Abstract
Background
Secretory myoepithelial carcinomas (SMCA) are rare, mucinous, signet ring predominant tumors with primitive myoepithelial features. While many mucinous salivary gland tumors have now been molecularly characterized, key drivers in SMCA have yet to be elucidated. Recently, NKX3.1, a homeodomain transcription factor implicated in salivary mucous acinar development was also shown in a subset of salivary mucinous neoplasms, salivary intraductal papillary mucinous neoplasms (SG-IPMN). To date, NKX3.1 expression has not been characterized in other mucinous salivary lesions. Here, we report molecular and extended immunophenotypic findings in SMCA and NKX3.1 expression in the context of other head and neck lesions.
Methods
We retrieved 4 previously reported SMCA, performed additional immunohistochemical and targeted next-generation sequencing (NGS). We also investigated the use of NKX3.1 as a marker for SMCA in the context of its prevalence and extent (using H-score) in a mixed cohort of retrospectively and prospectively tested head and neck lesions (n = 223) and non-neoplastic tissues (n = 66).
Results
NKX3.1 positivity was confirmed in normal mucous acini as well as in mucous acinar class of lesions (5/6, mean H-score: 136.7), including mucinous adenocarcinomas (3/4), SG-IPMN (1/1), and microsecretory adenocarcinoma (MSA) (1/1). All SMCA were positive. Fluorescence in situ hybridization for SS18 rearrangements were negative in all successfully tested cases (0/3). NGS was successful in two cases (cases 3 and 4). Case 3 demonstrated a PTEN c.655C>T p.Q219* mutation and a SEC16A::NOTCH1 fusion while case 4 (clinically aggressive) showed a PTEN c.1026+1G>A p.K342 splice site variant, aTP53 c.524G>A p.R175H mutation and a higher tumor mutation burden (29 per Mb). PTEN immunohistochemical loss was confirmed in both cases and a subset of tumor cells showed strong (extreme) staining for P53 in Case 4.
Conclusion
Despite a partial myoepithelial phenotype, SMCA, along with mucinous adenocarcinomas/SG-IPMN and MSA, provisionally constitute a mucous acinar class of tumors based on morphology and NKX3.1 expression. Like salivary mucinous adenocarcinomas/SG-IPMN, SMCA also show alterations of the PTEN/PI3K/AKT pathway and may show progressive molecular alterations. We document the first extramammary tumor with a SEC16A::NOTCH1 fusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mucin-producing salivary gland tumors are biologically diverse and now consist of numerous entities, extending well beyond mucoepidermoid carcinoma, the prototype for this category. Aside from variant morphologies of more common tumor types (e.g. salivary duct carcinoma [1,2,3,4], secretory carcinoma [5, 6]) that may show prominent mucin production, there are several newer entities that are now known to show characteristic molecular alterations (e.g., MEF2C::SS18 and SS18::ZBTZ7A fusions in microsecretory adenocarcinoma (MSA) [7] and microcribriform adenocarcinoma [8], respectively; and AKT mutations in mucinous adenocarcinomas [9, 10], and so-called salivary gland “intraductal papillary mucinous neoplasms” (SG-IPMN) [11]).
Secretory myoepithelial carcinoma (SMCA), also known as mucin-producing signet ring adenocarcinoma [12] and mucinous myoepithelioma [13], is a rare and nebulous entity with a primitive myoepithelial phenotype. It is characterized by a signet ring cell predominant mucin producing tumor arranged in cords and strands showing only partial myoepithelial marker expression, and rudimentary ultrastructural characteristics supportive of myoepithelial differentiation [14]. The molecular characteristics of SMCA were previously restricted to documentation of negative findings, namely the absence of fusions in EWSR1, ETV6, and ALK by fluorescence in situ hybridization (FISH), as our group has previously done [14]. With the availability of next-generation sequencing (NGS), SMCA then becomes an attractive target for an enhanced molecular characterization.
Another point of interest is the status of NKX3.1 expression as a potential phenotypic classifier for SMCA. NKX3.1 is an androgen regulated homeobox gene located on chromosome 8 in humans. It is most commonly known for its role in prostate development and as an immunohistochemical marker to indicate prostate origin. Interestingly, murine models have also shown a parallel role for NKX3.1 in minor salivary gland development and document constitutive expression in mucous cells in acini and ducts [15]. A decade later, NKX3.1 immunoexpression was confirmed in human salivary gland mucous acini [16]. Given the tie to androgen regulation, NKX3.1 was also subsequently queried and noted in a subset of salivary duct carcinomas [17, 18].
