Abstract
Objective
The purpose of this study was to explore the appropriate surgical procedure and clinical decision for appendiceal adenocarcinoma.
Methods
A total of 1,984 appendiceal adenocarcinoma patients from 2004 to 2015 were retrospectively identified from the Surveillance, Epidemiology, and End Results (SEER) database. All patients were divided into three groups based on the extent of surgical resection: appendectomy (N = 335), partial colectomy (N = 390) and right hemicolectomy (N = 1,259). The clinicopathological features and survival outcomes of three groups were compared, and independent prognostic factors were assessed.
Results
The 5-year OS rates of patients who underwent appendectomy, partial colectomy and right hemicolectomy were 58.3%, 65.5% and 69.1%, respectively (right hemicolectomy vs appendectomy, P < 0.001; right hemicolectomy vs partial colectomy, P = 0.285; partial colectomy vs appendectomy, P = 0.045). The 5-year CSS rates of patients who underwent appendectomy, partial colectomy and right hemicolectomy were 73.2%, 77.0% and 78.7%, respectively (right hemicolectomy vs appendectomy, P = 0.046; right hemicolectomy vs partial colectomy, P = 0.545; partial colectomy vs appendectomy, P = 0.246). The subgroup analysis based on the pathological TNM stage indicated that there was no survival difference amongst three surgical procedures for stage I patients (5-year CSS rate: 90.8%, 93.9% and 98.1%, respectively). The prognosis of patients who underwent an appendectomy was poorer than that of those who underwent partial colectomy (5-year OS rate: 53.5% vs 67.1%, P = 0.005; 5-year CSS rate: 65.2% vs 78.7%, P = 0.003) or right hemicolectomy (5-year OS rate: 74.2% vs 53.23%, P < 0.001; 5-year CSS rate: 65.2% vs 82.5%, P < 0.001) for stage II disease. Right hemicolectomy did not show a survival advantage over partial colectomy for stage II (5-year CSS, P = 0.255) and stage III (5-year CSS, P = 0.846) appendiceal adenocarcinoma.
Conclusions
Right hemicolectomy may not always be necessary for appendiceal adenocarcinoma patients. An appendectomy could be sufficient for therapeutic effect of stage I patients, but limited for stage II patients. Right hemicolectomy was not superior to partial colectomy for advanced stage patients, suggesting omission of standard hemicolectomy might be feasible. However, adequate lymphadenectomy should be strongly recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The malignant tumour of the appendix is an uncommon clinical entity, which mainly includes appendiceal carcinoid tumours and appendiceal adenocarcinoma. Appendiceal malignancy usually is accidentally detected during an appendectomy for acute appendicitis or postoperative histopathological examination [1, 2]. In other words, it is difficult to make a definite diagnosis for appendiceal tumours before surgery. It has been estimated that the incidence of primary appendiceal adenocarcinoma was 0.4–1.0% of all gastrointestinal malignancies and 50.5–65.0% of appendiceal neoplasms [3,4,5,6].
Due to extremely low prevalence, the demographic, clinicopathological characteristics and survival outcomes of appendiceal adenocarcinoma patients remain unclear. In terms of surgical procedure and clinical management, the optimal method for appendiceal adenocarcinoma also is still questionable. Some scholars believed that appendiceal adenocarcinoma should be treated as colon adenocarcinoma and proposed right hemicolectomy as the standard surgical procedure for this malignancy [7, 8]. However, this opinion has been challenged by increasing evidence. Accumulative studies have reported that right hemicolectomy could not improve the long-term oncologic outcome of appendiceal adenocarcinoma patients compared with an appendectomy [9,10,11]. To date, there is no clinical consensus on how to obtain the best outcome of surgical intervention. In the present study, we retrospectively reviewed the clinicopathological characteristics and survival data of appendiceal adenocarcinoma patients using the Surveillance, Epidemiology, and End Results (SEER) database in order to determine the appropriate surgical procedure and clinical decision for this rare malignancy.
Materials and methods
Study population
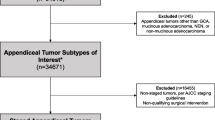
We used the International Classification of Disease for Oncology 3 (ICD-O-3) codes to identify appendiceal adenocarcinoma patients (site code: C18.1 histological code: 8020/3, 8480/3, 8140/3, 8144/3 and 8490/3) from the Surveillance, Epidemiology, and End Results (SEER) 18 Registries Research database (1973–2016). The eligible criteria of this analysis were as follows: (1). Patients received surgical treatment and were histopathologically diagnosed with appendiceal adenocarcinoma (including mucinous adenocarcinoma and signet-ring cell carcinoma); (2). No distant metastasis (M0 stage) at the initial diagnosis; (3). Patient age ≥ 18 years old; (4). Survival status was known and duration of follow-up was not less than one month.
