Abstract
Immune cells infiltrating the tumor microenvironment are physiologically important in controlling cancers. However, emerging studies have shown that cancer cells can evade immune surveillance and establish a balance in which these immune cells support tumor progression and therapeutic resistance. The signaling lymphocytic activation molecule family members have been recognized as mediators of tumor microenvironment interactions, and a promising therapeutic target for cancer immunotherapy. This review is focused on the role of SLAM family in tumor and immune cell interactions and discusses how such crosstalk affects tumor behavior. This will shed insight into the next step toward improving cancer immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer immunotherapies, particularly immune checkpoint inhibitors, have revolutionized the landscape of cancer treatment and are promising curative treatments across several cancer types. Nevertheless, a significant number of patients do not respond and even those who respond relapse with time leading to disease progression [1, 2]. Accordingly, studies exploring mechanisms of resistance and new strategies to boost the efficacy of immunotherapies have emerged [3,4,5].
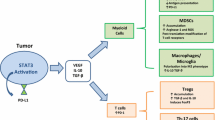
Components of the tumor microenvironment undergo complex interplay with cancer cells via cell-to-cell interaction or the release of soluble factors that can shape the tumor microenvironment [6,7,8]. Particularly, studies have shown that the dynamic and continuous interaction between tumor and their tumor immune microenvironment have a decisive role in dictating the fate of tumors and therapeutic outcomes [9,10,11,12]. Accumulating evidence has shown that the immunosuppressive milieu generated in the tumor microenvironment is associated with impaired anti-tumor response immunity leading to treatment failure [13,14,15]. As a result, increasing interest in understanding the immunosuppressive tumor microenvironment has begun to evolve as it could lead to the development of combined therapies that could enhance the efficacy of immunotherapies [16,17,18].
Accumulating evidence has shown that the signaling lymphocytic activation molecule (SLAM) family receptors are critically involved in the pathophysiology of several solid and hematologic malignancies [19,20,21]. Notably, SLAM family receptors can bridge tumor cells and their microenvironment [22, 23], revealing SLAM family receptors as communicators that can mediate possible crosstalk between tumors and their surrounding niche, particularly immune cells. Recently, studies have found that SLAM family receptors can regulate the expression of checkpoint molecules, suggesting the therapeutic potential of SLAM family receptors in cancer immunotherapy, though our understanding in this regard is incomplete [24]. Therefore, this review discusses the role of SLAM family in regulating the interaction between tumor and immune cells and describes how such interaction promotes tumor progression. Also, the potential of exploring SLAM family receptors in cancer immunotherapy will be discussed.
Structure and signaling of SLAM family receptors
SLAM family receptors, broadly expressed on immune cells, are type 1 membrane receptors composed of nine members: SLAMF1 (CD150), SLAMF2 (CD48), SLAMF3 (CD229, Ly9), SLAMF4 (CD244,2B4), SLAMF5 (CD84), SLAMF6 (CD352, NTB-A), SLAMF7 (CD319, CS1, CRACC), SLAMF8 (CD353) and SLAMF9 (CD84H1, CD2F10) [25,26,27,28]. Except for SLAMF4 and SLAMF2 which act reciprocal toward each other, SLAM family receptors are activated by homophilic interaction and, therefore act as self-ligand [29,30,31,32]. SLAM family receptors are composed of an extracellular segment with two Ig-like domains, a transmembrane segment and cytoplasmic tail-bearing immunoreceptor tyrosine-based signaling motifs (ITSMs) [33]. In contrast to this are SLAMF2, SLAMF8 and SLAMF9 which lack ITSMs [34,35,36]. Additionally, SLAMF3 has four Ig-like domains in its extracellular segment [37]. In SLAMFs with ITSMs in their cytoplasmic tail, SLAMF engages with SLAM-associated proteins (SAP) and Ewing sarcoma-associated transcript-2 (EAT-2) to directly interact with the Src family kinase Fyn and phospholipase C respectively, thus inducing immune cell activation [38, 39] (Fig. 1).
SLAM family receptors are instrumental in the modulation of immune responses of innate and adaptive immune systems[40, 41]. However, increasing evidence has implicated SLAM family receptors in the pathophysiology of both solid and hematologic malignancies. Notably, SLAMF activation has been linked with tumor metastasis[42, 43], therapeutic resistance [44,45,46], apoptosis resistance [45, 46], and tumor proliferation. Evidence strongly supports that SLAM family receptors secreted by tumor cells are in involved the polarization of immune cells toward pro-tumor phenotype to support tumor progression. On the other hand, documentation shows that SLAM family receptors secreted by immune cells can potentiate the immune evasion mechanisms of tumor cells, revealing SLAM family receptors as mediators of tumor microenvironment (TME) interactions.
