Abstract
The escalating incidence of squamous cell carcinoma of the oropharynx and base of the tongue (OPC) is associated with remote exposure to hrHPV and thereby indicates that the increasing numbers of these cancers will be seen in the clinical setting. HPV status in OPC impacts a range of clinical factors including primary site determination, extent of surgery, adjuvant treatment, and prognosis. Thus, accurate and reproducible methods to determine HPV status will be needed for an increasing volume of patients. For OPC, p16 is now considered to be a reliable surrogate for detection of hrHPV, but its use will likely wane as molecular testing such as in situ hybridization becomes more widespread and laboratories become more comfortable with application and interpretation of these methodologies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
The escalating incidence of squamous cell carcinoma of the oropharynx and base of the tongue (OPC) is associated with remote exposure to hrHPV and thereby indicates that the increasing numbers of these cancers will be seen in the clinical setting. HPV status in OPC impacts a range of clinical factors including primary site determination, extent of surgery, adjuvant treatment, and prognosis. Thus, accurate and reproducible methods to determine HPV status will be needed for an increasing volume of patients. For OPC, p16 is now considered to be a reliable surrogate for detection of hrHPV, but its use will likely wane as molecular testing such as in situ hybridization becomes more widespread and laboratories become more comfortable with application and interpretation of these methodologies.
10.1 Introduction
Traditional risk factors for head and neck squamous cell carcinoma (HNSCC) include tobacco and alcohol use. These factors acting synergistically increase the risk of cancer development by 26-fold [1].Since the 1980s, there has been a relatively modest decrease in the incidence of HNSCC that has generally paralleled the decrease in use of tobacco [2]. By contrast, over the past two decades, the emergence of a form of squamous cell carcinoma that occurs primarily in the oropharynx and base of tongue (OPC) has been noted that is linked biologically and epidemiologically to oncogenic forms of the human papillomavirus (HPV) [3]. While tobacco- and alcohol- linked HNSCC continues to be more common in older men, OPC is now seen in a group of younger men whose principal risk factor is sexual behaviors that facilitate the transmission of HPV. In this group, the greatest risk is associated with a high number of sex partners, a history of oral-genital and oral-anal sex, and marijuana use [4]. While tobacco use is not clearly linked to the causation of this form of cancer, its concurrent or former use is strongly linked to worsened response to therapy and 5-year survival [5].
10.2 Biology of HPV
HPV is a family of DNA viruses whose only known host is humans. There are over 130 unique subtypes of HPV that can infect the skin and mucosa. An important biological distinction is between the oncogenic or “high-risk” forms of HPV (subtypes 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82) and the non-oncogenic (“low-risk”) forms (6, 11, 13, 32, etc.) [6]. High-risk HPV (hrHPV) is causative in sexually transmitted high-grade squamous intraepithelial lesions and the vast majority of anogenital malignancies in the USA and in most parts of Europe (cervical, vaginal, vulvar, and anal carcinoma). In contrast, low-risk HPV is associated with anogenital condyloma acuminatum and benign oral lesions including squamous papilloma, oral warts, and focal epithelial hyperplasia (Heck’s disease) [7].
Definition
HPV16 is responsible for the vast majority of oropharyngeal cancers, with only a minority associated with other high-risk subtypes such as HPV18.
The circular HPV genome encodes DNA sequences for six early (E) proteins associated with viral gene regulation and cell transformation, two late (L) proteins which form the shell of the virus, and one region of regulatory DNA. In the pathogenesis of virally induced malignant disease, continued expression of E6 and E7 proteins is required to sustain a malignant phenotype. When the HPV genome is integrated into the host nuclear DNA, the E2 regulatory region is disrupted, leaving the expression of viral proteins E6 and E7 unregulated. In a normal cell, the wild-type p53 protein negatively regulates cell growth limiting cell cycle transition from G0/G1 to S phase and functioning as a tumor suppressor protein. Viral E6 specifically binds to p53 , resulting in its degradation. Similarly, the Rb protein normally inhibits the cell cycle through interaction with the E2F transcription factor. In response to DNA damage, Rb typically binds and inactivates E2F halting replication or inducing apoptosis. Viral E7 inhibits the function of Rb protein by disrupting the E2F/Rb protein complex allowing uncontrolled cell proliferation [8].