We had been aware of some of these concepts informally, and had evaluated a variety of head and neck tumors prospectively as part of clinical care for the past 8 years. Through this experience we did encounter a small subset of mucinous tumors that was strongly NKX3.1 positive, to the level seen in normal mucous acini, suggesting recapitulation of this mucous acinar phenotype, which to date has not been extensively explored in mucinous salivary tumors, including SMCA. Indeed, Nakaguro et al. [11] recently confirmed strong and diffuse expression in salivary gland intraductal papillary mucinous neoplasms (SG-IPMN) suggesting validity to this concept. We herein survey SMCA for NKX3.1 within the framework of our broader experience with this marker in head and neck, as well as providing extended molecular characterization of these tumors by targeted NGS.
Materials and Methods
This study was approved by the Institutional Review Board (IRB) through the University of Pittsburgh Human Research Protection Office (STUDY22010004).
SMCA Case Selection
Four previously reported cases (University of Pittsburgh Medical Center, Pittsburgh PA, 1983–2009) were retrieved [14]. One additional case was identified between 2009 and 2022, but could not be included as slides and blocks were not available for review. Clinical outcomes were updated as available.
NKX3.1 Case Framework
Overall, 254 head and neck cases were tested (2015–2022) for NKX3.1. 138 constituted an initial retrospective survey of cases (107 on previously characterized tissue microarrays [19, 20], and 31 whole sections), while the remainder (n = 116) were prospectively accrued as part of routine clinical work-up. Key clinical utilization patterns were as follows:
-
(1)
To evaluate for the possibility of metastasis from prostate (adenocarcinoma and neuroendocrine carcinoma).
-
(2)
To query androgen driven/ apocrine phenotype in salivary tumors.
-
(3)
To document infiltration of minor salivary tissue.
-
(4)
To assist in mesenchymal tumor classification.
-
(5)
To suggest mucous acinar phenotype in mucinous tumors.
Specific diagnoses from the diagnostic reports were converted into 55 diagnostic categories. Given the low numbers in most categories, diagnostic categories were combined into 12 diagnostic classes based on morphologic and immunohistochemical characteristics as follows: mucous acinar, salivary biphasic (non-apocrine), mesenchymal/epithelial mesenchymal, apocrine, squamoglandular, squamous, neuroendocrine/round blue cell, non-salivary glandular/non-squamous, non-mucinous monophasic salivary distal/acinar, sinonasal glandular type, and miscellaneous. For classification purposes, mucous acinar and apocrine features took precedence if a given lesion could fit into more than one category (e.g. apocrine epithelial myoepithelial carcinoma or MSA with spindle cell metaplasia).
The 254 cases tested included 238 lesions (including 24 metastases to the head and neck region) and 16 unpaired non-tumor normals. 51 lesions also demonstrated adjacent non-neoplastic tissue thus resulting in 67 non-neoplastic specimens. The immunostains for 15 lesions and 1 associated non-neoplastic were inadequately documented in the report and were unable to be retrieved and thus excluded, leaving 223 lesions and 66 non-neoplastic specimens. Regarding-specific diagnostic categories, salivary duct carcinoma (n = 46), mucoepidermoid carcinoma (n = 29), and adenosquamous carcinoma (n = 20) represented the most frequently tested tumors (Online Resource 1). When collapsing categories into class, apocrine (n = 57) and squamoglandular (n = 56) predominated (Online Resource 2), though non-mucinous monophasic salivary distal/acinar (which included: acinic cell carcinoma, polymorphous adenocarcinoma, non-apocrine intraductal carcinoma, secretory carcinoma, oncocytic carcinoma, and salivary carcinoma NOS), also had a high representation (n = 27). Only 6 cases (4 mucinous adenocarcinomas, 1 SG-IPMN, and 1 MSA) constituted the mucous acinar category.
Immunohistochemistry
The basic immunohistochemical profile of the SMCA is previously described [14]. Additional immunohistochemical stains for p53, PTEN, and Notch intracellular domain 1 (NICD1), NKX3.1 were also performed. Antibody characteristics summarized in Table 1. P53 interpretation was qualitatively categorized based on prior schema [20, 21] into “strong/extreme positivity”, “complete/extreme negativity”, or “non-extreme”, with the extent classified as “subset” or “diffuse”. PTEN was classified as “retained” or with “loss”. Loss was gauged by comparison with adjacent internal controls (i.e., vessels and stroma). NICD1 was evaluated for overexpression with an adenoid cystic carcinoma demonstrating an activating NOTCH1 mutation serving as a positive external control, and endothelial cells of small vessels serving as positive internal control. NKX3.1 was evaluated for intensity (scale: 0–3) and proportion of cells staining (0–100, rounded to nearest percentile from 1 to 10%, and 10th percentile above 10%) and qualitatively assessed for distribution of staining (e.g. diffuse, random, luminal, mucinous components). H-scores were generated as previously described (scale: 0–300) [22]. Prostatic adenocarcinoma was used as the positive external control, but 3+ intensity was preferentially calibrated with normal mucous acini internal controls. Scores were dichotomized into positive or negative based on visual binning (SPSS software ver.28.0.1.1, SPSS, Chicago, IL) resulting in an H-score cut-off = 15.
