Abstract
Benign fibro-osseous lesions (BFOLs) are a particularly challenging set of diagnoses for the pathologist. This diverse collection of diseases includes fibrous dysplasia, ossifying fibroma and cemento-osseous dysplasia. While all three conditions have similar microscopic presentations, their treatment and prognosis differ, demanding an accurate and definitive diagnosis. A practical and systematic approach considering the patient’s history, demographics, intraoperative presentation, and gross appearance with an emphasis on radiology and histology will be discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Benign Fibro-osseous Lesions
Benign fibro-osseous lesions (BFOLs) are a particularly challenging set of diagnoses for the pathologist. The three major forms of BFOLs, fibrous dysplasia (FD), ossifying fibroma (OF) and cemento-osseous dysplasia (COD) are a set of conditions that share many histological features, yet are diverse in their clinical, radiographic, intraoperative, and gross appearances [1,2,3,4,5,6]. Benign fibro-osseous lesions are characterized histologically by a fibrous stroma with various types and amounts of mineralized products [5]. While conveniently classified for the pathologist, the BFOL designation results in a situation in which the pathologist must look beyond the microscope and integrate a host of other factors to establish the most accurate diagnosis. Those who practice pathology of the head and neck, in particular, may find themselves a subject matter expert in the BFOLs as these entities have a predilection for these anatomic locations. Cemento-osseous dysplasia is exclusive to the tooth-bearing areas, while ossifying fibroma is a neoplasm of the craniofacial region. Finally, fibrous dysplasia, while affecting the entire skeletal system, is frequently found in the head and neck.
Given the fact that BFOLs have similar microscopic features, a systematic approach to evaluating such cases is essential to avoid diagnostic pitfalls and ensure the most appropriate diagnoses. The accurate and definitive diagnosis of BFOLs is necessary for proper treatment and an informative prognosis [1,2,3,4,5,6,7]. While it is tempting to apply a dogmatic algorithm or flowchart, the interpretative (if at times subjective) skills of the pathologist are compulsory and cannot be replaced. It is the goal of this brief review to discuss the diagnoses of BFOLs by way of a comprehensive, yet simplified approach. This approach integrates the patient’s history, demographics, intraoperative presentation, and gross appearance with an emphasis on radiology and histology.
Cemento-Osseous Dysplasia
Cemento-osseous dysplasia (COD) is a non-neoplastic change to bone associated with the tooth-bearing areas of the gnathic bones, generally affecting the mandible over the maxilla [8]. It is the most common BFOL of the jaws [9]. It may be under-represented in pathology services as many lesions are clinically and radiographically diagnosed without the need for biopsy. Cemento-osseous dysplasia is further sub-classified into three categories: periapical cemento-osseous dysplasia (Fig. 1), when areas affected are localized to the periapical regions of the mandibular incisors; focal cemento-osseous dysplasia (Fig. 2), when a single tooth is affected, and finally the term florid cemento-osseous dysplasia (Fig. 3) is appropriate when multi-quadrant lesions are identified [9].
Pathogenesis
Cemento-osseous dysplasia is non-neoplastic and likely a reactive process. It is associated with only tooth-bearing areas and possibly originates within the periodontal ligament [9].
Clinical
Cemento-osseous dysplasia is a common finding, characteristically affecting middle-aged black women [9]. Since COD affects the tooth-bearing areas of the jaws exclusively, it may be incidentally detected on routine dental radiographs. Patients (with the exception of florid COD) are asymptomatic. All associated teeth should test vital. Those affected by the florid variant may develop osteomyelitis with bone sequestration [10]. These patients may present with pain, discharge and delayed healing.