Finally, a total of 1984 consecutive patients who underwent surgical treatment for appendiceal adenocarcinoma between 2004 and 2015 were included in this retrospective analysis. Ethical approval and informed consent were waived since the data of all patients were obtained from a publicly available database. This study was completed following the STROBE reporting checklist.
Variables and outcomes
The SEER database was accessed via free public website at www.seer.cancer.gov, and relevant data were extracted using the SEER*Stat software (version 8.3.6). Investigators received permission from the SEER programme to access the original data. Data collection included demographic (age, sex and race), clinicopathologic features (year of diagnosis, surgical procedure, histological classification, histological grade, tumour size, pT stage, pN stage, pathological TNM stage, lymph node yield, and serum level of carcinoembryonic antigen at the time of diagnosis) and follow-up information (follow-up duration and vital status). The pathological stage of appendiceal adenocarcinoma was determined according to the 7th edition of the TNM classification of the American Joint Commission on Cancer (AJCC). This was defined as the intra-mucosal carcinoma, pT1 stage was defined as the submucosal invasion of tumour, pT2 stage was defined as the invasion of muscularis propria, pT3 stage was described as the lesion that invaded through muscularis and/or subserosa and pT4 stage was the invasion of mesoappendix, adjacent tissue and/or organ. The patients with 1–3 positive lymph nodes were classified as pN1 stage, and ≥ 4 positive nodes were classified as pN2 stage.
The extent of surgical resection for appendiceal adenocarcinoma was identified by Surgery Codes Manual of SEER Programme. In the SEER database, right hemicolectomy is described as the removal of right colon and a portion of transverse colon (Code 40 and 41). According to the Surgery Codes Manual, Code 30 included appendectomy (for an appendix primary only), cecectomy, ileocolectomy and partial colectomy. To further distinguish an appendectomy from partial colectomy but less than hemicolectomy, patients with Surgery code 30 were subdivided based on the number of lymph node harvest. If more than 2 lymph nodes were harvested, the extent of surgical resection was defined as partial colectomy. If not, code 30 designates an appendectomy [11].
The primary event of survival analysis was overall survival (OS), which defined as the time interval from the date of diagnosis to the date of death owing to any causes. The other observation was cancer-specific survival (CSS), which was defined as the period from the date of diagnosis to the date of death owing to cancer-related causes. The observations of patients who died of other causes or were alive at the end of follow-up were treated as censored events.
Statistical analysis
Categorical variables were summarised by frequency distributions in a descriptive table and were compared by Pearson’s chi-square test or Fisher’s exact test as appropriate. Continuous variables were expressed as median with interquartile range (IQR), and non-parametric test was used to compare the statistical difference between groups. Survival analysis was conducted by Kaplan–Meier method with log-rank test. The prognostic significance of each clinicopathologic factor was tested by the univariate Cox regression analysis, and the variables with a significant level were included in the multivariate analysis to determine independent prognostic factors for appendiceal adenocarcinoma patients. The data for univariate and multivariate Cox regression analysis were expressed as hazard ratio (HR) with 95% confidence intervals (CIs). The predictive factors for lymph node metastasis in appendiceal adenocarcinoma were assessed using the Logistic regression model. The statistical package for Windows SPSS 23.0 version (IBM Inc, New York, USA) was used to implement data processing and statistical analysis, and a two-sided P value of less than 0.05 was considered statistically significant.
Results
Patient characteristics of study cohort
From 2004 to 2015, a total of 1984 appendiceal adenocarcinoma patients were identified from the SEER database. The proportion of appendiceal adenocarcinoma patients presented a sustained rising tendency over the last decade (2004–2007: 28.1%, 2008–2011: 33.6%, and 2012–2015: 38.3%) (Table 1). The entire cohort consisted of 1040 males (52.4%) and 944 females (47.6%), and the proportion of patients aged 60 or older was 55.6% (1109/1984). The vast majority of patients were Caucasian (81.1%). The moderately differentiated adenocarcinoma accounted for 45.4% of all patients. In terms of histological type, the proportion of mucinous adenocarcinoma and signet-ring cell carcinoma was 46.3% and 10.1%, respectively. In addition, elevated CEA level was detected in 216 appendiceal adenocarcinoma patients (10.9%) at the time of diagnosis.
