and one with marked myxoid matrix. The latter case occurred in the neck of a 3-year-old female and exhibited prominent vascular pattern reminiscent of “chicken wire” vasculature seen in myxoid liposarcoma, although this is a less likely diagnostic consideration at age 3. Additionally, this case had the fusion panel performed documenting the presence of a HAS2::PLAG1 gene fusion. Overall, we believe that a combination of PLAG1 and desmin immunohistochemical staining may be of particular utility when LPB is encountered in an unusual clinical setting (i.e., older children/young adult) or exhibiting unusual morphologic features such as maturation or metaplastic elements. Positivity for PLAG1 within the chondroid component of our case supports the divergent differentiation of tumor cells theory leading to over stimulation of stromal resident multipotent undifferentiated mesenchymal cells [33]. We noted one case with hibernoma-like foci in line with a previously described pattern in a cytogenetically confirmed case [21].
Similar to any other site, the differential diagnosis of LPB in the head and neck region is based on histologic subtypes. LPB with a predominant mature lipomatous morphology mimics lipoma but the latter lacks the lobular growth and PLAG1 rearrangement [34]. A patient with PIK3CA-related overgrowth spectrum (PROS) disorder that exhibits features similar to those lipoma-like LPB with focal fibrous septa has been described [35, 36]. That case occurred in the posterior neck of 3-month-old male and presented as a solitary mass involving the subcutaneous tissue [36]. Thus, molecular testing to look for potential PIK3CA mutation is important to confirm the diagnosis in patients with localized fatty overgrowth instead of an isolated lipomatous tumor [36]. LPB with predominantly myxoid morphology should be distinguished from myxoid liposarcoma, which is extremely rare in children and exhibits distinctive delicate vasculature in a “chicken wire” pattern and lacking prominent lobulation [37]. Recently, the detection of gene t(12;16)(q13;p11) involving the DDIT3 and FUS genes can aid in confirming the diagnosis of myxoid liposarcoma [38]. Predominantly myxoid LPB with metaplastic elements represents a potential diagnostic pitfall by mimicking pleomorphic adenoma with chondroid stroma [39]. The mesenchymal component produced by myoepithelial cells may be myxoid/mucoid, osseous/cartilaginous, or hyalinized. The lipomatous stromal component varies from tumor to tumor and if it constitutes > 90% of tumor tissue, it is considered a lipomatous pleomorphic adenoma [40, 41]. Molecular alterations involving the PLAG1 gene are found in LPB and pleomorphic adenoma. Unlike LPB, pleomorphic adenoma consists of a proliferation of ductal structures and myoepithelial cells [42, 43]. For LPB with a predominantly primitive and fibroblastic component, the differential diagnosis includes fibrous hamartoma of infancy and lipofibromatosis. Fibrous hamartoma of infancy is less circumscribed and displays triphasic morphology with variable percentages of fat, fibroblastic fascicles, and primitive mesenchyme [44]. While lipofibromatosis often presents as a growing mass of the distal extremities and consists of an infiltrative admixture of mature fat, lipoblast-like cells, and fascicles of bland uniform fibroblastic/myofibroblastic cells [45].
In summary, we have reported the clinicopathological and immunohistochemical features of the largest series of head and neck LPBs to date. Our results confirm and extend prior observations about this pediatric adipocytic tumor. LPBs arising in the head and neck region are not uncommon and occurred at a rate of 9% in our cohort. They showed typical morphologic features of lobules of fat in various stages of maturation surrounded by thick fibrous bands. LPB should be kept in the differential diagnosis when a fatty tumor is encountered in an older child or occurring at an unusual location. In general, the diagnosis can be rendered based on histologic features alone. However, on rare occasions, particularly in tumors occurring outside the classic clinical setting, ancillary studies may be of value.
Data Availability
N/A.
Code Availability
Data availability statements provide a statement about where data supporting the results reported in a published article can be found including, where applicable, hyperlinks to publicly archived datasets analyzed or generated during the study: N/A.
Consent for Publication
N/A.
