Abstract
Human papillomavirus (HPV)-related head and neck carcinoma (HNC) represents an important subgroup of head and neck cancer that is characterized by a consistent microscopic appearance and a favorable prognosis. A growing experience with HPV testing, however, has uncovered variants that deviate from the prototypic HPV-HNC with respect to morphology. While these HPV-HNCs may deviate morphologically from the prototype, they do not appear to stray far from the favorable clinical outcome assigned to HPV-positive status. In effect, HPV positivity trumps traditional prognostic features predicated on morphology such as tumor grade and histologic subtype when it comes to predicting clinical behavior. For the diagnostic pathologist, the pedestrian task of tumor grading and subtyping would seem to be of little prognostic or therapeutic relevance when it comes to HPV-HNC. Recognition and documentation of neuroendocrine differentiation is a most notable exception. Forms of HPV-HNC have now been reported that morphologically resemble small cell carcinoma (SCC) and large cell neuroendocrine carcinoma (LCNEC) of other sites, and that immunohistochemically exhibit neuroendocrine differentiation. Despite the presence of HPV, these SCCs and LCNECs share the same aggressive clinical behavior of their counterparts in the lung and other sites where the high grade neuroendocrine phenotype is associated with early distant spread and poor overall survival. Consequently, the high grade neuroendocrine phenotype should be regarded as an aggressive form of HPV-HNC where tumor morphology displaces HPV positivity as the most important prognostic feature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
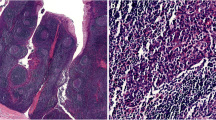
High grade neuroendocrine carcinomas (NECs) are usually encountered in the lungs, but they can arise in extrapulmonary sites including the head and neck. In the head and neck, these high grade NECs most commonly arise in the larynx, but they have also been reported in the sinonasal tract, salivary glands, trachea, oral cavity and oropharynx [1, 2]. Like their grouping in the lungs, high grade NECs of the head and neck are subclassified based on their morphologic features. SCC grows as nests, sheets and trabeculae of small anaplastic cells with a high nuclear to cytoplasmic ratio, hyperchromatic nuclei lacking visible nuclei, and scant cytoplasm (Figs. 1, 2). Tumor necrosis, abundant mitotic figures, and numerous apoptotic cells are consistent features. LCNECs are grow as nests and trabeculae of medium to large polygonal cells with abundant cytoplasm, coarse to vesicular chromatin, prominent nucleoli, and nuclear palisading around the periphery of the tumor nests (Figs. 3b, 4). Like SCC, LCNEC exhibits a high mitotic rate, abundant apoptotic cells and tumor necrosis.
HPV-related large cell neuroendocrine carcinoma (a hematoxylin and eosin stain). The tumor cells are positive for the neuroendocrine marker synaptophysin (b) and INSM1 (b inset), and they are negative for the squamous marker p40 (c). An RNA in-situ hybridization assay for high risk HPV show intense hybridization signals within the tumor cells (d)
In 2011, Bishop and Westra first reported five cases of HPV-related oropharyngeal carcinomas with well-developed features of SCC [3]. The following year, Kraft et al. [4] reported an additional six SCCs of the oropharynx that were HPV-positive. These HPV-SCCs of the head and neck were found to share features with SCC of the uterine cervix including an association with high risk HPV, expression of neuroendocrine markers by immunohistochemistry, and frequent co-existence with a non-small cell carcinoma including squamous cell carcinoma [5]. Many of the head and neck cases also arose in association with a synchronous or metachronous HPV-associated squamous cell carcinoma (Fig. 2). High risk HPV has now also been reported in LCNECs of the head and neck. In their report of ten LCNECs, Thompson et al. [6] noted the presence of high risk HPV in three. Two of these had arisen in the oropharynx, and one in the sinonasal tract.
The finding of high risk HPV in a form of head and neck carcinoma that deviates from the usual appearance of HPV-HNC is nothing new. Most HPV-HNCs arise from tonsillar crypts and infiltrate as sheets, lobules or ribbons of non-keratinized squamous cells; but the list of HPV-HNCs that deviate from the prototype is now rather substantial. The entire morphologic spectrum of HPV-HNSCC includes variants with papillary, adenosquamous, lymphoepithelioma-like, sarcomatoid, basaloid and ciliated features [7]. Although these phenotypic variations may be diagnostically relevant for the way they cause confusion with other cancer types, they do not appear to impact prognosis. The presence of HPV consistently imparts a favorable prognosis, even when detected in more phenotypes that have been associated with more aggressive behavior such as the basaloid variant of squamous cell carcinoma [8, 9].