10.3 HPV and Carcinogenesis
HPV-associated malignancies within the anogenital tract have clinically and histologically recognized dysplastic precursor lesions with a well-understood risk of progression to invasive carcinoma. In contrast within the head and neck, and particularly within the base of tongue and oropharynx , there are few recognized precursor lesions associated with oncogenic HPV infection. An exception appears to be the recently described form of oral epithelial dysplasia associated with HPV16 [9]. Sometimes reported using the terms koilocytic dysplasia or HPV-associated oral intraepithelial neoplasia, the natural history of this form of dysplasia is not fully defined although a proportion have been reported to progress to oral squamous cell carcinoma [10].
Over 75% of oropharyngeal carcinomas (OPC) are linked to hrHPV. HPV16 is responsible for the vast majority of these tumors; a minority is associated with other high-risk subtypes such as HPV18.
10.4 Pathology
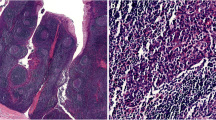
HPV-associated oropharyngeal cancers have distinct phenotypic and molecular features [11]. Pathologically, hrHPV+ OPC are presumed to arise from the tonsillar crypts without evidence of a noninvasive precursor lesion analogous to epithelial dysplasia for traditional SCC. The majority of the HPV-related tumors have a characteristic lobular or nested growth pattern with central necrosis and are intimately permeated by lymphocytes (◘ Fig. 10.1 panel a). The cytomorphology of hrHPV+ OPC is typically basaloid with limited keratinization, resembling the reticulated epithelium of the tonsillar crypt [12]. The nomenclature of the hrHPV+ tumors has evolved with several names having been proposed including “poorly differentiated,” “nonkeratinizing,” and “basaloid” squamous cell carcinoma . The term “basaloid” is particularly problematic since there is a histologically similar entity, “basaloid squamous cell carcinoma, ” that can arise in the oropharynx [13]. Basaloid squamous cell carcinoma has long been recognized as a poorly differentiated tumor mainly caused by smoking and alcohol , the traditional risk factors for squamous cell carcinoma, and, unlike the HPV-associated tumors, has a poor prognosis [14].Given the prognostic significance of HPV status in the oropharynx , and the potential for confusion arising from histologic descriptors, the current WHO Classification advocates the term “squamous cell carcinoma” for OPC with a modifier indicating the HPV status. Given the disconnect between the apparent lack of differentiation of the HPV-associated tumors and the relatively good prognosis, histologic grading for hrHPV+ squamous cell carcinoma arising in the oropharynx is discouraged [15].
Panel a: An example of a hrHPV+ SCC from the oropharynx showing the characteristic lobular or nested growth pattern intimately permeated by lymphocytes (H&E 20×). Panel b: An example of a hrHPV+ SCC with papillary architecture and keratinization (H&E 40×). Panel c: RNA in situ hybridization using probes to detect E6 and E7 mRNA of hrHPV showing punctate positive signals (H&E 40×). Panel d: An example of a p16-positive SCC from the oropharynx showing greater than 70% of tumor cells with nuclear and cytoplasmic staining
Important
The cytomorphology of high-risk HPV-positive OPC is typically basaloid with limited keratinization, resembling the reticulated epithelium of the tonsillar crypt.
A small percentage of hrHPV+ squamous cell carcinomas show divergent histology. This group includes keratinizing tumors with papillary architecture (◘ Fig. 10.1 panel b) and hrHPV+ high-grade neuroendocrine/small cell carcinoma. Occasionally hrHPV+ tumors with histomorphology similar to the typical oropharyngeal type can occur in the nasopharynx . These HPV+ nasopharynx tumors show overlapping features with the typical EBV-associated nasopharyngeal carcinoma but are more frequent in white men, unlike EBV+ nasopharyngeal carcinoma that is most common in people of Chinese descent [16].