SLAM family receptors-mediated interaction between tumor cells and macrophages in TME
Tumor-associated macrophages can be activated and polarized by factors in the tumor microenvironment to promote tumor progression [1, 10]. It has been demonstrated that cancer expressing SLAM family can directly promote the immunosuppressive functions of macrophages by inducing M2 macrophages with high expression of SLAMF [47]. According to Dolt et al., interferon-gamma and macrophage colony-stimulating factor secreted by melanoma tumor-conditioned media can upregulate SLAMF9 expression in bone marrow-derived macrophages and human peripheral blood monocytes. Additionally, melanoma-induced SLAMF9 + macrophages impaired the wound-healing capacity of RAW 264.7 cells [47]. Li et al. injected lymphoma cells expressing SLAMF3 and SLAMF4 into mice and found significant tumor growth. In these mice, downregulation of SLAMF3 and SLAMF4 potentiated macrophage-induced phagocytosis leading to tumor regression [48] suggesting that SLAMF3 and SLAMF4 expressed by tumor cells are associated with impaired phagocytosis of macrophages.
A mechanistic study has demonstrated that SLAMF6 expression in hepatocellular carcinoma (HCC) cells can induce M2 macrophage polarization with high expression of SLAMF6 to enhance the migration, invasion and growth of HCC. Notably, silencing SLAMF6 suppressed M2 macrophage polarization which in turn impaired migration, invasion and growth of HCC [49]. In the same study, SLAMF6 levels in CD14 + monocytes were higher in HCC patients compared to healthy donors, and this increase was associated with worse clinical outcomes [49]. Tumor-associated macrophage expressing SLAMF7 can upregulate PD-1 and TOX expression on CD8 + T cells, implying an exhausted phenotype. Furthermore, deficiency of SLAMF7 in tumor-bearing mice rejuvenated CD8 + T cells, leading to inhibition of tumor growth [50]. In breast cancer, high expression of SLAMF7 is associated with impaired phagocytic activities of macrophages [51]. A recent study has shown that SLAMF4 downregulation on macrophages could be used as a potential combination therapeutic strategy with checkpoint inhibitors to restore antitumor immunity [52]. Kim et al. found that high expression of SLAMF4 on monocytes infiltrating melanoma tumors impaired the maturation of antitumor macrophages and dampened antigen-specific action of T cells leading to tumor progression. Furthermore, antitumor immunity was restored in mice lacking SLAMF4 + macrophages, increasing the sensitivity of tumors to anti-PD-L1 therapies [52].
Documentations have shown that increased expression of SLAMF can boost immunogenicity, hence improving treatment response. For example, SLAMF7 expressed on macrophages and tumor cells in lymphoma has been found to play a critical role in mediating anti-CD47-induced macrophage phagocytosis. Specifically, in the presence of anti-CD47, SLAMF7 synergizes with Mac-1 expressed on macrophages to promote phagocytosis of tumor cells [53]. Similarly, high expression of SLAMF8 on macrophages in gastric cancer was associated with enhanced cytotoxic capacity of T cells, resulting in an improved response to anti-PD-1 immunotherapy [54]. Using liver-specific knockout mice, SLAMF7 deficiency has been shown to induce immunosuppressive tumor microenvironment by increasing M2 macrophage infiltration and polarization, enhancing PD-1 expression on CD8 + T cells leading to immune checkpoint blockade resistance and HCC growth and metastasis [55] (Fig. 2).