Radiographic
Cemento-osseous dysplasia affects the associated periapical bone of any tooth or teeth including edentulous areas. As previously mentioned, the presentation can be focal or multifocal. The margins of COD are well-defined and sclerotic. The shape is mildly irregular but roughly ovoid and centric to the root apex [8, 11]. The internal characteristics of cemento-osseous dysplasia vary along a spectrum and reflect the maturity of the lesion. Characteristically, lesions present with an outer, irregular zone of relative radiolucency and amorphous central radiopacity [12]. Early lesions may be completely radiolucent whereas mature lesions appear predominantly radiopaque. The effects of cemento-osseous dysplasia on the adjacent dentition may include the loss of the normal lamina dura or a widened periodontal ligament space with occasional hypercementosis. Cortical expansion, generally only associated with large lesions, is uncommon and mild. The thin outer cortical bone will always be intact [11]. As previously stated, cemento-osseous dysplasia may be diagnosed based on the radiographic and clinical features alone. When lesions present atypically, however, a biopsy may be considered to confirm the diagnosis. Therefore, the pathologist likely reviews a disproportionate number of cases that are not radiographically classic, and perhaps disproportionately difficult [7].
Intraoperative Findings
Upon reflection of the overlying tissue the lesion may or may not be readily identifiable. The outer cortex may require removal to reveal lesions that are difficult to excise and generally require curettage.
Pathologic Features
Macroscopic
The submitted specimen is usually hemorrhagic, brown, gritty fragments of tissue [7].
Histology
Regardless of the variant, cemento-osseous dysplasia has the same microscopic features. The lesions consist of a fibrous stroma with loose collagen and varying cellularity in association with mineralized tissues [9]. Cemento-osseous dysplasia’s mineralized portion is composed of woven or lamellar bone, osteoid and cementum-like material. As lesions mature they become denser and less cellular. Osteoblastic rimming is uncommon or focal [9]. The tissue is generally vascular contributing to its brown appearance both intraoperatively and macroscopically. The numerous extravasated red blood cells seen microscopically are surgical artifact. Cemento-osseous dysplasia is not encapsulated and biopsies may show little interface with normal bone, in part due to curettage of the lesion. (Fig. 4) Cases of florid COD may show signs of associated osteomyelitis and infection. A simple bone cyst may also be seen in this variant [5, 13,14,15].
Differential Diagnosis
For early presentations of periapical and focal COD, the clinical and radiographic differential diagnosis includes pulpal pathology, either a radicular cyst or a radicular granuloma [16]. These are common diseases of the dentition and may be the first diagnostic consideration in this setting. It is therefore essential that the provider, the dentist in most cases, determines the vitality of the affected tooth. A non-vital tooth generally indicates pulpal pathology and generally, a biopsy is not indicated. Conversely a vital tooth is not consistent pulpal inflammatory disease, and a radiolucent, mixed density or radiopaque lesions of the apex of vital teeth (especially with the appropriate demographics) points to COD. Furthermore, if lesions are found outside the tooth-bearing areas, COD can immediately be excluded. The intraoperative and gross appearance of gritty fragmented hemorrhagic tissue should quickly distinguish COD from fibrous dysplasia and ossifying fibroma. Histologically, COD shares many features with the other BFOLs. However, it is notable that usually COD lacks osteoblastic rimming, or it is found only focally. Distinguishing florid cemento-osseous dysplasia from osteomyelitis may be complicated by their radiographic and clinical similarities, and in fact the two diagnoses may co-exist. Biopsy of the florid variant of COD may indeed show areas of inflammation. In this context, the diagnosis of both COD and secondary osteomyelitis should be considered. As stated previously, due to similarities, all clinical and radiologic information must be reviewed in conjunction with the microscopic findings (Table 1).
Prognosis and Treatment
Periapical and focal cemento-osseous dysplasia do not require treatment. Within the proper clinical setting, the diagnosis of COD may be rendered chairside and documented in the patient record [17]. Routine follow-up is recommended, with dental radiographs showing a maturation of the lesions over time. Florid COD may be an exception. Patients diagnosed with this variant may become symptomatic and develop osteomyelitis. Osteomyelitis can be difficult to manage and may require surgical treatment [17, 18].