With regard to the extent of surgical resection, 16.9% (N = 335) patients received an appendectomy, 19.7% (N = 390) had a partial colectomy and 63.4% (N = 1259) underwent right hemicolectomy for appendiceal adenocarcinoma. The frequency of lymph node metastasis was 21.9% (435/1984), and the median of positive lymph nodes was 2 (IQR: 1–5). The median of lymph nodes harvest was 16 (IQR: 12–22). According to the 7th edition of the TNM classification of the AJCC, the proportion of stage I, stage II and stage III were 20.7% (410/1984), 57.6% (1143/1984) and 21.7% (431/1984), respectively.
For the purpose of this study, we further divided appendiceal adenocarcinoma patients into three research groups based on the surgical procedure: appendectomy, partial colectomy (but less than hemicolectomy) and right hemicolectomy group. The comparisons of demographic and clinicopathological characteristics of three groups are summarised in Table 1. Compared with appendectomy group, patient age was younger (P = 0.007), the proportion of poorly differentiated/undifferentiated adenocarcinoma (P < 0.001), pT4 stage (P < 0.001) and TNM stage III (P < 0.001) was higher, lymph node metastasis (P < 0.001) was more frequent, and tumour size (P < 0.001) was large in partial colectomy/right hemicolectomy group. There were no distribution differences between right hemicolectomy and partial colectomy group except for the number of lymph node yield (P < 0.001).
Comparisons of survival outcomes of appendiceal adenocarcinoma patients based on the extent of surgical resection
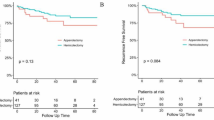
In this study cohort, the median follow-up period was 46 months (IQR: 25–84 months). The 5-year OS and 5-year CSS rates of appendiceal adenocarcinoma patients were 77.6% and 66.6%, respectively. Figure 1 showed the survival curves of appendiceal adenocarcinoma patients who underwent different surgical procedures. The 5-year OS rates of patients who underwent appendectomy, partial colectomy and right hemicolectomy were 58.3%, 65.5% and 69.1%, respectively. The 5-year CSS rates of patients who underwent appendectomy, partial colectomy and right hemicolectomy were 73.2%, 77.0% and 78.7%, respectively. The survival analysis showed that patients who underwent right hemicolectomy had a better OS (P < 0.001) and CSS (P = 0.046) than those who underwent appendectomy (Fig. 1). In addition, patients who underwent partial colectomy had a slightly better OS than those who underwent appendectomy (P = 0.045). There were no significant survival differences between right hemicolectomy and partial colectomy for appendiceal adenocarcinoma (5-year OS, P = 0.285; 5-year CSS, P = 0.545).
The subgroup analysis based on the pathological TNM stage indicated that 5-year OS rate of patients who underwent right hemicolectomy was superior to that of those who underwent appendectomy for stage I (83.5% vs 68.9%, P = 0.002) and stage II (74.2% vs 53.23%, P < 0.001) disease. However, right hemicolectomy did not show a survival advantage over partial colectomy for appendiceal adenocarcinoma patients (stage I: 83.5% vs 86.9%, P = 0.537; stage II: 74.2% vs 67.1%, P = 0.052; stage III: 42.2% vs 48.6%, P = 0.554). In terms of CSS, there was no survival difference amongst three surgical procedures for stage I patients. The 5-year CSS rate of appendectomy, partial colectomy and right hemicolectomy group was 90.8%, 93.9% and 98.1%, respectively (P > 0.05). For stage II patients, the prognosis of patients who underwent an appendectomy was poorer than that of those who underwent partial colectomy (5-year CSS rate: 65.2% vs 78.7%, P = 0.003) or right hemicolectomy (65.2% vs 82.5%, P < 0.001). However, the survival outcome of patients who underwent right hemicolectomy was not significantly different from that of those who underwent partial colectomy for stage II (P = 0.255) and stage III (P = 0.846) appendiceal adenocarcinoma (Fig. 2).