SLAM family receptors mediate interaction between tumor cells and macrophages in TME. A, B Tumor cells induced M1 macrophages to express SLAMF7 and SLAMF8 to improve the efficacy of anti-PD-1 and anti-CD47 respectively. C M2 macrophages induced by tumor cells express SLAMF3 and SLAMF4 to suppress phagocytosis. D Tumor cell-induced M2 macrophage expressed SLAMF4 to foster immunosuppression. D, E Also, SLAMF6 and SLAMF7 derived from tumor cells induced M2 macrophages to express SLAMF6 and SLAMF7, leading to tumor growth and immunosuppression respectively
SLAM family receptors-mediated interaction between tumor cells and Myeloid-derived suppressor cells (MDSCs) in TME
MDSCs are regarded as one of the potent immunosuppressive cells known to promote tumor progression by suppressing the antitumor functions of T cells [3, 56]. This immunosuppressive capacity of MDSCs is in part attributed to the expression of SLAMF in the tumor microenvironment [57, 58]. In breast cancer, high expression of SLAMF5 was detected on MDSCs derived from human peripheral blood mononuclear cells (PBMCs) and various organs of tumor-bearing mice [59]. Functionally, high SLAMF5 + MDSCs impaired the proliferation of CD8 + T cells [59]. In multiple myeloma (MM), macrophage inhibitory factor (MIF) secreted by MM cells enhanced SLAMF5 expression in the tumor microenvironment to facilitate tumor progression [22]. Delving further, upregulation of SLAMF5 induced high expression of PD-L1 expression on MDSCs, which in turn impaired the cytotoxic capacity of CD8 + T cells. Additionally, interfering with SLAMF5 functions using anti-SLAMF5 antibody blocking in vivo suppressed MDSCs infiltration, and enhanced the anti-tumor functions of CD8 + T cells, thus attenuating tumor growth [22]. In clinical samples from neck and head squamous cell carcinoma (HNSCC), high expression of SLAMF4 corresponded to high expression of PD-1 + T cells demonstrating, an exhausted phenotype [60]. Additionally, SLAMF4 was found to be highly expressed on MDSCs and DC cells, and this was associated with increased expression of PD-L1 and immunosuppression [60]. In established syngeneic tumors, therapeutic inhibition of SLAMF4 using monoclonal SLAMF4 antibody increased CD8 + T cell infiltration which resulted in impaired tumor growth [60]. Using a colon cancer mouse model, Sugita et al. showed that SLAMF2 + polymorphonuclear neutrophils (PMNs)-MDSCs contributed significantly to tumor dissemination to the peritoneal and that in vivo depletion of PMN-MDSCs using anti-Ly6G monoclonal antibody increased antitumor capacity of CD4 + and CD8 + T cells, hence inhibiting peritoneal dissemination [61].
SLAM family receptors-mediated interaction between tumor cells and Natural Killer Cells (NK cells) in TME
NK-mediated cytotoxicity against tumor cells can be regulated by SLAMFs expressed on either tumor cells or NK cells. This has not only revealed the critical role of SLAMFs in NK immunity regulation but has provided the rationale for evaluating SLAMFs as biomarkers for NK cell-based cancer immunotherapies [62].
Accumulating studies have shown that upregulation of SLAMFs in the tumor cells improves the killing abilities of NK cells, and this has been shown across several cancer types. In non-small cell lung cancer cells (NSCLC), high expression of SLAMF4 in cancer cells renders them more susceptible to NK-mediated killing [63]. Mechanistically, SLAMF4 expression in cancer cells mediated a stable contact between NSCLC and NK cells which enhanced the efficient killing of cancer cells [63]. Furthermore, inhibiting SLAMF4 impaired the killing of tumor cells by NK cells [63]. Consistently, low levels of SLAMF1 and SLAMF7 in chronic lymphocytic leukemia (CLL) patients were found to be associated with decreased degradation of NK cells suggesting anti-tumor suppression. Delving further, overexpression of SLAMF1 and SLAMF7 in CLL cells boosted NK-mediated cytotoxicity against CLL, hence reducing their proliferation [64]. According to Sun et al. transmembrane 4 L six family member 5 (TM4SF5) can promote HCC by inducing NK cell exhaustion [65]. Notably, TM4SF5 expression in HCC cells downregulated stimulatory ligands and receptors associated with NK cell cytotoxicity including SLAMF6, SLAMF7 and major histocompatibility complex 1 related chain (MICA) leading to tumor progression [65]. Furthermore, TM4SF5 suppression recovered these receptor ligands and boosted the cytotoxicity capacity of NK cells against HCC cells [65]. In adult T cell leukemia/lymphoma (ATLL), IL2/STAT5-mediated downregulation of SLAMF4 rendered cancer cells resistant to NK cytotoxicity [62]. Similarly, Huang et al. showed that TGF-β derived from leukemia can downregulate SLAMF4 expression on the surface of leukemia cells to promote their escape from NK cell-killing [66]. Furthermore, choriocarcinoma cells lacking SLAMF4 can escape killing by NK cells [67]. Furthermore, AML1-ETO/P300-mediated acetylation can increase the expression of SLAMF2 in acute myeloid leukemia cells (AML) to boost NK cell-killing of AML cells [68], supporting the notion that downregulation of SLAMF2 on the surface of AML cells can promote their escape from NK-mediated immune surveillance [69].
On the other hand, the upregulation of SLAMFs in tumor cells impairs the cytotoxic capacity of NK cells. According to Hosen et al. high expression of SLAMF2 in multiple myeloma cells promotes tumor growth. Additionally, treating mice with anti-SLAMF2 enhanced antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) of NK cells against MM cells [70]. The cytotoxic capacity of NK cells can be impaired by monocytes expressing SLAMF2 [71]. According to Wu et al. monocytes infiltrating HCC cells express high levels of SLAMF2 and the exposure of these monocytes to NK cells induced their exhaustion. Furthermore, in vitro experiments showed that blocking SLAMF2 ligand SLAMF4 on NK cells attenuated NK cell dysfunction [71]. Consistent with the above, clinical samples from clear cell renal carcinoma patients showed overexpression of inhibitory phenotype including PD-1 and SLAMF2 [72] in both circulating and tumor-infiltrating NK cells, suggesting anti-tumor suppression (Fig. 3).