SS18 FISH
Dual color breakapart FISH for SS18 rearrangements (3′ orange and 5′ green, Abbott, Abbott Park, Illinois) using a Leica Biosystems (CytoVision FISH Capture and Analysis Workstation, Buffalo Grove, IL, USA). In all cases, at least 60 nonoverlapping nuclei were included in the analysis. The translocation was denoted by a combination of separate/split orange and green signals and 1 intact SS18, indicated by juxtaposed orange and green probes and resulting in a yellow signal. Cases that showed disruption of SS18 in > 10% of tumor cells were considered positive for a translocation.
NGS
NGS was performed using a targeted Oncomine™ Comprehensive Assay v3 using an Ion Torrent™ next-generation sequencing (NGS) platform (both ThermoFisher Scientific, Waltham, MA, USA) according to manufacturer’s instructions. A detailed list of genes tested is available online (https://mgp.upmc.com/Home/Print/Oncomine). This panel evaluates 161 cancer relevant driver genes to include 760 fusion genes. Briefly, total DNA and mRNA that is reverse transcribed into cDNA are subjected to multiplex PCR to amplify the regions of interest. Amplicons were barcoded, ligated with specific adapters, and purified. RNA library quantity and quality check were performed using the 4200 TapeStation (Agilent Technologies, Santa Clara, CA). The Ion Chef was used to prepare and enrich templates and enable testing via Ion Sphere Particles on a semiconductor chip. Massive parallel sequencing was carried out on an Ion GeneStudio S5 Prime System according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA) and data were analyzed with variant explorer (UPMC) for SNV, insertions, deletions, copy number alterations, and RNA fusion genes. The limit of detection of this DNA/RNA assay was 3–5% allelic frequency, roughly translating to 10–20% neoplastic cellularity.
Statistical Analysis
All statistical analyses were performed using SPSS software, version 28.0.1.1 (SPSS, Chicago, IL). Proportions of H-score positive cases were compared across classes using the Fisher–Freeman–Halton Exact test. Normality of H-score distribution was evaluated both by graphical methods (Q–Q plot, Histograms) and by Kolmogorov–Smirnov test, skewness, and kurtosis. Based on these findings, the Kruskal–Wallis test was applied to calculate differences in rank-sum across classes. For this, Monte Carlo Estimate (based on 10,000 samples with starting seed 2,000,000) was applied for the p value. All p values < 0.05 were considered significant.
Results
Recap of Clinicopathologic Features of SMCA
Clinical and demographic features as previously described [14] are reiterated in Table 2. Case 4 was updated with additional details on follow-up and nodal status. The mean patient age was 56.3 years (range 18–81 years). There was a male predilection of 3:1. All but one cases were of minor salivary gland origin; case 2 was in the left deep parotid lobe. A prior history of repeated trauma was noted in case 1, while a history of irradiation for dermatophytosis was noted in case 3. The mean tumor size was 2.7 cm (n = 3; range 1.5–4.5 cm). Only in case 4 were bilateral neck dissections performed, demonstrating node positivity in 10/78 lymph nodes, including extranodal extension on the right side. Cases were otherwise resected with negative margins. Three out of four patients showed no evidence of disease with a mean follow-up of 38.3 months (range 24–52 months). However, the patient in case 4 died 7 months after resection.
Histologically, all cases showed a proliferation of univacuolated/signet ring cells arranged in cords, trabeculae and occasional pseudocribriform patterns embedded in a virtually a cellular mucin rich stroma with variable fibrous septation. As noted previously all cases showed some immunophenotypic and/or ultrastructural evidence of myoepithelial differentiation [14].
Revisiting NKX3.1 Expression in Non-neoplastic Tissues
Non-neoplastic specimens included parotid (24), submandibular gland (6), sublingual gland (1), minor salivary gland (15), upper aerodigestive tract seromucinous glands (11), ectopic salivary tissue (2), and other (7), which included upper aerodigestive tract respiratory surface epithelium, and thyroglossal duct remnants. Three basic patterns of expression were noted. The most notable pattern as previously described [11, 16], was the expression in mucous acini which was confirmed to be diffuse and strong regardless of location (Fig. 1A). Surprisingly, respiratory type goblet cells in the ciliated pseudostratified columnar epithelium of the upper aerodigestive tract were also positive, though more variably so with weak to moderate intensity (Fig. 1B). Both these findings were not infrequently applied to confirm infiltration (Fig. 1C) or colonization. In a small proportion (8/66, 12.1%) of normal non-neoplastic salivary tissues, non-mucinous intercalated ducts transitioning to acini showed weak staining, even in parotid tissue (Fig. 1D).