10.5 Determining HPV Status
Determining and reporting of HPV status for OPC are now considered the standard of care, with the majority of head and neck pathologists recognizing the significance of evaluating all tumors [17]. HPV status is proven to be a strong, independent prognostic factor for OPC [18] predicting responsiveness to induction chemotherapy with cisplatin [19] and radiotherapy [20]. HPV status can also aid in the confirmation of the diagnosis of cystic neck lesions [21] and locating a clinically occult primary tumor since an HPV-positive tumor in cervical levels 2 and 3 of the neck is likely to have arisen from an oropharyngeal primary [21]. An increasing number of organizations now recommend HPV testing and the determination of HPV status including the National Comprehensive Cancer Network (NCCN) [22], the College of American Pathologists, and the Collaborative Stage Data Collection System, utilized by several US groups including the American Joint Committee on Cancer and the Surveillance Epidemiology and End Results (SEER) program. Outside the USA, the Royal College of Pathologists (UK) and the Australasian College of Pathologists also recommend the determination of HPV status for OPC. The problem remains that there is no uniform standard for validation or interpretation of HPV detection assays, although a consensus statement for reporting HPV status was issued by the College of American Pathologists in an attempt to standardize the application and interpretation of hrHPV testing [23]. The ideal test for hrHPV is technically reliable, reproducible, and easily interpreted. For histologic specimens, the test should ideally localize hrHPV within tumor cells. Currently used methodologies include PCR, HPV16 DNA in situ hybridization (ISH), hrHPV E6/E7 mRNA ISH, and p16 immunohistochemistry (IHC).
10.5.1 PCR-Based Techniques
PCR-based testing for HPV is problematic because it fails to identify transcriptionally active virus within tumor cells. This is particularly an issue for DNA based testing; some studies have reported that close to 50% of OPC positive for hrHPV DNA by PCR are negative for E6/E7 mRNA expression [24]. In a comprehensive analysis of different methodologies Jordan et al. reported 14% of hrHPV DNA-positive OPCs were negative for hrHPV E6/E7 mRNA expression and had very low HPV DNA viral load, concluding that PCR may detect “bystander” virus and arguing against the application of PCR alone for classifying HPV status [25]. Sample contamination, especially in DNA-based testing protocols, is a well-known limitation of PCR .
Warning
Use of PCR technique alone is not recommended for classification of HPV status of tumors due to low specificity of the technique.
10.5.2 In Situ Hybridization (ISH)
Type-specific HPV probes or “cocktails” can be applied on formalin-fixed, paraffin-embedded tissues for in situ hybridization (ISH), and the method allows localization topographically in tumor cells. Although the HPV16 DNA ISH assay can detect single-copy HPV with good sensitivity and high performance in comparison to hrHPV E6/E7 mRNA expression, it is type-specific, nonautomated, technically demanding to perform and has limited commercial availability. As reported by Schlecht et al., commercially distributed assays by Ventana (INFORM HPV-III Fam16B) and Dako (HPV16/18) have lower test performance standards in comparison to analysis of HPV16 E6/E7 mRNA expression (AUC 0.48–0.69) [26]. The test that may well become the standard for hrHPV detection is hrHPV E6/E7 mRNA ISH that is commercially available and can be performed on the automated platforms utilized for routine immunohistochemistry. One currently marketed kit (Advanced Cell Diagnostics) detects E6 and E7 mRNA of 18 hrHPV subtypes and has excellent performance showing punctate signal (◘ Fig. 10.1 panel c) [27].