Inclusion of the neuroendocrine phenotype within a growing list of morphologic variants of HPV-HNC may be more than just a pedantic exercise that carries little practical implications. Indeed, the opposite is true. A limited experience with HPV-SCC of the head and neck suggests that it shares the same aggressive clinical features of its counterpart in the uterine cervix where the small cell phenotype has been associated with early distant spread and poor overall survival [10, 11]. In the study by Bishop, 3 of the 5 patients died within 15 months of diagnosis (mean, 10 months) of widely disseminated disease [3]. In the Kraft series, HPV-related high grade neuroendocrine carcinoma was associated with progressive disease and distant spread [4]. Patients with HPV-LCNEC did not fare any better. The HPV-LCNECs reported by Thompson et al. [6] were also extremely aggressive, presenting with either widely disseminated or locally advanced disease. Following diagnosis, two patients rapidly died of disease and the third directly entered hospice care. In effect, the aggressive behavior of these HPV-related high grade NECs represents a sharp departure from that typically expected for HPV-HNC where HPV positivity generally carries an expectation of long-term survival [12, 13].
For patients with HPV-HNC, recognition of NEC and its distinction from HPV-related squamous cell carcinoma is important but not straightforward. Both tumor types share certain morphologic features including some degree of a basaloid appearance, a high nuclear to cytoplasmic ratio, an elevated mitotic rate, zones of necrosis and peripheral nuclear palisading (Fig. 3). Accordingly, a low threshold for immunohistochemical analysis is warranted whenever there is some concern of a high grade neuroendocrine component that cannot be reliably resolved on morphologic grounds. Like non-keratinizing squamous cell carcinoma, SCC and LCNEC are consistently cytokeratin positive (particularly low molecular weight cytokeratins), but the distribution of cytokeratin positivity in a perinuclear dot fashion suggests neuroendocrine differentiation. P40 and cytokeratin 5/6 are consistently expressed in HPV-related non-keratinizing carcinomas, but show loss of expression or diminished expression in high grade NECs. Markers of neuroendocrine differentiation are routinely positive in high grade NECs, and are consistently negative in HPV-related non-keratinizing squamous cell carcinomas [14]. To enhance the sensitivity of this immunohistochemical approach, it is common practice to use a panel of neuroendocrine markers in an attempt to discern some combination of staining for synaptophysin, chromogranin and CD56. Insulinoma-associated protein 1 (INSM1) is a novel transcription factor that has recently demonstrated excellent sensitivity and specificity for neuroendocrine differentiation in various anatomic sites. In the thoracic cavity, for example, INSM1 demonstrates superior sensitivity and specificity to traditional neuroendocrine markers and may serve as a standalone first-line marker of neuroendocrine differentiation [15]. Initial observations suggest that INSM1 may likewise be used for neuroendocrine tumors of the head and neck including SCC and LCNEC in a way that may renders the traditional approach using extended immunohistochemical panels obsolete (unpublished observation).
P16 immunohistochemistry is now widely recommended as the detection method of choice for determining the HPV status of oropharyngeal carcinomas [16]. When it comes to high grade NECs of the head and neck, P16 staining is not a suitable surrogate marker for HPV. Both LCNEC and SCC in the lung and other sites frequently express p16 via mechanisms independent of viral infection i.e. inactivation of retinoblastoma protein (RB) [17, 18]. Given the unreliability of p16 staining and the absence of any prognostic relevance of HPV status, there is no compelling reason to perform HPV analysis of high grade NECs of the head and neck, even for those arising in the oropharynx [19].
When our group first drew attention to an HPV-form of oropharyngeal SCC in 2011, we speculated that this might be a tumor with an incidence on the rise—a conjecture based on the dramatic upsurge in HPV-related oropharyngeal carcinoma in general over the past 2 decades [3]. Neither our practice experience nor the recent literature has validated this predicted increase in the sightings of HPV-related high grade NEC in the head and neck. In our large consultation practice, HPV-positive high grade NEC remains a rare bird, and the three sentinel reports cited in this paper have been substantiated by only a few additionally reported cases [3, 4, 20]. HPV-related high grade NEC has been and remains a very rare form of head and neck cancer. It is the rarity of this entity along with its paradoxical behavior that places the unwary pathologist and clinician at risk of erroneously branding it as just another form of HPV-related carcinoma. It is not.