Distribution of NKX3.1 expression in normal tissues. A As previously described NKX3.1 shows strong and diffuse staining of mucous acini in salivary and seromucinous gland tissues (H&E, 10×; Inset: NKX3.1, 20×). B Interestingly NKX3.1 was variably but consistently positive in respiratory epithelial goblet cells in the upper aerodigestive tract (H&E, 10×; Inset: NKX3.1, 20×). C The strong NKX3.1 reactivity in mucous acini facilitates documentation of infiltration by this polymorphous adenocarcinoma ((H&E, 10×; Inset: NKX3.1, 20×; arrows highlight tumor nests surrounding a mucous acinus). D A small proportion of normal intercalated ducts transitioning to acini in parotid tissue, showed weak staining (H&E, 10×; Inset: NKX3.1, 20×, arrows showing positive intercalated ducts)
NKX3.1 Distribution in Lesions
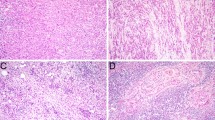
The proportion of positive cases, and mean H-score by class is summarized in Table 3 along with select specific categories (proportion of positive cases and mean H-score for all categories is presented in Online Resource 3). As predicted, the mucous acinar class showed the highest proportion (5/6, 83.3%) of tumors with NKX3.1 positivity (3/4 mucinous adenocarcinomas, 1/1 SG-IPMN, and 1/1 MSA). Additionally, this class demonstrated the highest mean H-score of 136.7 (range 0–300) (Fig. 2A–C). The other notable classes with a relatively high proportion of positivity were the mesenchymal/epithelial-mesenchymal class and the sinonasal type (3/8, 37.5%). The mesenchymal/epithelial-mesenchymal positivity was attributed solely to mesenchymal chondrosarcoma (3/3, 100%, Fig. 3A). Sinonasal type tumors with NKX3.1 expression consisted of seromucinous/non-intestinal type adenocarcinomas, with the positive tumors tending to be more mucinous (Fig. 3B). The apocrine class on the other hand had a lower prevalence of NKX3.1 positivity (5/57, 8.8%), with salivary duct carcinoma itself also showed an even lower prevalence of positivity (2/46, 4.3%) (Fig. 3C). Interestingly, other apocrine lesions (3/11, 27.2%), often lower grade, had a higher prevalence of NKX3.1 positivity. Staining distribution was haphazard or random within apocrine components. Squamoglandular lesions showed a slightly higher proportion of NKX3.1 positive cases (6/56, 10.7%), though the mean H-score of 6.9 (range 0–160) was on the lower end. Expressivity was restricted to mucous cell components for the most part. Adenosquamous carcinomas (3/20, 15.0%) and mucoepidermoid carcinomas (2/29, 6.9%) tended to be negative (Fig. 3D). Aside from the pure squamous class and neuroendocrine/round blue cell tumor class of lesions, which were uniformly negative, the other classes did show a small fraction with low levels of NKX3.1 expression. Only one metastatic prostatic adenocarcinoma was encountered, but it was diffusely, strongly positive (H-score = 300).
NKX3.1 expression in other lesional classes. A Mesenchymal chondrosarcoma consistently expressed NKX3.1 (H&E, 10×; Inset: NKX3.1, 20×). B A subset of sinonasal non-intestinal type adenocarcinomas also showed NKX3.1 positivity. The depicted case is on par with the mucous acinar class ((H&E, 10×; Inset: NKX3.1, 20×). C Apocrine lesions such as salivary duct carcinoma were only rarely positive in this series (H&E, 10×; Inset: NKX3.1, 20×). D Squamoglandular lesions such as this mucoepidermoid carcinoma metastasis tended to be negative (H&E, 10×; Inset: NKX3.1, 20×)
NKX3.1 Expression and Molecular Findings in SMCA
New immunohistochemical and molecular findings are summarized in Table 4. All four SMCA showed NKX3.1 staining (mean H-score: 217.5, range 140–300), on par with that of the mucous acinar class established above. One case (case 2) failed to hybridize for FISH testing, but the remaining three were negative for SS18 translocations. Only cases 3 (Fig. 4A) and 4 (Fig. 5A) showed adequate quality for NGS. Both these cases also showed the best immunohistochemical viability as reflected by the highest H-scores for NKX3.1 expression (Figs. 4B, 5B). Both cases demonstrated PTEN mutations (case 3: c.655C>T p.Q219; case 4: c.1026+1G>A p.K342 splice site variant) and were confirmed to show loss of PTEN expression (Figs. 4C, 5C). Case 3 demonstrated a SEC16A::NOTCH1 fusion with respective exon breakpoints at exon 1 for SEC16A and exon 27 for NOTCH1. However, this case did not overexpress NICD1 (Fig. 4D). Case 4 demonstrated a higher tumor mutation burden than Case 3, and also showed a TP53 mutation (c.524G>A p.R175H) (c. / p. annotation). Correlative p53 immunoexpression showed a strong/extreme pattern in a subset (~ 10%) of tumor cells (Fig. 5D).