Diagnostic Checklist
-
Tooth bearing-areas only, associated teeth should be vital
-
May be diagnosed without biopsy based on clinical and radiographic presentation
-
No treatment required (except in florid cases with osteomyelitis)
Ossifying Fibroma
Ossifying fibroma is a benign bone neoplasm that affects the jaws and bones of the craniofacial region. Ossifying fibroma is divided into three variants: cemento-ossifying fibroma (COF), often referred to as simply “ossifying fibroma”; juvenile trabecular ossifying fibroma (JTOF) and juvenile psammomatoid ossifying fibroma (JPOF). These additional classifications are an attempt to reflect each entities’ different demographics, histologic features and prognosis [9].
Pathogenesis
Ossifying fibromas are neoplastic. Some tumors have shown significant growth potential [9].
Clinical
Cemento-ossifying fibroma is a rare tumor that primarily affects patients in the third to fourth decade of life and has a female predilection (5:1). It overwhelmingly shows preference for the mandible, favoring the molar and pre-molar regions. Smaller tumors are often asymptomatic and may be detected incidentally on routine dental radiographs [5, 7, 19, 20]. As lesions enlarge, facial swelling may become clinically apparent. JTOF and JPOF, less common than conventional COF, generally present in the second decade but can be found in a broad age range [9]. The juvenile variants do not show gender predilection. JPOF overwhelmingly affects the sinuses, with the ethmoid sinus being most prevalent. JTOF favors the gnathic bones and is more common in the maxilla than the mandible [7, 9]. Smaller tumors are usually painless but the juvenile variants are subject to rapid growth and expansion, at times causing dramatic facial asymmetry and disfigurement. Additionally, because the juvenile variants tend to affect the maxilla, sinuses and other craniofacial bones, they may cause signs and symptoms associated with mass effect and involvement of adjacent vital structures including visual changes and sinus dysfunction.
Radiographic
Radiographically, cemento-ossifying fibroma is well-defined and concentric. In comparison to COD, the lesion presents a narrow more uniform, partial or complete, radiolucent border representing soft tissue encapsulation [21, 22]. A reactive front of sclerosis may be present at the interface with the adjacent normal bone, especially in the slower growing COF [23, 24]. The internal density and pattern is variable dependent on the maturity of the lesion and the amount and type of calcification. (Fig. 5) Cemento-ossifying fibroma may displace adjacent teeth or cause root resorption. When cortical expansion is present in COF, the outer cortices usually remain intact. The juvenile variants, which have a more aggressive growth pattern, may present with dehiscence along the expanded outer cortices (Figs. 6, 7). Radiographic evidence of impingement of nearby structures correlating with the patients’ symptoms may be seen. Large tumors may affect the brain [21, 22].
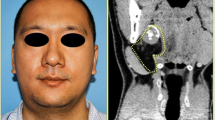
Coronal CT-sinus with bone algorithm. The image demonstrates a well-defined, mixed density lesion within the left anterior maxilla. There is a peripheral radiolucent rim suggesting soft tissue encapsulation. The radiographic features and location are consistent with juvenile trabecular ossifying fibroma (JTOF)
Coronal CT-Sinus with bone algorithm image. The image presents radiographic features similar to Fig. 6. The epicenter of growth is within the left frontal sinus with expansion into the adjacent orbit. The epicenter of growth within the frontal sinus favors juvenile psammomatoid ossifying fibroma (JPOF)
Intraoperative Findings
Ossifying fibromas are readily identified intraoperatively. Tumors are well-demarcated but may infiltrate associated bone. Surgeons often describe the tumors as “shelling out” [7].
Pathologic Features
Macroscopic
Grossly, as tumors may shell out, they may appear as an intact mass. An excisional biopsy may render a rounded mass on the slide, while an incisional biopsy may have an artificial shape such as a wedge. Usually the specimen is adherent, but may be fragmented due to the surgical procedure. The cut surface is yellow-white with a gritty consistency on sectioning [7].