SLAM family receptors mediate interaction between tumor cells and NK cells in TME. A Downregulation of SLAMF1, SLAMF2, SLAMF4, SLAMF6 and SLAMF7 in tumor cells impairs the cytotoxic capacity of natural killer cells. B Tumor cells can upregulate SLAMF2 on monocytes to impair the cytotoxic capacity of natural killer cells
SLAM family receptors-mediated interaction between tumor cells and T cells in the TME
Evidence has shown that SLAM family receptors expressed either by tumor cells or T cells can suppress antitumor function, hence promoting tumor progression. Specifically, SLAMFs are critically involved in the exhaustion of T cells.
In melanoma, leukemia, and lymphoma cells, inhibition of SLAMF6 reversed the exhausted phenotype of PD1 + T cells resulting in tumor regression [73]. Delving deeper, a study has found the constitutive expression of SLAMF6 on CD8 + T cells to be associated with impaired CD8 + T cell-mediated killing of tumors, thus promoting tumor growth [74]. Similarly, Hajaj et al. have showed that CD8 + T cells expressing SLAMF4 produce low levels of IFNγ and IL-2, demonstrating an exhausted phenotype [75]. In support of this, Chen et al. administered PD-1 blockade therapy and anti-SLAMF4 in lung cancer sepsis model and found anti-SLAMF4 but not PD-1 blockade to be associated with improved survival [76]. According to Binsky et al. SLAMF5 activation protects CLL cells from apoptosis through upregulation of anti-apoptotic genes Bcl-2 and Mcl-1[20]. Through in vitro and in vivo studies, SLAMF5 activation induced PD-L1 expression, attenuating antitumor activity of CD8 + T cells. Furthermore, incubation of CD8 + T cells with CLL cells deficient in SLAMF5 restored the cytotoxic capacity of CD8 + T cells [77]. A bioinformatic analysis in glioma has shown that high expression of SLAMF8 is associated with reduced overall survival and chemoresistance. Additionally, high expression of SLAMF8 correlated positively with T cell suppressive markers, such as PD-1, T cell immunoglobulin and mucin domain 3 (TIM-3), cytotoxic T lymphocyte-associated protein (CTLA-4), B7 Homolog 3 (B7-H3), and PD-L2 [78].
On the other hand, SLAMF signaling can act as a T cell activator to promote tumor rejection. Using a human colon cancer model, Mehrle et al. found the adoptive transfer of SLAMF1 overexpressing lymphocytes can increase Th1 response to suppress tumor growth [79]. In melanoma and hepatocellular carcinoma tumor bed, Zhang et al. found PD-1 + SLAMF6 + TIM3 to be associated with improved efficacy to anti-PD-1 blockade compared with terminally exhausted tumor-infiltrating lymphocytes which are defined as PD-1 + SLAMF6-TIM3 + [80] (Fig. 4).
SLAM family receptors mediate interaction between tumor cells and T cells in TME. A SLAMF5 and SLAMF6 released by tumor cells directly suppressed the cytotoxic capacity of T cells. Also, Tumor cell-induced SLAMF4 and SLAMF6 on T cells suppressed their cytotoxic capacity. B Tumor cell-induced SLAMF1 and SLAMF6 on T cells enhanced their cytotoxic capacity
Concluding remarks
This review has elucidated the role of SLAM family receptors in tumor microenvironment interactions, particularly between tumor and immune cells. The insights have demonstrated that some members of the SLAM family receptors can act as inhibitory immune checkpoints and can serve as a biomarker associated with the pathophysiology of solid and hematologic malignancies. Given the fact that current immune checkpoint blockade therapies are designed to target receptor-ligand interaction, SLAM family receptors represent an appealing strategy that can be combined with other immune checkpoint therapies to overcome tumor immune evasive mechanisms and inform the generation of novel immunotherapy approaches that can translated into the clinic.
References
Kwantwi LB. Overcoming anti-PD-1/PD-L1 immune checkpoint blockade resistance: the role of macrophage, neutrophils and mast cells in the tumor microenvironment. Clin Exp Med. 2023;23(7):3077–91.
Galli F, Aguilera JV, Palermo B, Markovic SN, Nisticò P, Signore A. Relevance of immune cell and tumor microenvironment imaging in the new era of immunotherapy. J Exp Clin Cancer Res. 2020;39(1):89.
Kwantwi LB. Genetic alterations shape innate immune cells to foster immunosuppression and cancer immunotherapy resistance. Clin Exp Med. 2023;23(8):4289–96.