SMCA case 3. A The tumor is composed of mucin rich signet ring cells in a mucinous background, and demonstrates a solid to corded infiltrative border permeating normal minor salivary tissue (bottom right, H&E, 10×). As with other tested tumors SS18 break apart fluorescence in situ hybridization was negative (inset). B This tumor shows diffuse strong NKX3.1 positivity (14×). The tumor demonstrated a PTEN c.655C>T; p.Q219* mutation and C PTEN immunohistochemical loss (20×). While the tumor did demonstrate a SEC16A::NOTCH1 fusion, NOTCH1 activation was unable to be confirmed by NICD1 immunohistochemistry (20×, arrow highlights internal endothelial cell control; inset: adenoid cystic carcinoma with known activating NOTCH1 mutation, 20×)
SMCA case 4. A This tumor demonstrates similar cytonuclear features and stroma but also shows pseudoglandular cribriform spaces (H&E, 10×). B This tumor was also NKX3.1 diffusely strongly positive (14×). This tumor showed a PTEN c.1026+1G>A; p.K342 splice variant and C also showed PTEN immunohistochemical loss (20×). Additionally, the tumor also had a TP53 c.524G>A; p.R175H mutation and D showed a subset of tumor cells with strong/extreme positivity (20×)
Discussion
While admittedly a “refurbished” cohort, the rarity of SMCA justifies re-examination using techniques and concepts that had since evolved following our initial publication [14]. As noted previously, the tumor shows a mucinous appearance with an incomplete or primitive myoepithelial phenotype. However, key questions remain unanswered:
-
(1)
Is there a distinctive “normal” phenotypic correlate for SMCA?
-
(2)
With the formalization of mucinous adenocarcinoma, Ref. [9] does SMCA fit on this spectrum?
NKX3.1 as a Mucous Acinar Differentiation Marker
With the recognition of NKX3.1 expression as a requisite for mucinous salivary gland development, Ref. [15] and its consistent immunoexpression in mucous acini, [11, 16] the first question can be addressed. And if the criteria for designating a salivary or seromucinous tumor as “mucous acinar type” are simply a highly mucinous morphology and strong, consistent NKX3.1 expression, then the answer for SMCA is a resounding ‘yes’. However, to do this concept justice, one approach would be to examine NKX3.1 across a variety of lesions.
Initially not a focal point of our study, we document the spectrum of NKX3.1 expression in head and neck lesions over an 8-year period, anticipating the eventuality of a mucous acinar phenotype in a subset of lesions. The prospective and clinically applied nature of over half the tested cases, as well as the bias of the retrospective “arm” of this study towards salivary duct carcinoma and mucoepidermoid carcinoma TMAs limits the balance in representation of head and neck lesions, but nonetheless points to a class with mucous acinar phenotype showing shared mucinous morphology and significantly more frequent and prominent NKX3.1 expression. This group provisionally consists of mucinous adenocarcinoma, so-called SG-IPMN (verified recently [11]), MSA, and now, SMCA.
We did confirm the viability of NKX3.1 as a marker of mucous acinar differentiation, given the consistent strong and diffuse expression of NKX3.1 in non-neoplastic mucous acini (often stronger and more consistent than our prostatic adenocarcinoma control). More practically, we demonstrate how this finding can be leveraged to confirm/document infiltration. But we also noted an unanticipated variable expression in respiratory epithelial goblet cells and occasional weak expression in intercalated ducts transitioning to acini (regardless of whether they were serous or mucous acini). Given these findings, it is thus not surprising that a small fraction of other tumor types shows some, albeit weaker and more focal, NKX3.1 positivity.
We also noted, however, that it is an inconsistent weak determinant of androgen receptor positive apocrine phenotype, only being variably expressed in lesions such as salivary duct carcinoma and apocrine epithelial myoepithelial carcinoma. The 4.3% prevalence in salivary duct carcinoma noted here is considerably lower than that previously described (13–36%) [17, 18]. One caveat is that the bulk of the salivary duct carcinomas tested were represented by TMA cores rather than whole sections. To that point, other apocrine lesions (e.g. apocrine epithelial myoepithelial carcinoma) that were represent by whole sections showed a higher proportion of positivity (27.1%).
One slight surprise was the sinonasal class, specifically non-intestinal type adenocarcinomas. The understanding now is that a significant proportion (if not most) of these are in fact seromucinous type adenocarcinomas whose classification is slowly evolving [23,24,25]. While only 8 cases were queried here, over a third shows NKX3.1 expression, with one case (shown in Fig. 3B) showing staining equivalent to that of the mucous acinar class of salivary gland lesions. This offers yet another line of evidence supporting the term seromucinous adenocarcinoma over non-intestinal type adenocarcinoma.