Histology
Cemento-ossifying fibromas consist of variably hypercellular fibrous tissue with mineralized tissue. As suggested by the intraoperative and radiographic appearance, COF is well defined and may be encapsulated. Generally, the juvenile variants are not encapsulated [9]. The mineralized tissue associated with the COF may consist of trabeculae of bone or osteoid with a woven and lamellar pattern. The mineralization may also present as lobulated collections of basophilic cementum-like material with a distinctive “brush boarder” that interfaces with the surrounding stroma. The amount and type of mineralization may vary among tumors or even within the same tumor. Osteoblastic rimming is a feature of COF (Fig. 8). While the stroma may be cellular and hyperchromatic, there is generally no mitosis. In contrast, the stroma of JTOP is cellular with occasional mitotic figures. The mineralized component is a cellular osteoid trabeculae, which is focally mineralized often at the center of these trabeculae [25]. The immature bone formation is characteristic for its lack of osteoblastic rimming. However, collections of osteoclastic giant cells may be seen (Fig. 9). Juvenile psammomatoid ossifying fibroma is perhaps the most recognizable and shows numerous small ossicles that are referred to as psammomatoid bodies (Fig. 10). These small structures may coalesce to form large areas of mineralization [26]. Both juvenile types have had cystic degeneration reported in relation to the tumors [5].
Differential Diagnosis
The differential diagnosis for ossifying fibroma based on imaging studies may be broad. Any mixed density lesion may be considered. Additionally, if little calcification is present, a radiographic differential diagnosis of radiolucent lesions may be considered. Histologically, as expected, the tumor is firmly in the BFOL category. Radiographically and intraoperatively COF’s well demarcated nature will generally exclude FD. Furthermore, if the lesion is outside of the tooth-bearing areas, cemento-osseous dysplasia is not a diagnostic consideration. More diagnostic difficulty may lie in distinguishing ossifying fibromas presenting within tooth-bearing areas. However, the intraoperative characteristic of “shelling out” is very different from cemento-osseous dysplasia, with its gritty fragmented nature. Histologically, COF is characterized by osteoblastic rimming and it is considered a hallmark of the lesion’s microscopic presentation. Osteoblastic rimming, however, notably is not a feature of the juvenile variants. Since JTOF and JTOP affect a wide age range, demographic considerations are only so helpful (Table 1).
Treatment and Prognosis
Ossifying fibromas, regardless of variant, are neoplastic and require treatment. Complete surgical excision is the treatment of choice for all ossifying fibromas. Incomplete removal results in increased recurrences. Those tumors in close approximation to vital structures pose treatment challenges. Large resections, more common in the juvenile variants, may require extensive reconstruction [22].
Diagnostic Checklist
-
Affects the jaws and craniofacial regions
-
Well-defined on radiographs
-
May appear to “shell out” to surgeons
-
Histology for COD shows “brush borders” and osteoblastic rimming
-
Neoplasm that requires surgical excision
Fibrous Dysplasia
Fibrous dysplasia is a skeletal condition in which normal bone is replaced by poorly organized bone and fibrous tissue. The disease may affect a single bone, monostotic fibrous dysplasia, or multiple bones, polyostotic fibrous dysplasia. When multiple adjacent bones of the craniofacial region are affected the term craniofacial fibrous dysplasia is preferred [9]. Polyostotic fibrous dysplasia is associated with a number of syndromes, most prevalent being McCune-Albright syndrome [4,5,6, 9].
Pathogenesis
Fibrous dysplasia is caused by post-zygotic activating missense mutations in the GNAS gene, which encode the alpha subunit of the stimulatory G protein (GS alpha). GS alpha stimulates adenylyl cyclase activity causing over expression of cAMP. This results in cellular property changes of the bone osteoprogenitor cells, leading to abnormal bone formation [5, 9].