References
Coffin CM, Lowichik A, Putnam A (2009) Lipoblastoma (LPB): a clinicopathologic and immunohistochemical analysis of 59 cases. Am J Surg Pathol 33(11):1705–1712. https://doi.org/10.1097/PAS.0b013e3181b76462. (Epub 9 Oct 2009)
Gisselsson D, Hibbard MK, Dal Cin P, Sciot R, Hsi BL, Kozakewich HP et al (2001) PLAG1 alterations in lipoblastoma: involvement in varied mesenchymal cell types and evidence for alternative oncogenic mechanisms. Am J Pathol 159(3):955–962. https://doi.org/10.1016/S0002-9440(10)61771-3. (Epub 11 Sep 2001)
The WHO Classification of Tumours Editorial Board (2020) WHO classification of tumours soft tissue and bone tumours, 5th edn. IARC Press, Lyon
Collins MH, Chatten J (1997) Lipoblastoma/lipoblastomatosis: a clinicopathologic study of 25 tumors. Am J Surg Pathol 21(10):1131–1137. https://doi.org/10.1097/00000478-199710000-00002. (Epub 23 Oct 1997)
Fritchie K, Wang L, Yin Z, Nakitandwe J, Hedges D, Horvai A et al (2020) Lipoblastomas presenting in older children and adults: analysis of 22 cases with identification of novel PLAG1 fusion partners. Mod Pathol. https://doi.org/10.1038/s41379-020-00696-4. (Epub 25 Oct 2020)
Lopez-Nunez O, Alaggio R, Ranganathan S, Schmitt L, John I, Church AJ et al (2020) New molecular insights into the pathogenesis of lipoblastomas: clinicopathologic, immunohistochemical, and molecular analysis in pediatric cases. Hum Pathol 104:30–41. https://doi.org/10.1016/j.humpath.2020.07.016. (Epub 22 July 2020)
Pedeutour F, Deville A, Steyaert H, Ranchere-Vince D, Ambrosetti D, Sirvent N (2012) Rearrangement of HMGA2 in a case of infantile lipoblastoma without Plag1 alteration. Pediatr Blood Cancer 58(5):798–800. https://doi.org/10.1002/pbc.23335. (Epub 10 Jan 2012)
Lomoro P, Simonetti I, Nanni AL, Corsani G, Togni G, Fichera V et al (2021) Imaging of head and neck lipoblastoma: case report and systematic review. J Ultrasound 24(3):231–239. https://doi.org/10.1007/s40477-020-00439-w. (Epub 7 March 2020)
Gupta P, Potti TA, Wuertzer SD, Lenchik L, Pacholke DA (2016) Spectrum of fat-containing soft-tissue masses at MR imaging: the common, the uncommon, the characteristic, and the sometimes confusing. Radiographics 36(3):753–766. https://doi.org/10.1148/rg.2016150133. (Epub 11 May 2016)
Pham NS, Poirier B, Fuller SC, Dublin AB, Tollefson TT (2010) Pediatric lipoblastoma in the head and neck: a systematic review of 48 reported cases. Int J Pediatr Otorhinolaryngol 74(7):723–728. https://doi.org/10.1016/j.ijporl.2010.04.010. (Epub 18 May 2010)
Jandali D, Heilingoetter A, Ghai R, Jeffe J, Al-Khudari S (2018) Large parotid gland lipoblastoma in a teenager. Front Pediatr 6:50. https://doi.org/10.3389/fped.2018.00050. (Epub 30 March 2018)
Anantharajan N, Ravindranathan N (2010) Parotid lipoblastoma in a child: rare presentation as huge infratemporal mass with cervical extension. Indian J Plast Surg 43(1):84–87. https://doi.org/10.4103/0970-0358.63961. (Epub 7 Oct 2010)
Fernandez-Ferro M, Lopez-Betancourt A, Santos-Armentia E, Mosteiro-Cervino MJ, Fernandez-Sanroman J, Costas-Lopez A (2020) Rapidly growing facial tumor in a 5-year-old girl. Ann Maxillofac Surg 10(1):267–271. https://doi.org/10.4103/ams.ams_200_19. (Epub 29 Aug 2020)
Abijay C, Miller S, Booth T, Mitchell RB, Liu C (2022) A cheek mass in a 5-year-old child. Ear Nose Throat J 101(6):365–367. https://doi.org/10.1177/0145561320964270. (Epub 2 Oct 2020)
Wan MH, Tengku Nun Ahmad TE, Naicker MS, Abu Bakar MZ (2021) Congenital tongue base lipoblastoma causing neonatal airway compromise. BMJ Case Rep 14:1. https://doi.org/10.1136/bcr-2020-239554. (Epub 20 Jan 2021)
Zalzal GH, Patterson K, Cotton RT (1994) Congenital tumors of the dorsum of the tongue. Int J Pediatr Otorhinolaryngol 28(2–3):219–227. https://doi.org/10.1016/0165-5876(94)90015-9. (Epub 1 Jan 1994)