Tangential to our objectives but documented here nonetheless is the utility of NKX3.1 in the diagnosis of mesenchymal chondrosarcoma [26, 27]. Other spindle cell lesions of the head and neck were negative, though it must be noted that while our one case of MSA that was NKX3.1 positive did have spindle cell change (featured in Bishop et al. [28], seen prior to this report by us for SS18 FISH testing), its mucous acinar phenotype took precedence in terms of classification.
SMCA in the Spectrum of Mucous Acinar Type Lesions
Based on morphology and NKX3.1 expression, SMCA can also be considered a mucous acinar-type tumor with myoepithelial features. Its relationship to other entities in this provisional group then remains a question of relevance here. Ultimately the limited number of cases overall, and even more limited molecular data precludes a conclusive statement on this, however, we document the presence of PTEN alterations in both cases for which NGS was feasible. Interestingly, Nakaguro et al. [11] show one case of (sic) SG-IPMN with a PTEN p.G36Dfs*18 frameshift deletion. Aside from this, the bulk of SG-IPMN and mucinous adenocarcinomas of salivary gland show AKT1 p.E17K mutations [9,10,11] thus indicative of shared alterations of the PTEN/PI3K/AKT pathway. Whether this is enough to justify placement of SMCA on the same spectrum as these other lesions remains to be seen.
Case 4 was notable for its aggressiveness from a histologic, clinical, and molecular standpoint. This tumor showed a TP53 mutation and a higher tumor mutation burden. Of interest, cases labeled in the literature as mucinous adenocarcinoma do appear to have a higher prevalence of TP53 mutations, suggesting that they are indeed high-grade counterparts to so-called SG-IPMN (which likely should be designated as low-grade mucinous adenocarcinomas at this point given their NKX3.1 positive mucous acinar rather than ductal phenotype and lack of conclusive morphologic evidence for an intraductal origin).
Case 3 showed a novel SEC16A::NOTCH1 fusion which represents the first extramammary occurrence of this translocation, to our knowledge. To date, this fusion has only been recognized in a small subset (~ 2%) of triple negative breast carcinomas [29,30,31]. Based on cell lines and xenografts, the fusion is expected to result in high levels of NOTCH1 activation as manifested by increased NICD1 overexpression. However, on the tissue sections stained here with adequate controls, we were unable to verify this. Interest in NOTCH1 activation in salivary gland tumors centers on adenoid cystic carcinoma (ACC), for which 12–15% show activating NOTCH1 mutations [32], and up to a half show NICD1 overexpression [33]. Recently, NOTCH1 inhibitors have shown response in anecdotal reports of patients with ACC as well as in patients enrolled in clinical trials [34], The presence of a SEC16A::NOTCH1 fusion in case 3 raises a brief consideration for a relationship between ACC and SMCA, particularly since a rare signet ring variant of ACC has been described [35]. However, based on current findings, this would be a tenuous association at best. Unlike SMCA, ACC has prototypical angulated hyperchromatic nuclei features and is a biphasic tumor with distinct arrangements of ductal and myoepithelial cells. Furthermore, while follow-up is limited, only case 4 showed histologic and clinical aggression within the range seen in ACC. Finally, our NGS panel does cover MYB::NFIB, and MYBL1::NFIB fusions as well as NOTCH1 mutations commonly seen in ACC; these were all negative.
In conclusion, we establish, based on morphologic features and a prospectively established framework of NKX3.1 expression in head and neck lesions and normal tissues, that SMCA along with other select diagnostic categories can be justifiably placed in a mucous acinar category. Two cases studies demonstrated PTEN gene mutations suggesting commonality with salivary mucinous adenocarcinomas and so-called SG-IPMN, which also have alterations in the PTEN/PI3K/AKT pathway. One aggressive case also showed a higher tumor mutation burden and TP53 mutation, and we also, document the first extramammary occurrence of SEC16A::NOTCH1 fusion in another case, though the significance here is unclear since we were unable to verify NOTCH1 activation by NICD1 immunohistochemistry.
Data Availability
Not Applicable.
Code Availability
Not Applicable.