Clinical
Fibrous dysplasia is generally diagnosed in patients in their first or second decade of life. There is no gender predilection and those affected often present with the chief complaint of a painless swelling (Fig. 11). Changes in the bone can range from mild expansion, being primarily a cosmetic issue, to large expansions that affect adjacent structures. The bones in the craniofacial region, especially the gnathic bones are among the sites most commonly affected [9]. The maxilla is favored over the mandible with a predominately unilateral presentation.
Radiographic
The density of fibrous dysplasia range along a spectrum that is related to the maturity of the lesions. Immature areas are predominantly radiolucent with more mature lesions appearing more sclerotic [27]. Several common descriptors are widely used to characterize the variable internal structure of the lesion: ground-glass, orange-peel and cotton wool (Fig. 12). The pattern tends to be more uniform and subtle in the maxilla. In mature lesions of fibrous dysplasia cyst-like spaces may be apparent. Importantly, the peripheral margin of fibrous dysplasia is usually indistinct. There is no clear point of demarcation between the adjacent normal bone and the lesion [4,5,6]. The outer cortical bone may be expanded, with significant thinning, but remains intact. If teeth are directly involved in the lesion the associated lamina dura may be lost and the periodontal ligament space may be narrowed. Tooth displacement and root resorption are not common features of fibrous dysplasia [28, 29].
Axial bone algorithm CT-Maxillofacial image. Unilateral fibrous dysplasia (FD) involving the left maxilla. With bilateral comparison the altered trabecular pattern, right to left, is striking and characteristic of altered bone metabolism. The longitudinal pattern of expansion enlarges the left maxilla preserving its general contours
Intraoperative
The area affected may show expansion, however, the bone may not be visibly different from surrounding normal bone (Fig. 13). Surgeons may use a bur or a trephine to obtain the specimen for biopsy.
Pathologic Features
Macroscopic
The submitted specimens may have an artificial shape like a cylindrically shaped core of bone, the result of acquiring the specimen by use of a trephine. Specimens may be cube shaped if a bur is used to obtain the biopsy. The tissue is gray-white with a rubbery and compressible texture. Upon sectioning, the tissue may feel gritty [9].
Histology
Tissue samples show a cellular fibrous stroma composed of fibroblasts and collagen. Within the stroma is a mineralized component of fine, curvilinear trabeculae of woven bone. Fibrous dysplasia of the gnathic bones may show areas of lamellar bone. The boney trabeculae of the lesion merges with the surrounding bone, reflecting the indistinguishable margins described in the corresponding radiographs. The buzz words commonly used for the histologic picture (Fig. 14) of fibrous dysplasia is “alphabet soup” or “Chinese characters.” There is little to no osteoblastic rimming [2,3,4, 9].
Differential Diagnosis
All the other BFOLs must be considered, as previously stated (Table 1). The radiographic appearance is distinctly different from the other BFOLs and should assist in directing the diagnosis to FD. The margins of the lesion being indistinct and the intraoperative appearance of a lesion that is difficult to localize is supportive of fibrous dysplasia. Aside from the other BFOLs, osteosarcoma or osteomyelitis may be considered. Osteosarcoma should generally show some atypia, may invade into the surrounding soft tissue and perforate the cortex, features not consistent with FD. Osteomyelitis should show areas of inflammatory infiltrate.
Prognosis and Treatment
Patients with fibrous dysplasia generally do not require treatment. Surgery is considered for cosmetic reasons and the disease tends to slow after skeletal maturation is complete. On rare occasions FD, when it affects nearby vital structures, can cause significant deformity and become more than a cosmetic issue. The most significant disability being blindness [27]. Cases of FD that cause signs and symptoms may require surgical intervention.