References
Hatoum GF, Patton B, Takita C, et al. Small cell carcinoma of the head and neck: the university of Miami experience. Int J Radiat Oncol Biol Phys. 2009;74(2):477–81.
Renner G. Small cell carcinoma of the head and neck: a review. Semin Oncol. 2007;34(1):3–14.
Bishop JA, Westra WH. Human papillomavirus-related small cell carcinoma of the oropharynx. Am J Surg Pathol. 2011;35(11):1679–84.
Kraft S, Faquin WC, Krane JF. HPV-associated neuroendocrine carcinoma of the oropharynx: a rare new entity with potentially aggressive clinical behavior. Am J Surg Pathol. 2012;36(3):321–30.
Crowder S, Tuller E. Small cell carcinoma of the female genital tract. Semin Oncol. 2007;34(1):57–63.
Thompson ED, Stelow EB, Mills SE, Westra WH, Bishop JA. Large cell neuroendocrine carcinoma of the head and neck: a clinicopathologic series of 10 cases with an emphasis on HPV status. Am J Surg Pathol. 2016;40(4):471–8.
Westra WH. The pathology of HPV-related head and neck cancer: implications for the diagnostic pathologist. Semin Diagn Pathol. 2015;32(1):42–53.
Begum S, Westra WH. Basaloid squamous cell carcinoma of the head and neck is a mixed variant that can be further resolved by HPV status. Am J Surg Pathol. 2008;32(7):1044–50.
Thariat J, Badoual C, Faure C, Butori C, Marcy PY, Righini CA. Basaloid squamous cell carcinoma of the head and neck: role of HPV and implication in treatment and prognosis. J Clin Pathol. 2010;63(10):857–66.
Wang KL, Yang YC, Wang TY, et al. Neuroendocrine carcinoma of the uterine cervix: a clinicopathologic retrospective study of 31 cases with prognostic implications. J Chemother. 2006;18(2):209–16.
Abeler VM, Holm R, Nesland JM, Kjorstad KE. Small cell carcinoma of the cervix. A clinicopathologic study of 26 patients. Cancer. 1994;73(3):672–7.
Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35.
Wuerdemann N, Wittekindt C, Sharma SJ, et al. Risk factors for overall survival outcome in surgically treated human papillomavirus-negative and positive patients with oropharyngeal cancer. Oncol Res Treat. 2017;40(6):320–7.
Lewis JS Jr, Chernock RD, Bishop JA. Squamous and neuroendocrine specific immunohistochemical markers in head and neck squamous cell carcinoma: a tissue microarray study. Head Neck Pathol. 2017. https://doi.org/10.1007/s12105-017-0825-y.
Rooper LM, Sharma R, Li QK, Illei PB, Westra WH. INSM1 demonstrates superior performance to the individual and combined use of synaptophysin, chromogranin and CD56 for diagnosing neuroendocrine tumors of the thoracic cavity. Am J Surg Pathol. 2017;41(11):1561–9.
Westra WH, Boy S, E-M SK, et al. HPV-positive squamous cell carcinoma of the oropharynx. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of head and neck tumours. Lyon: International Agency for Research on Cancer; 2017.
Beasley MB, Lantuejoul S, Abbondanzo S, et al. The P16/cyclin D1/Rb pathway in neuroendocrine tumors of the lung. Hum Pathol. 2003;34(2):136–42.
Dosaka-Akita H, Cagle PT, Hiroumi H, et al. Differential retinoblastoma and p16(INK4A) protein expression in neuroendocrine tumors of the lung. Cancer. 2000;88(3):550–6.
Alos L, Hakim S, Larque AB, et al. p16 overexpression in high-grade neuroendocrine carcinomas of the head and neck: potential diagnostic pitfall with HPV-related carcinomas. Virchows Arch. 2016;469(3):277–84.
Misawa K, Kawasaki H, Matsuo R, et al. Human papillomavirus-associated small cell carcinoma/neuroendocrine carcinoma of the oropharynx: a report of two cases. Springerplus. 2016;5(1):1847.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
William Westra declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Westra, W.H. Human Papillomavirus-Related Neuroendocrine Carcinomas of the Head and Neck. Head and Neck Pathol 12, 9–12 (2018). https://doi.org/10.1007/s12105-018-0886-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-018-0886-6