Dutton JJ, Escaravage GK Jr, Fowler AM, Wright JD (2011) Lipoblastomatosis: case report and review of the literature. Ophthalmic Plast Reconstr Surg 27(6):417–421. https://doi.org/10.1097/IOP.0b013e318221118c. (Epub 12 July 2011)
Hwang S, Kim JW, Shin SA, Siapno DL, Suh YL, Woo KI et al (2017) A case of orbital lipoblastoma: temporal evolution of imaging findings. J Pediatr Ophthalmol Strabismus 54:e67–e70. https://doi.org/10.3928/01913913-20170907-04. (Epub 11 Oct 2017)
Hibbard MK, Kozakewich HP, Dal Cin P, Sciot R, Tan X, Xiao S et al (2000) PLAG1 fusion oncogenes in lipoblastoma. Cancer Res 60(17):4869–4872 (Epub 15 Sep 2000)
Yoshida H, Miyachi M, Ouchi K, Kuwahara Y, Tsuchiya K, Iehara T et al (2014) Identification of COL3A1 and RAB2A as novel translocation partner genes of PLAG1 in lipoblastoma. Genes Chromosomes Cancer 53(7):606–611. https://doi.org/10.1002/gcc.22170. (Epub 5 April 2014)
Brandal P, Bjerkehagen B, Heim S (2006) Rearrangement of chromosomal region 8q11-13 in lipomatous tumours: correlation with lipoblastoma morphology. J Pathol 208(3):388–394. https://doi.org/10.1002/path.1879. (Epub 26 Nov 2005)
Brcic I, Igrec J, Halbwedl I, Viertler C, Liegl-Atzwanger B (2022) Expanding the spectrum of PLAG1-rearranged lipoblastomas arising in patients over 45, with identification of novel fusion partners. Mod Pathol 35(2):283–285. https://doi.org/10.1038/s41379-021-00888-6. (Epub 18 Aug 2021)
Morerio C, Rapella A, Rosanda C, Tassano E, Gambini C, Romagnoli G et al (2005) PLAG1-HAS2 fusion in lipoblastoma with masked 8q intrachromosomal rearrangement. Cancer Genet Cytogenet 156(2):183–184. https://doi.org/10.1016/j.cancergencyto.2004.04.017. (Epub 12 Jan 2005)
Deen M, Ebrahim S, Schloff D, Mohamed AN (2013) A novel PLAG1-RAD51L1 gene fusion resulting from a t(8;14)(q12;q24) in a case of lipoblastoma. Cancer Genet 206(6):233–237. https://doi.org/10.1016/j.cancergen.2013.05.019. (Epub 31 July 2013)
Nitta Y, Miyachi M, Tomida A, Sugimoto Y, Nakagawa N, Yoshida H et al (2019) Identification of a novel BOC-PLAG1 fusion gene in a case of lipoblastoma. Biochem Biophys Res Commun 512(1):49–52. https://doi.org/10.1016/j.bbrc.2019.02.154. (Epub 13 March 2019)
Giovannoni I, Barresi S, Rossi S, Stracuzzi A, Argentieri MG, Alaggio R (2021) Pediatric lipoblastoma with a novel EEF1A1-PLAG1 fusion. Genes Chromosomes Cancer 60(7):525–526. https://doi.org/10.1002/gcc.22945. (Epub 10 March 2021)
Meloni-Ehrig AM, Riggott L, Christacos NC, Mowrey PN, Johal J (2009) A case of lipoblastoma with seven copies of chromosome 8. Cancer Genet Cytogenet 190(1):49–51. https://doi.org/10.1016/j.cancergencyto.2008.12.007. (Epub 7 March 2009)
de Saint Aubain Somerhausen N, Coindre JM, Debiec-Rychter M, Delplace J, Sciot R (2008) Lipoblastoma in adolescents and young adults: report of six cases with FISH analysis. Histopathology 52(3):294–298. https://doi.org/10.1111/j.1365-2559.2007.02954.x. (Epub 14 Feb 2008)
Robb A, Rogers T, Nicholls G (2010) A tale of 3 testes? A rare presentation of lipoblastoma with a novel karyotype. J Pediatr Surg 45(1):E29-31. https://doi.org/10.1016/j.jpedsurg.2009.10.093. (Epub 29 Jan 2010)
Suzuki K, Yasuda T, Watanabe K, Hori T, Kanamori M, Kimura T (2017) Myxoid liposarcoma with cartilaginous differentiation showing DDIT3 rearrangement. Oncol Lett 14(6):6789–6794. https://doi.org/10.3892/ol.2017.7056. (Epub 29 Nov 2017)
Gigis I, Gigis P (2012) Fibrolipoma with osseous and cartilaginous metaplasia of Hoffa’s fat pad: a case report. Case Rep Orthop 2012:547963. https://doi.org/10.1155/2012/547963. (Epub 22 Dec 2012)
Dogan R, Kara M, Firat P, Gedikoglu G (2007) An unusual tumor of the neck and mediastinum: lipoblastomatosis resulting in paraparesis. Eur J Cardiothorac Surg 31(2):325–327. https://doi.org/10.1016/j.ejcts.2006.11.012. (Epub 13 Dec 2006)
Debras R, Sciot R, Hompes D, Sinnaeve F, Wafa H (2021) An egg in the leg: case report of an osteochondrolipoma. SICOT-J. https://doi.org/10.1051/sicotj/2021059