Tang T, Huang X, Zhang G, Hong Z, Bai X, Liang T. Advantages of targeting the tumor immune microenvironment over blocking immune checkpoint in cancer immunotherapy. Signal Transduct Target Ther. 2021;6(1):72.
Simoncello F, Piperno GM, Caronni N, Amadio R, Cappelletto A, Canarutto G, et al. CXCL5-mediated accumulation of mature neutrophils in lung cancer tissues impairs the differentiation program of anticancer CD8 T cells and limits the efficacy of checkpoint inhibitors. OncoImmunology. 2022;11(1):2059876.
Sheng Y, Peng W, Huang Y, Cheng L, Meng Y, Kwantwi LB, et al. Tumor-activated neutrophils promote metastasis in breast cancer via the G-CSF-RLN2-MMP-9 axis. J Leukoc Biol. 2023;113(4):383–99.
Cai Z, Zhang M, Boafo Kwantwi L, Bi X, Zhang C, Cheng Z, et al. Breast cancer cells promote self-migration by secreting interleukin 8 to induce NET formation. Gene. 2020;754: 144902.
Kwantwi LB, Wang S, Sheng Y, Wu Q. Multifaceted roles of CCL20 (C-C motif chemokine ligand 20): mechanisms and communication networks in breast cancer progression. Bioengineered. 2021;12(1):6923–34.
Kwantwi LB, Wang S, Zhang W, Peng W, Cai Z, Sheng Y, et al. Tumor-associated neutrophils activated by tumor-derived CCL20 (C-C motif chemokine ligand 20) promote T cell immunosuppression via programmed death-ligand 1 (PD-L1) in breast cancer. Bioengineered. 2021;12(1):6996–7006.
Kwantwi LB. Exosome-mediated crosstalk between tumor cells and innate immune cells: implications for cancer progression and therapeutic strategies. J Cancer Res Clin Oncol. 2023;149(11):9487–503.
Peng W, Sheng Y, Xiao H, Ye Y, Kwantwi LB, Cheng L, et al. Lung adenocarcinoma cells promote self-migration and self-invasion by activating neutrophils to upregulate notch3 expression of cancer cells. Front Mole Biosci. 2022;8:762729.
Salemme V, Centonze G, Cavallo F, Defilippi P, Conti L. The crosstalk between tumor cells and the immune microenvironment in breast cancer: implications for immunotherapy. Front Oncol. 2021;11: 610303.
Barnestein R, Galland L, Kalfeist L, Ghiringhelli F, Ladoire S, Limagne E. Immunosuppressive tumor microenvironment modulation by chemotherapies and targeted therapies to enhance immunotherapy effectiveness. OncoImmunology. 2022;11(1):2120676.
Petitprez F, Meylan M, de Reyniès A, Sautès-Fridman C, Fridman WH. The tumor microenvironment in the response to immune checkpoint blockade therapies. Front Immunol. 2020;11:784.
Meng Y, Ye F, Nie P, Zhao Q, An L, Wang W, et al. Immunosuppressive CD10+ALPL+ neutrophils promote resistance to anti-PD-1 therapy in HCC by mediating irreversible exhaustion of T cells. J Hepatol. 2023;79(6):1435–49.
Mukherjee D, Romano E, Walshaw R, Zeef LAH, Banyard A, Kitcatt SJ, et al. Reprogramming the immunosuppressive tumor microenvironment results in successful clearance of tumors resistant to radiation therapy and anti-PD-1/PD-L1. OncoImmunology. 2023;12(1):2223094.
Han Z, Wu X, Qin H, Yuan YC, Schmolze D, Su C, et al. Reprogramming of PD-1+ M2-like tumor-associated macrophages with anti-PD-L1 and lenalidomide in cutaneous T cell lymphoma. JCI insight. 2023. https://doi.org/10.1172/jci.insight.163518.
Nielsen SR, Strøbech JE, Horton ER, Jackstadt R, Laitala A, Bravo MC, et al. Suppression of tumor-associated neutrophils by lorlatinib attenuates pancreatic cancer growth and improves treatment with immune checkpoint blockade. Nat Commun. 2021;12(1):3414.
Sugimoto A, Kataoka TR, Ito H, Kitamura K, Saito N, Hirata M, et al. SLAM family member 8 is expressed in and enhances the growth of anaplastic large cell lymphoma. Sci Rep. 2020;10(1):2505.
Binsky-Ehrenreich I, Marom A, Sobotta MC, Shvidel L, Berrebi A, Hazan-Halevy I, et al. CD84 is a survival receptor for CLL cells. Oncogene. 2014;33(8):1006–16.
Zhang Y, Zhang Q, Han X, Han L, Wang T, Hu J, et al. SLAMF8, a potential new immune checkpoint molecule, is associated with the prognosis of colorectal cancer. Trans Oncol. 2023;31:101654.