Diagnostic Checklist
-
Generally first diagnosed in the first or second decade
-
No clear demarcation between lesion and surrounding bone
-
“Ground glass” appearance on radiograph
-
Histology shows bone with shapes similar to “alphabet soup or Chinese charcters” and a general lack of osteoblastic rimming
Conclusion
A practical approach to the definitive diagnosis of benign fibro-osseous lesions requires analysis of all the correlating factors in a case. The demographics, clinical history, intraoperative appearance, gross appearance and most importantly the histology and radiology are essential for an accurate diagnosis. The pathologist’s judgement is still paramount to rendering a definitive diagnosis that will result in the appropriate treatment of the condition. A broad diagnosis of benign fibrous osseous-lesion, while at times necessary due to a lack of clinical and radiologic history, may result in the improper treatment and management of the patient. It is essential that the pathologist collaborate, when possible, with the provider and radiologist to attain the complete picture of the patient’s disease process. The goal is to provide the patient with the most accurate and definitive diagnosis to facilitate proper care.
References
Mainville GN, Turgeon DP, Kauzman A. Diagnosis and management of benign fibro-osseous lesions of the jaws: a current review for the dental clinician. Oral Dis. 2017;23(4):440–50.
Waldron CA, Giansanti JS. Benign fibro-osseous lesions of the jaws: a clinical-radiologic-histologic review of sixty-five cases. II. Benign fibro-osseous lesions of periodontal ligament origin. Oral Surg Oral Med Oral Pathol. 1973;35(3):340–50.
Waldron CA, Giansanti JS. Benign fibro-osseous lesions of the jaws: a clinical-radiologic-histologic review of sixty-five cases. Oral Surg Oral Med Oral Pathol. 1973;35(2):190–201.
Abramovitch K, Rice DD. Benign fibro-osseous lesions of the jaws. Dent Clin North Am. 2016;60(1):167–93. https://doi.org/10.1016/j.cden.2015.08.010.
El-Mofty SK. Fibro-osseous lesions of the craniofacial skeleton: an update. Head Neck Pathol. 2014;8(4):432–44.
Eversole R, Su L, ElMofty S. Benign fibro-osseous lesions of the craniofacial complex. A review. Head Neck Pathol. 2008;2(3):177–202.
Brannon RB, Fowler CB. Benign fibro-osseous lesions: a review of current concepts. Adv Anat Pathol. 2001;8(3):126–43.
Pereira DL, Pires FR, Lopes MA, Carlos R, Wright JM, Patel P, van Heerden W, Uys A, Vargas PA. Clinical, demographic, and radiographic analysis of 82 patients affected by florid osseous dysplasia: an international collaborative study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(2):250–7.
Mofty E. WHO classification of head and neck tumours. 4th ed., vol. 9. Lyon: IARC; 2017. p. 251–5.
Cavalcante MB, de Oliveira Lima AL, Júnior MA, Santos MB. Florid cemento-osseous dysplasia simultaneous the chronic suppurative osteomyelitis in mandible. J Craniofac Surg. 2016;27(8):2173–6.
White SC, Pharoah MJ. Oral radiology principles and interpretation. 7th ed. St. Louis: Mosby; 2014. p. 402–426.
Cavalcanti PHP, Nascimento EHL, Pontual MLDA, Pontual ADA, Marcelos PGCL, Perez DEDC, Ramos-Perez FMM. Cemento-osseous dysplasias: imaging features based on cone beam computed tomography scans. Braz Dent J. 2018;29(1):99–104.
Jacomacci WP, Veloso Perdigão JP, Veltrini VC, Farah GJ, Tolentino ES, Vessoni Iwaki LC, Iwaki Filho L. Associated aneurysmal bone cyst and cemento-osseous dysplasia: a case report and review of the literature. Gen Dent. 2017;65(1):28–32.
Fernandes DT, Pereira DL, Santos-Silva AR, Vargas PA, Lopes MA. Florid osseous dysplasia associated with multiple simple bone cysts: a patient with 22 years of follow-up. Gen Dent. 2016;64(2):21–5.