References
Williams L, Thompson LD, Seethala RR, Weinreb I, Assaad AM, Tuluc M et al (2015) Salivary duct carcinoma: the predominance of apocrine morphology, prevalence of histologic variants, and androgen receptor expression. Am J Surg Pathol 39(5):705–713
Kusafuka K, Maeda M, Honda M, Nakajima T (2012) Mucin-rich salivary duct carcinoma with signet-ring cell feature ex pleomorphic adenoma of the submandibular gland: a case report of an unusual histology with immunohistochemical analysis and review of the literature. Med Mol Morphol 45(1):45–52
Yakirevich E, Sabo E, Klorin G, Alos L, Cardesa A, Ellis GL et al (2010) Primary mucin-producing tumours of the salivary glands: a clinicopathological and morphometric study. Histopathology 57(3):395–409
Simpson RH, Prasad AR, Lewis JE, Skalova A, David L (2003) Mucin-rich variant of salivary duct carcinoma: a clinicopathologic and immunohistochemical study of four cases. Am J Surg Pathol 27(8):1070–1079
Seethala RR, Chiosea SI (2016) MAML2 status in mucoepidermoid carcinoma can no longer be considered a prognostic marker. Am J Surg Pathol 40(8):1151–1153
Petersson F, Michal M, Kazakov DV, Grossmann P, Michal M (2016) A new hitherto unreported histopathologic manifestation of mammary analogue secretory carcinoma: “masked MASC” associated with low-grade mucinous adenocarcinoma and low-grade in situ carcinoma components. Appl Immunohistochem Mol Morphol 24(9):e80–e85
Bishop JA, Weinreb I, Swanson D, Westra WH, Qureshi HS, Sciubba J et al (2019) Microsecretory adenocarcinoma: a novel salivary gland tumor characterized by a recurrent MEF2C-SS18 fusion. Am J Surg Pathol 43(8):1023–1032
Weinreb I, Hahn E, Dickson BC, Rooper LM, Rupp NJ, Freiberger SN et al (2022) Microcribriform adenocarcinoma of salivary glands: a unique tumor entity characterized by an SS18::ZBTB7A fusion. Am J Surg Pathol. https://doi.org/10.1097/PAS.0000000000001980
Rooper LM, Argyris PP, Thompson LDR, Gagan J, Westra WH, Jordan RC et al (2021) Salivary mucinous adenocarcinoma is a histologically diverse single entity with recurrent AKT1 E17K mutations: clinicopathologic and molecular characterization with proposal for a unified classification. Am J Surg Pathol 45(10):1337–1347
Agaimy A, Mueller SK, Bumm K, Iro H, Moskalev EA, Hartmann A et al (2018) Intraductal papillary mucinous neoplasms of minor salivary glands with AKT1 p.Glu17Lys mutation. Am J Surg Pathol 42(8):1076–1082
Nakaguro M, Sadow PM, Hu R, Hattori H, Kuwabara K, Tsuzuki T et al (2022) NKX3.1 Expression in salivary gland “intraductal” papillary mucinous neoplasm: a low-grade subtype of salivary gland mucinous adenocarcinoma. Head Neck Pathol 16:1114
Ghannoum JE, Freedman PD (2004) Signet-ring cell (mucin-producing) adenocarcinomas of minor salivary glands. Am J Surg Pathol 28(1):89–93
Gnepp DR (2013) Mucinous myoepithelioma, a recently described new myoepithelioma variant. Head Neck Pathol 7(Suppl 1):S85–S89
Bastaki JM, Purgina BM, Dacic S, Seethala RR (2014) Secretory myoepithelial carcinoma: a histologic and molecular survey and a proposed nomenclature for mucin producing signet ring tumors. Head Neck Pathol 8(3):250–260
Schneider A, Brand T, Zweigerdt R, Arnold H (2000) Targeted disruption of the Nkx3.1 gene in mice results in morphogenetic defects of minor salivary glands: parallels to glandular duct morphogenesis in prostate. Mech Dev 95(1–2):163–174
Gurel B, Ali TZ, Montgomery EA, Begum S, Hicks J, Goggins M et al (2010) NKX3.1 as a marker of prostatic origin in metastatic tumors. Am J Surg Pathol 34(8):1097–1105
Yang RK, Zhao P, Lu C, Luo J, Hu R (2019) Expression pattern of androgen receptor and AR-V7 in androgen-deprivation therapy-naive salivary duct carcinomas. Hum Pathol 84:173–182
Takada N, Nishida H, Oyama Y, Kusaba T, Kadowaki H, Arakane M et al (2020) Immunohistochemical reactivity of prostate-specific markers for salivary duct carcinoma. Pathobiology 87(1):30–36
Seethala RR, Dacic S, Cieply K, Kelly LM, Nikiforova MN (2010) A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol 34(8):1106–1121
Chiosea SI, Williams L, Griffith CC, Thompson LD, Weinreb I, Bauman JE et al (2015) Molecular characterization of apocrine salivary duct carcinoma. Am J Surg Pathol 39(6):744–752
Boyle DP, McArt DG, Irwin G, Wilhelm-Benartzi CS, Lioe TF, Sebastian E et al (2014) The prognostic significance of the aberrant extremes of p53 immunophenotypes in breast cancer. Histopathology 65(3):340–352