Lewinsky H, Gunes EG, David K, Radomir L, Kramer MP, Pellegrino B, et al. CD84 is a regulator of the immunosuppressive microenvironment in multiple myeloma. JCI insight. 2021. https://doi.org/10.1172/jci.insight.141683.
Agresta L, Hoebe KHN, Janssen EM. The emerging role of CD244 signaling in immune cells of the tumor microenvironment. Front Immunol. 2018;9:2809.
Farhangnia P, Ghomi SM, Mollazadehghomi S, Nickho H, Akbarpour M, Delbandi AA. SLAM-family receptors come of age as a potential molecular target in cancer immunotherapy. Front Immunol. 2023;14:1174138.
Fouquet G, Marcq I, Debuysscher V, Bayry J, Rabbind Singh A, Bengrine A, et al. Signaling lymphocytic activation molecules Slam and cancers: friends or foes? Oncotarget. 2018;9(22):16248–62.
Choe U, Pham Q, Kim YS, Yu L, Wang TTY. Identification and elucidation of cross talk between SLAM family member 7 (SLAMF7) and Toll-like receptor (TLR) pathways in monocytes and macrophages. Sci Rep. 2023;13(1):11007.
Radhakrishnan SV, Bhardwaj N, Luetkens T, Atanackovic D. Novel anti-myeloma immunotherapies targeting the SLAM family of receptors. OncoImmunology. 2017;6(5): e1308618.
Kwantwi LB, Rosen ST, Querfeld C. The role of signaling lymphocyte activation molecule family receptors in hematologic malignancies. Curr Opin Oncol. 2024;36(5):449–55.
Falco M, Marcenaro E, Romeo E, Bellora F, Marras D, Vély F, et al. Homophilic interaction of NTBA, a member of the CD2 molecular family: induction of cytotoxicity and cytokine release in human NK cells. Eur J Immunol. 2004;34(6):1663–72.
Kumaresan PR, Lai WC, Chuang SS, Bennett M, Mathew PA. CS1, a novel member of the CD2 family, is homophilic and regulates NK cell function. Mol Immunol. 2002;39(1–2):1–8.
Martin M, Romero X, De la Fuente MA, Tovar V, Zapater N, Esplugues E, et al. CD84 functions as a homophilic adhesion molecule and enhances IFN-γ secretion: adhesion is meditated by Ig-like domain 1. J Immunol. 2001;167(7):3668–76.
Veillette A. Immune regulation by SLAM family receptors and SAP-related adaptors. Nat Rev Immunol. 2006;6(1):56–66.
Wu N, Veillette A. SLAM family receptors in normal immunity and immune pathologies. Curr Opin Immunol. 2016;38:45–51.
Fraser CC, Howie D, Morra M, Qiu Y, Murphy C, Shen Q, et al. Identification and characterization of SF2000 and SF2001, two new members of the immune receptor SLAM/CD2 family. Immunogenetics. 2002;53(10):843–50.
Calpe S, Wang N, Romero X, Berger SB, Lanyi A, Engel P, et al. The SLAM and SAP gene families control innate and adaptive immune responses. Adv Immunol. 2008;97:177–250.
Kingsbury GA, Feeney LA, Nong Y, Calandra SA, Murphy CJ, Corcoran JM, et al. 2001 Cloning, expression, and function of BLAME, a novel member of the CD2 family. J Immunol. 2001;166(9):5675–80.
Zhou T, Guan Y, Sun L, Liu W. A review: Mechanisms and molecular pathways of signaling lymphocytic activation molecule family 3 (SLAMF3) in immune modulation and therapeutic prospects. Int Immunopharmacol. 2024;133: 112088.
Dong Z, Veillette A. How do SAP family deficiencies compromise immunity? Trends Immunol. 2010;31(8):295–302.
Chen S, Dong Z. NK cell recognition of hematopoietic cells by SLAM-SAP families. Cell Mol Immunol. 2019;16(5):452–9.
Veillette A, Latour S. The SLAM family of immune-cell receptors. Curr Opin Immunol. 2003;15(3):277–85.
Ishibashi M, Morita R, Tamura H. Immune functions of signaling lymphocytic activation molecule family molecules in multiple myeloma. Cancers. 2021;13(2):279.
Fang X, Bai Y, Zhang L, Ding S. Silencing circSLAMF6 represses cell glycolysis, migration, and invasion by regulating the miR-204–5p/MYH9 axis in gastric cancer under hypoxia. Biosci Rep. 2020;40(6):20201275.
Fouquet G, Marié C, Collet L, Vilpoux C, Ouled-Haddou H, Nguyen-Khac E, et al. Rescuing SLAMF3 expression restores sorafenib response in hepatocellular carcinoma cells through the induction of mesenchymal-to-epithelial transition. Cancers (Basel). 2022;14(4):910.