10.5.3 p16 Immunohistochemistry
Immunohistochemistry for the endogenous cell cycle protein p16 has proven a simple and practical surrogate marker for hrHPV in the setting of OPC. P16 is overexpressed in tumor tissue with transcriptionally active hrHPV . Therefore, detection of p16 protein by immunohistochemistry (IHC) is an acceptable and a satisfactory alternative to HPV ISH [26]. In HPV-induced cancers, overexpression of p16 is due to inactivation of pRB by the hrHPV E7 oncoprotein. During the natural history of cervical cancer, p16 expression progressively increases with the severity of dysplasia and is commonly used for triage of low-grade vs. high-grade dysplasia [28]. Standardization of testing methods and interpretation of positive/negative status of p16 IHC has recently been achieved for HPV-associated squamous intraepithelial lesions and superficially invasive anogenital squamous carcinomas [29].
Important
p16 staining must be both nuclear and cytoplasmic to be considered positive in reporting HPV status of an oropharyngeal carcinoma.
The p16 immunohistochemical staining protocol has been standardized with a single monoclonal antibody (E6H4, MTM Labs) used in the vast majority of laboratories. High inter-rater agreement has been reported [30], confirming the competency of pathologists in interpretation and reporting of IHC assays. In tissue biopsies from the oropharynx , p16 IHC is interpreted as positive when there is moderate to strong staining in at least 70% of tumor cells. Both nuclear and cytoplasmic staining should be present to be considered positive for HPV (◘ Fig. 10.1 panel D). When interpreted correctly, the statistical correlation between p16 IHC status and HPV-specific tests such as hrHPVE6/E7 mRNA ISH is very high [31]. A reproducible and validated H-score has also been described and used in large clinical trials for p16 scoring [25]. The H-score is the cross product of staining intensity (0, 1, 2, or 3) and the percentage positive cells that show that intensity. On a scale of 0–300, an optimal cut-off point has been set at 60. This score contributes to an average sensitivity of 91.6% and specificity of 90.4% for hrHPV oncogene expression. P16 IHC is likely the most practical test for resource-poor settings, although the specificity of this marker as a surrogate for hrHPV drops precipitously when applied to tumors arising outside of the oropharynx . This is reflected in current testing guidelines published by the College of American Pathologists that discourages p16 testing for carcinomas outside of the oropharynx [23].
A comparison of the performance characteristics of the different methods described here is shown in ◘ Table 10.1.
10.6 Fine-Needle Aspiration (FNA) for HPV Testing
HPV-associated squamous cell carcinomas frequently present as an enlarged cervical lymph node with a small, clinically occult primary tumor within the palatine tonsils or base of the tongue . As many hrHPV+ OPC initially present with an enlarged upper cervical lymph node, HPV testing of fine-needle aspirate (FNA) specimens obtained from a cervical metastasis is of considerable clinical value as it may direct the clinician to perform close examination of the oropharynx . The presence of hrHPV is so specific for oropharyngeal origin that a diagnostic tonsillectomy and base of the tongue resection may be performed in order to identify a microscopic primary tumor. Prior to recognizing the frequency of occult oropharyngeal primary tumors, pathologists previously rendered a diagnosis of “squamous carcinoma arising in a branchial cleft cyst” for those cases where a primary tumor was not clinically evident. HPV testing of unknown primary SCCs has proven that the majority of these represent HPV-driven malignancies, likely of oropharyngeal origin . [32]
OPC characteristically metastasizes to the upper and mid jugular chain (cervical levels 2 and 3). Aspiration of these enlarged nodes can be performed with a narrow-gauge needle (23 or 25 gauge, ◘ Fig. 10.2 panel a), allowing for immediate diagnosis of malignancy. Specimens can be preserved in alcohol -based preservative solutions (◘ Fig. 10.2 panel b) and/or formalin for hrHPV testing, in addition to traditional cytologic preparations (◘ Fig. 10.2 panel c).The cytomorphology of the hrHPV+ squamous cell carcinoma recapitulates the histomorphology of these tumors. Aspirate smears contain cohesive clusters of hyperchromatic, “basaloid” epithelium and scattered keratinizing cells (◘ Fig. 10.2 panel d). While incisional biopsy of the primary tumor or metastasis can yield tissue for HPV testing, testing of FNA specimens has the advantage of not contaminating the neck or disrupting the surgical field within the oropharynx . Furthermore, highly sensitive and specific hrHPV testing platforms developed for cervical cancer screening are available in most clinical laboratories and can be utilized in testing of FNA samples held in the appropriate liquid preservative .