Chadwick JW, Alsufyani NA, Lam EW. Clinical and radiographic features of solitary and cemento-osseous dysplasia-associated simple bone cysts. Dentomaxillofac Radiol. 2011;40(4):230–5.
Resnick CM, Novelline RA. Cemento-osseous dysplasia, a radiological mimic of periapical dental abscess. Emerg Radiol. 2008;15(6):367–74.
Aiuto R, Gucciardino F, Rapetti R, Siervo S, Bianch AE. Management of symptomatic florid cemento-osseous dysplasia: literature review and a case report. J Clin Exp Dent. 2018;10(3):e291–5.
Fenerty S, Shaw W, Verma R, Syed AB, Kuklani R, Yang J, Ali S. Florid cemento-osseous dysplasia: review of an uncommon fibro-osseous lesion of the jaw with important clinical implications. Skeletal Radiol. 2017;46(5):581–90.
Liu JJ, Thompson LD, Janisiewicz AM, Shibuya TY, Keschner DB, Garg R, Lee JT. Ossifying fibroma of the maxilla and sinonasal tract: case series. Allergy Rhinol(Providence). 2017;8(1):32–6.
Eversole LR, Leider AS, Nelson K. Ossifying fibroma: a clinicopathologic study of sixty-four cases. Oral Surg Oral Med Oral Pathol. 1985;60(5):505–11.
Owosho AA, Hughes MA, Prasad JL, Potluri A, Bransletter B. Psammomatoid and trabecular juvenile ossifying fibroma: two distinct radiologic entities. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118(6):732–6.
Han J, Hu l, Zhang C, Tian Z, Wang Y, Zhu L, Yang C, Sun J, Zhang C, Li J, Xu L. Juvenile ossifying fibroma of the jaw: a retrospective study of 15 cases. Int J Oral Maxillofac Surg. 2016;45(3):368–76.
Eversole LR, Merrell PW, Strub D. Radiographic characteristics of central ossifying fibroma. Oral Surg Oral Med Oral Pathol. 1985 May;59(5):522–7.
Kawaguchi M, Kato H, Miyazaki T, Kato K, Hatakeyama D, Mizuta K, Aoki M, Matsuo M. CT and MR imaging characteristics of histological subtypes of head and neck ossifying fibroma. Dentomaxillofac Radiol. 2018;47(6):20180085.
Sultan AS, Schwartz MK, Caccamese JF Jr, Papadimitriou JC, Basile J, Foss RD, Younis RH. Juvenile trabecular ossifying fibroma. Head Neck Pathol. 2017. https://doi.org/10.1007/s12105-017-0862-6.
Rao S, Nandeesh BN, Arivazhagan A, Moiyadi AV, Yasha TC. Psammomatoid juvenile ossifying fibroma: report of three cases with a review of literature. J Pediatr Neurosci. 2017;12(4):363–6.
Gupta D, Garg P, Mittal A. Computed tomography in craniofacial fibrous dysplasia: a case series with review of literature and classification update.Open Dent J. 2017;11:384–403.
Unal Erzurumlu Z, Celenk P, Bulut E, Barıs YS. ct imaging of craniofacial fibrous dysplasia. Case Rep Dent. 2015;2015:134123.
MacDonald-Jankowski D. Fibrous dysplasia: a systematic review. Dentomaxillofac Radiol. 2009;38(4):196–215.
Funding
This study has no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Brenda L. Nelson declares that she has no conflict of interest. Billy J. Philips declares that he has no conflict of interest.
Research Involving Human Participants and/or Animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Disclaimer
The views expressed herein are our own and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. Government.
Rights and permissions
About this article
Cite this article
Nelson, B.L., Phillips, B.J. Benign Fibro-Osseous Lesions of the Head and Neck. Head and Neck Pathol 13, 466–475 (2019). https://doi.org/10.1007/s12105-018-0992-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-018-0992-5