Abdul-Ghafar J, Ahmad Z, Tariq MU, Kayani N, Uddin N (2018) Lipoblastoma: a clinicopathologic review of 23 cases from a major tertiary care center plus detailed review of literature. BMC Res Notes 11(1):42. https://doi.org/10.1186/s13104-018-3153-8. (Epub 19 Jan 2018)
Oh KS, Bahmad HF, Stoyanov KV, Amjad IH, Brathwaite C (2023) Recurrent PIK3CA H1047R-mutated congenital infiltrative facial lipomatosis: a case report and review of literature. Curr Issues Mol Biol 45(2):1712–1719. https://doi.org/10.3390/cimb45020110. (Epub 25 Feb 2023)
Sudduth CL, Konczyk DJ, Al-Ibraheemi A, Smits PJ, Greene AK (2021) Lipoblastoma phenotype contains a somatic PIK3CA mutation. Pediatr Dermatol 38(1):299–300. https://doi.org/10.1111/pde.14406. (Epub 10 Oct 2020)
Aman P, Ron D, Mandahl N, Fioretos T, Heim S, Arheden K et al (1992) Rearrangement of the transcription factor gene CHOP in myxoid liposarcomas with t(12;16)(q13;p11). Genes Chromosomes Cancer 5(4):278–285. https://doi.org/10.1002/gcc.2870050403. (Epub 1 Nov 1992)
Zullow HJ, Sankar A, Ingram DR, Same Guerra DD, D’Avino AR, Collings CK et al (2022) The FUS::DDIT3 fusion oncoprotein inhibits BAF complex targeting and activity in myxoid liposarcoma. Mol Cell 82(9):1737–50.e8. https://doi.org/10.1016/j.molcel.2022.03.019. (Epub 8 April 2022)
Aigner T, Neureiter D, Volker U, Belke J, Kirchner T (1998) Epithelial–mesenchymal transdifferentiation and extracellular matrix gene expression in pleomorphic adenomas of the parotid salivary gland. J Pathol 186(2):178–185. https://doi.org/10.1002/(SICI)1096-9896(1998100)186:2<178::AID-PATH161>3.0.CO;2-2(Epub 30 Jan 1999)
Seifert G, Donath K, Schafer R (1999) Lipomatous pleomorphic adenoma of the parotid gland. Classification of lipomatous tissue in salivary glands. Pathol Res Pract 195(4):247–252. https://doi.org/10.1016/S0344-0338(99)80042-9. (Epub 25 May 1999)
Shah SS, Moustafa TZ (2018) An unusual variant of a common palatal salivary gland tumor: case report of a pleomorphic adenoma with significant lipomatous metaplasia. Case Rep Dent 2018:2052347. https://doi.org/10.1155/2018/2052347. (Epub 25 Jan 2019)
Bishop JA, Thompson LDR, Wakely PE, Weinreb I (2021) Tumors of the salivary glands. American Registry of Pathology
Martins C, Fonseca I, Roque L, Pereira T, Ribeiro C, Bullerdiek J et al (2005) PLAG1 gene alterations in salivary gland pleomorphic adenoma and carcinoma ex-pleomorphic adenoma: a combined study using chromosome banding, in situ hybridization and immunocytochemistry. Mod Pathol 18(8):1048–1055. https://doi.org/10.1038/modpathol.3800386
Al-Ibraheemi A, Martinez A, Weiss SW, Kozakewich HP, Perez-Atayde AR, Tran H et al (2017) Fibrous hamartoma of infancy: a clinicopathologic study of 145 cases, including 2 with sarcomatous features. Mod Pathol 30(4):474–485. https://doi.org/10.1038/modpathol.2016.215
Fetsch JF, Miettinen M, Laskin WB, Michal M, Enzinger FM (2000) A clinicopathologic study of 45 pediatric soft tissue tumors with an admixture of adipose tissue and fibroblastic elements, and a proposal for classification as lipofibromatosis. Am J Surg Pathol 24(11):1491–1500. https://doi.org/10.1097/00000478-200011000-00004
Funding
No funding obtained.
Author information
Authors and Affiliations
Contributions
ZA conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript; AA-I reviewed the pathology slides, revised the manuscript, created the figures, and critically reviewed the manuscript for important intellectual content; all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest to disclose.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
N/A.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aldawood, Z., Al-Ibraheemi, A. Lipoblastoma Arising in the Head and Neck: A Clinicopathologic Analysis of 20 Cases. Head and Neck Pathol 17, 768–774 (2023). https://doi.org/10.1007/s12105-023-01575-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-023-01575-5