Panel a: Percutaneous aspiration of an enlarged node is performed with a narrow-gauge needle attached to a syringe and an aspirator. Panel b: FNA samples can be held in alcohol -based preservative solutions and/or fixed in formalin for hrHPV testing. Panel c: Conventional cytologic smears and cell block stained prior to microscopic examination. Panel d: Cytologic preparation from a hrHPV+ squamous cell carcinoma containing cohesive clusters of hyperchromatic, “basaloid” epithelium, and scattered keratinizing cells
10.7 Testing Saliva for HPV Infection
Salivary tests for HPV in asymptomatic patients are commercially available but have several significant limitations including high false positives, low specificity , and inability to differentiate persistent from transient infection. most importantly, the action prompted by a positive result is unclear since a clinically visible precancerous lesion is usually not present. Because of these important limitations, their incorporation into clinical practice is currently not recommended .
10.8 Conclusions
The escalating incidence of OPC associated with remote exposure to hrHPV means that increasing numbers of these cancers will be seen in the clinical setting. HPV status in OPC impacts a range of clinical factors including primary site determination, extent of surgery, adjuvant treatment, and prognosis. Thus, accurate and reproducible methods to determine HPV status will be needed for an increasing volume of patients. Although p16 has proven to be a reliable surrogate for detection of hrHPV, its use will likely wane as molecular testing such as in situ hybridization becomes more widespread and laboratories become more comfortable with the application and interpretation of these methodologies.
References
Castellsagué X, Quintana MJ, Martínez MC, et al. The role of type of tobacco and type of alcoholic beverage in oral carcinogenesis. Int J Cancer. 2004;108(5):741–9.
Elashoff D, Zhou H, Reiss J, et al. Prevalidation of salivary biomarkers for oral cancer detection. Cancer Epidemiol Biomarkers Prev. 2012;21(4):664–72.
Gillison ML, Shah KV. Human papillomavirus-associated head and neck squamous cell carcinoma: mounting evidence for an etiologic role for human papillomavirus in a subset of head and neck cancers. Curr Opin Oncol. 2001;13(3):183–8.
D’Souza G, Gross ND, Pai SI, et al. Oral Human Papillomavirus (HPV) infection in HPV-positive patients with oropharyngeal cancer and their partners. J Clin Oncol. 2014;32(23):2408–15.
Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35.
Guo M, Lin CY, Gong Y, et al. Human papillomavirus genotyping for the eight oncogenic types can improve specificity of HPV testing in women with mildly abnormal Pap results. Mod Pathol. 2008;21(8):1037–43.
Praetorius F. HPV-associated diseases of oral mucosa. Clin Dermatol. 1997;15(3):399–413.
Delius H, Saegling B, Bergmann K, Shamanin V, de Villiers EM. The genomes of three of four novel HPV types, defined by differences of their L1 genes, show high conservation of the E7 gene and the URR. Virology. 1998;240(2):359–65.
Woo S-B, Cashman EC, Lerman MA. Human papillomavirus-associated oral intraepithelial neoplasia. Mod Pathol. 2013;26(10):1288–97.
Lerman MA, Almazrooa S, Lindeman N, Hall D, Villa A, Woo S-B. HPV-16 in a distinct subset of oral epithelial dysplasia. Mod Pathol. 2017;30(12):1646–54.
Shi W, Kato H, Perez-Ordonez B, et al. Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol. 2009;27(36):6213–21.
Westra WH. The changing face of head and neck cancer in the 21st century: the impact of HPV on the epidemiology and pathology of oral cancer. Head Neck Pathol. 2009;3(1):78–81.
Wain SL, Kier R, Vollmer RT, Bossen EH. Basaloid-squamous carcinoma of the tongue, hypopharynx, and larynx: report of 10 cases. Hum Pathol. 1986;17(11):1158–66.