McClelland RA, Finlay P, Walker KJ, Nicholson D, Robertson JF, Blamey RW et al (1990) Automated quantitation of immunocytochemically localized estrogen receptors in human breast cancer. Cancer Res 50(12):3545–3550
Patel S, Snyderman CH, Muller SK, Agaimy A, Seethala RR (2022) Sinonasal mixed transitional epithelial-seromucinous papillary glandular neoplasms with BRAF p.V600E mutations—sinonasal analogues to the sialadenoma papilliferum family tumors. Virchows Arch. 481(4):565–574
Purgina B, Bastaki JM, Duvvuri U, Seethala RR (2015) A subset of sinonasal non-intestinal type adenocarcinomas are truly seromucinous adenocarcinomas: a morphologic and immunophenotypic assessment and description of a novel pitfall. Head Neck Pathol 9(4):436–446
Rooper LM, Thompson LDR, Gagan J, Hwang JSG, London NR, Mikula MW et al (2022) Low-grade non-intestinal-type sinonasal adenocarcinoma: a histologically distinctive but molecularly heterogeneous entity. Mod Pathol 35(9):1160–1167
Yoshida KI, Machado I, Motoi T, Parafioriti A, Lacambra M, Ichikawa H et al (2020) NKX3-1 Is a useful immunohistochemical marker of EWSR1-NFATC2 sarcoma and mesenchymal chondrosarcoma. Am J Surg Pathol 44(6):719–728
Yoshida A, Hashimoto T, Ryo E, Yoshida KI, Motoi T, Yatabe Y et al (2021) Confirmation of NKX3-1 expression in EWSR1-NFATC2 sarcoma and mesenchymal chondrosarcoma using monoclonal antibody immunohistochemistry, RT-PCR, and RNA in situ hybridization. Am J Surg Pathol 45(4):578–582
Bishop JA, Sajed DP, Weinreb I, Dickson BC, Bilodeau EA, Agaimy A et al (2021) Microsecretory adenocarcinoma of salivary glands: an expanded series of 24 cases. Head Neck Pathol 15(4):1192–1201
Stoeck A, Lejnine S, Truong A, Pan L, Wang H, Zang C et al (2014) Discovery of biomarkers predictive of GSI response in triple-negative breast cancer and adenoid cystic carcinoma. Cancer Discov 4(10):1154–1167
Robinson DR, Kalyana-Sundaram S, Wu YM, Shankar S, Cao X, Ateeq B et al (2011) Functionally recurrent rearrangements of the MAST kinase and Notch gene families in breast cancer. Nat Med 17(12):1646–1651
Edwards PA, Howarth KD (2012) Are breast cancers driven by fusion genes? Breast Cancer Res 14(2):303
Ho AS, Kannan K, Roy DM, Morris LG, Ganly I, Katabi N et al (2013) The mutational landscape of adenoid cystic carcinoma. Nat Genet 45(7):791–798
Ferrarotto R, Mitani Y, Diao L, Guijarro I, Wang J, Zweidler-McKay P et al (2017) Activating NOTCH1 mutations define a distinct subgroup of patients with adenoid cystic carcinoma who have poor prognosis, propensity to bone and liver metastasis, and potential responsiveness to Notch1 inhibitors. J Clin Oncol 35(3):352–360
de Sousa LG, Jovanovic K, Ferrarotto R (2022) Metastatic adenoid cystic carcinoma: genomic landscape and emerging treatments. Curr Treat Options Oncol 23(8):1135–1150
Altemani A, Costa AF, Montalli V, Mosqueda-Taylor A, de Almeida OP, Leon JE et al (2012) Signet-ring cell change in adenoid cystic carcinoma: a clinicopathologic and immunohistochemical study of four cases. Histopathology 62:531
Funding
Trainee Research Advancement Award (Department of Pathology at University of Pittsburgh, Pittsburgh, PA).
Author information
Authors and Affiliations
Contributions
Conceptualization: SP, RRS; Methodology: AIW, JMB, SIC, ADS, RRS; Formal analysis and investigation: SP, RRS; Writing—original draft preparation: SP; Writing—review and editing: JMB, SIC, ADS, RRS; Funding acquisition: SP, RRS; Resources: AIW, SIC, ADS, RRS; Supervision: RRS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to Participate
This study has obtained IRB approval from (University of Pittsburgh Human Research Protection Office) and the need for informed consent was waived.
Consent for Publication
For this type of study consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Part of this study was awarded a Leon Barnes award as a poster at the USCAP 2022 Annual Meeting in Los Angeles (Abstracts from USCAP 2022: Head and Neck Pathology (833) Mod Pathol 2022; 35: 897). Dr. Seethala is currently a member of the Editorial Board for Head and Neck Pathology Journal.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patel, S., Wald, A.I., Bastaki, J.M. et al. NKX3.1 Expression and Molecular Characterization of Secretory Myoepithelial Carcinoma (SMCA): Advancing the Case for a Salivary Mucous Acinar Phenotype. Head and Neck Pathol 17, 467–478 (2023). https://doi.org/10.1007/s12105-023-01524-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-023-01524-2