Fouquet G, Debuysscher V, Ouled-Haddou H, Eugenio MS, Demey B, Singh AR, et al. Hepatocyte SLAMF3 reduced specifically the multidrugs resistance protein MRP-1 and increases HCC cells sensitization to anti-cancer drugs. Oncotarget. 2016;7(22):32493–503.
Jun F, Peng Z, Zhang Y, Shi D. Quantitative proteomic analysis identifies novel regulators of methotrexate resistance in choriocarcinoma. Gynecol Oncol. 2020;157(1):268–79.
Shi D, Zhang Y, Tian Y. SLAMF1 promotes methotrexate resistance via activating autophagy in choriocarcinoma cells. Cancer Manag Res. 2020;12:13427–36.
Dollt C, Michel J, Kloss L, Melchers S, Schledzewski K, Becker K, et al. The novel immunoglobulin super family receptor SLAMF9 identified in TAM of murine and human melanoma influences pro-inflammatory cytokine secretion and migration. Cell Death Dis. 2018;9(10):939.
Li D, Xiong W, Wang Y, Feng J, He Y, Du J, et al. SLAMF3 and SLAMF4 are immune checkpoints that constrain macrophage phagocytosis of hematopoietic tumors. Sci Immunol. 2022;7(67):eabj5501.
Meng Q, Duan X, Yang Q, Xue D, Liu Z, Li Y, et al. SLAMF6/Ly108 promotes the development of hepatocellular carcinoma via facilitating macrophage M2 polarization. Oncol Lett. 2022;23(3):83.
O’Connell P, Hyslop S, Blake MK, Godbehere S, Amalfitano A, Aldhamen YA. SLAMF7 signaling reprograms T cells toward exhaustion in the tumor microenvironment. J Immunol. 2021;206(1):193–205.
Wang SH, Chou WC, Huang HC, Lee TA, Hsiao TC, Wang LH, et al. Deglycosylation of SLAMF7 in breast cancers enhances phagocytosis. Am J Cancer Res. 2022;12(10):4721–36.
Kim J, Kim TJ, Chae S, Ha H, Park Y, Park S, et al. Targeted deletion of CD244 on monocytes promotes differentiation into anti-tumorigenic macrophages and potentiates PD-L1 blockade in melanoma. Mol Cancer. 2024;23(1):45.
Chen J, Zhong MC, Guo H, Davidson D, Mishel S, Lu Y, et al. SLAMF7 is critical for phagocytosis of haematopoietic tumour cells via Mac-1 integrin. Nature. 2017;544(7651):493–7.
Zhang Q, Cheng L, Qin Y, Kong L, Shi X, Hu J, et al. SLAMF8 expression predicts the efficacy of anti-PD1 immunotherapy in gastrointestinal cancers. Clin Transl Immunology. 2021;10(10): e1347.
Weng J, Wang Z, Hu Z, Xu W, Sun JL, Wang F, et al. Repolarization of immunosuppressive macrophages by targeting SLAMF7-regulated CCL2 signaling sensitizes hepatocellular carcinoma to immunotherapy. Cancer Res. 2024;84(11):1817–33.
Ma T, Renz BW, Ilmer M, Koch D, Yang Y, Werner J, et al. Myeloid-derived suppressor cells in solid tumors. Cells. 2022;11(2):310.
Sun L, Gang X, Li Z, Zhao X, Zhou T, Zhang S, et al. Advances in understanding the roles of CD244 (SLAMF4) in immune regulation and associated diseases. Front Immunol. 2021;12: 648182.
Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;91(1):167–81.
Alshetaiwi H, Pervolarakis N, McIntyre LL, Ma D, Nguyen Q, Rath JA, et al. Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics. Sci Immunol. 2020;5(44):6017.
Agresta L, Lehn M, Lampe K, Cantrell R, Hennies C, Szabo S, et al. CD244 represents a new therapeutic target in head and neck squamous cell carcinoma. J Immunother Cancer. 2020;8(1): e000245.
Sugita Y, Yamashita K, Fujita M, Saito M, Yamada K, Agawa K, et al. CD244(+) polymorphonuclear myeloid-derived suppressor cells reflect the status of peritoneal dissemination in a colon cancer mouse model. Oncol Rep. 2021;45(6):1–3.
Chiba M, Shimono J, Ishio T, Takei N, Kasahara K, Ogasawara R, et al. Genome-wide CRISPR screens identify CD48 defining susceptibility to NK cytotoxicity in peripheral T-cell lymphomas. Blood. 2022;140(18):1951–63.