Begum S, Westra WH. Basaloid squamous cell carcinoma of the head and neck is a mixed variant that can be further resolved by HPV status. Am J Surg Pathol. 2008;32(7):1044–50.
El-Naggar AK, Chan JKC, Takata T, Grandis JR, Slootweg PJ. The fourth edition of the head and neck World Health Organization blue book: editors’ perspectives. Hum Pathol. 2017;66:10–2.
Punwaney R, Brandwein MS, Zhang DY, et al. Human papillomavirus may be common within nasopharyngeal carcinoma of Caucasian Americans: investigation of Epstein-Barr virus and human papillomavirus in eastern and western nasopharyngeal carcinoma using ligation-dependent polymerase chain reaction. Head Neck. 1999;21(1):21–9.
Ahmed A, Cascarini L, Sandison A, Clarke P. Survey of the use of tests for human papilloma virus and epidermal growth factor receptor for squamous cell carcinoma of the head and neck in UK head and neck multidisciplinary teams. Br J Oral Maxillofac Surg [Internet] 2011; Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21450378.
Ang KK. Larynx preservation clinical trial design: summary of key recommendations of a consensus panel. Oncologist. 2010;15(Suppl 3):25–9.
Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–9.
Cantley RL, Gabrielli E, Montebelli F, Cimbaluk D, Gattuso P, Petruzzelli G. Ancillary studies in determining human papillomavirus status of squamous cell carcinoma of the oropharynx: a review. Pathol Res Int. 2011;2011:138469.
Cao D, Begum S, Ali SZ, Westra WH. Expression of p16 in benign and malignant cystic squamous lesions of the neck. Hum Pathol. 2010;41(4):535–9.
Pfister DG, Ang KK, Brizel DM, et al. Head and neck cancers. J Natl Compr Cancer Netw. 2011;9(6):596–650.
Lewis JS, Beadle B, Bishop JA, et al. Human papillomavirus testing in head and neck carcinomas: guideline from the College of American Pathologists. Arch Pathol Lab Med. 2017;
Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24(5):736–47.
Jordan RC, Lingen MW, Perez-Ordonez B, et al. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 2012;36(7):945–54.
Schlecht NF, Brandwein-Gensler M, Nuovo GJ, et al. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Mod Pathol. 2011;24(10):1295–305.
Mirghani H, Casiraghi O, Amen F, et al. Diagnosis of HPV-driven head and neck cancer with a single test in routine clinical practice. Mod Pathol. 2015;28(12):1518–27.
Klaes R, Friedrich T, Spitkovsky D, et al. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92(2):276–84.
Darragh TM, Colgan TJ, Cox JT, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136(10):1266–97.
Thavaraj S, Stokes A, Guerra E, et al. Evaluation of human papillomavirus testing for squamous cell carcinoma of the tonsil in clinical practice. J Clin Pathol. 2011;64(4):308–12.
Begum S, Gillison ML, Ansari-Lari MA, Shah K, Westra WH. Detection of human papillomavirus in cervical lymph nodes: a highly effective strategy for localizing site of tumor origin. Clin Cancer Res. 2003;9(17):6469–75.
Zengel P, Assmann G, Mollenhauer M, et al. Cancer of unknown primary originating from oropharyngeal carcinomas are strongly correlated to HPV positivity. Virchows Arch. 2012;461(3):283–90.
Schache AG, Liloglou T, Risk JM, et al. Validation of a novel diagnostic standard in HPV-positive oropharyngeal squamous cell carcinoma. Br J Cancer. 2013;108(6):1332–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
van Zante, A., Jordan, R.C. (2020). Detection Methods for Human Papillomavirus (HPV) in Head and Neck Cancers. In: Warnakulasuriya, S., Greenspan, J. (eds) Textbook of Oral Cancer. Textbooks in Contemporary Dentistry. Springer, Cham. https://doi.org/10.1007/978-3-030-32316-5_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-32316-5_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-32315-8
Online ISBN: 978-3-030-32316-5
eBook Packages: MedicineMedicine (R0)