Park EJ, Jun HW, Na IH, Lee HK, Yun J, Kim HS, et al. CD48-expressing non-small-cell lung cancer cells are susceptible to natural killer cell–mediated cytotoxicity. Arch Pharmacal Res. 2022;45(1):1–10.
von Wenserski L, Schultheiß C, Bolz S, Schliffke S, Simnica D, Willscher E, et al. SLAMF receptors negatively regulate B cell receptor signaling in chronic lymphocytic leukemia via recruitment of prohibitin-2. Leukemia. 2021;35(4):1073–86.
Sun H, Kim E, Ryu J, Lee H, Shin E-A, Lee M, et al. TM4SF5-mediated liver malignancy involves NK cell exhaustion-like phenotypes. Cell Mol Life Sci. 2021;79(1):49.
Huang C-H, Liao Y-J, Chiou T-J, Huang H-T, Lin Y-H, Twu Y-C. TGF-β regulated leukemia cell susceptibility against NK targeting through the down-regulation of the CD48 expression. Immunobiology. 2019;224(5):649–58.
Avril T, Iochmann S, Brand D, Bardos P, Watier H, Thibault G. Human choriocarcinoma cell resistance to natural killer lysis due to defective triggering of natural killer cells1. Biol Reprod. 2003;69(2):627–33.
Wang Z, Guan W, Wang M, Chen J, Zhang L, Xiao Y, et al. AML1-ETO inhibits acute myeloid leukemia immune escape by CD48. Leuk Lymphoma. 2021;62(4):937–43.
Wang Z, Xiao Y, Guan W, Wang M, Chen J, Zhang L, et al. Acute myeloid leukemia immune escape by epigenetic CD48 silencing. Clin Sci (Lond). 2020;134(2):261–71.
Hosen N, Ichihara H, Mugitani A, Aoyama Y, Fukuda Y, Kishida S, et al. CD48 as a novel molecular target for antibody therapy in multiple myeloma. Br J Haematol. 2012;156(2):213–24.
Wu Y, Kuang D-M, Pan W-D, Wan Y-L, Lao X-M, Wang D, et al. Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology. 2013;57(3):1107–16.
Ziblat A, Iraolagoitia XLR, Nuñez SY, Torres NI, Secchiari F, Sierra JM, et al. Circulating and tumor-infiltrating NK cells from clear cell renal cell carcinoma patients exhibit a predominantly inhibitory phenotype characterized by overexpression of CD85j, CD45, CD48 and PD-1. Front Immunol. 2021;12: 681615.
Yigit B, Wang N, ten Hacken E, Chen S-S, Bhan AK, Suarez-Fueyo A, et al. SLAMF6 as a regulator of exhausted CD8+ T cells in cancer. Cancer Immunol Res. 2019;7(9):1485–96.
Hajaj E, Eisenberg G, Klein S, Frankenburg S, Merims S, Ben David I, et al. SLAMF6 deficiency augments tumor killing and skews toward an effector phenotype revealing it as a novel T cell checkpoint. Elife. 2020;9:e52539.
Mittal R, Chen C-W, Lyons JD, Margoles LM, Liang Z, Coopersmith CM, et al. Murine lung cancer induces generalized T-cell exhaustion. J Surg Res. 2015;195(2):541–9.
Chen CW, Xue M, Zhang W, Xie J, Coopersmith CM, Ford ML. 2B4 but not PD-1 blockade improves mortality in septic animals with preexisting malignancy. JCI Insight. 2019. https://doi.org/10.1172/jci.insight.127867.
Lewinsky H, Barak AF, Huber V, Kramer MP, Radomir L, Sever L, et al. CD84 regulates PD-1/PD-L1 expression and function in chronic lymphocytic leukemia. J Clin Invest. 2018;128(12):5465–78.
Zou C-Y, Guan G-F, Zhu C, Liu T-Q, Guo Q, Cheng W, et al. Costimulatory checkpoint SLAMF8 is an independent prognosis factor in glioma. CNS Neurosci Ther. 2019;25(3):333–42.
Mehrle S, Schmidt J, Büchler MW, Watzl C, Märten A. Enhancement of anti-tumor activity in vitro and in vivo by CD150 and SAP. Mol Immunol. 2008;45(3):796–804.
Zhang R, Chen K, Gong C, Wu Z, Xu C, Li XN, et al. Abnormal generation of IL-17A represses tumor infiltration of stem-like exhausted CD8(+) T cells to demote the antitumor immunity. BMC Med. 2023;21(1):315.
Funding
The author declares that no funds, grants, or other support was received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
LBK conceived the idea, designed, and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author has no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kwantwi, L.B. SLAM family-mediated crosstalk between tumor and immune cells in the tumor microenvironment: a promising biomarker and a potential therapeutic target for immune checkpoint therapies. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03675-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03675-2