Univariate and multivariate analysis of prognostic factors for appendiceal adenocarcinoma patients
The data of univariate and multivariate Cox analysis showed that age (HR for ≥ 60 years old: 1.730, 95% CI 1.481–2.021, P < 0.001), histological grade (HR for moderately differentiated adenocarcinoma: 1.744, 95% CI 1.400–2.173, P < 0.001; HR for poorly differentiated/undifferentiated adenocarcinoma: 2.012, 95% CI 1.560–2.594, P < 0.001), pT stage (HR for pT3 stage: 1.458, 95% CI: 1.065–1.996, P = 0.019; HR for pT4 stage: 2.269, 95% CI 1.654–3.112, P < 0.001), lymph node metastasis (HR for pN1 stage: 1.600, 95% CI 1.299–1.972, P < 0.001; HR for pN2 stage: 3.023, 95% CI 2.372–3.854, P < 0.001), the extent of surgical resection (HR for partial colectomy: 0.535, 95% CI 0.416–0.689, P < 0.001; HR for right hemicolectomy: 0.508, 95% CI 0.413–0.624, P < 0.001), lymph nodes yield (HR for < 12 nodes: 1.694, 95% CI 1.414–2.028, P < 0.001) and CEA level (HR: 1.814, 95% CI 1.401–2.350, P < 0.001) were independent predictors of OS for appendiceal adenocarcinoma patients (Table 2).
Moreover, histological grade (HR for moderately differentiated adenocarcinoma: 1.597, 95% CI 1.194–2.137, P = 0.002; HR for poorly differentiated/undifferentiated adenocarcinoma: 1.934, 95% CI 1.394–2.684, P < 0.001), pT stage (HR for pT3 stage: 3.444, 95% CI 1.856–6.394, P < 0.001; HR for pT4 stage: 6.255, 95% CI 3.375–11.591, P < 0.001), lymph node metastasis (HR for pN1 stage: 1.921, 95% CI 1.476–2.500, P < 0.001; HR for pN2 stage: 4.402, 95% CI 3.240–5.979, P < 0.001), the extent of surgical resection (HR for partial colectomy: 0.578, 95% CI 0.403–0.830, P = 0.003; HR for right hemicolectomy: 0.576, 95% CI 0.419–0.793, P = 0.001), lymph nodes yield (HR for < 12 nodes: 1.468, 95% CI 1.153–1.870, P = 0.002) and CEA level (HR: 2.104, 95% CI 1.519–2.915, P < 0.001) were identified as independent predictors of CSS for appendiceal adenocarcinoma patients (Table 3).
Univariate and multivariate analysis of predictive factors for lymph node metastasis in appendiceal adenocarcinoma
The results of univariate and multivariate logistic regression analysis demonstrated that moderately differentiated (OR: 1.665, 95% CI 1.166–2.376, P = 0.005) and poorly differentiated adenocarcinoma (OR: 3.721, 95% CI 2.483–5.575, P < 0.001), tumour size > 4 cm (OR: 1.923, 95% CI 1.341–2.758, P < 0.001) and the depth of tumour invasion (OR for T2 stage: 2.415, 95% CI 1.014–5.753, P = 0.046; OR for T3 stage: 5.420, 95% CI 2.570–11.430, P < 0.001; OR for T4 stage: 10.056, 95% CI 4.765–21.223, P < 0.001) were independent predictive factors for lymph node metastasis (Table 4). Interestingly, we found that mucinous adenocarcinoma had a relatively low frequency of lymph node metastasis in comparison to other histological types (OR: 0.478, 95% CI 0.365–0.627, P < 0.001).
Discussion
As a rare neoplasm occurred at the gastrointestinal tract, the standard surgical management and therapeutic strategy for appendiceal adenocarcinoma have been not well established yet. Appendiceal adenocarcinoma usually was treated as colon counterparts and it is common practice to perform a right hemicolectomy. However, whether right hemicolectomy had more advantages over an appendectomy or partial colectomy for appendiceal adenocarcinoma in terms of the long-term oncological outcome remains debatable. Several reports have shown that an appendectomy could be sufficient to attain a satisfactory treatment effect for early-stage appendiceal adenocarcinoma with favourable biological features [10, 12, 13]. A retrospective analysis of 2487 patients from National Cancer Database reported that the incidence of lymph node metastasis in appendiceal adenocarcinoma was 26.2%, but was 1.8% in early-stage (pT1 stage) appendiceal adenocarcinoma with well/moderately differentiated type [14]. The study by AlMasri found that appendectomy is oncologically equivalent to right hemicolectomy for early-stage appendiceal adenocarcinoma with well-differentiated type [13]. In current patient series, lymph node metastasis was detected in 21.9% of all appendiceal adenocarcinoma patients and 3.0% of pT1 stage patients, which was in accordance with previous data [8, 14, 15]. Survival analysis indicated that right hemicolectomy seems to be associated with better OS of appendiceal adenocarcinoma patients with stage I, but it did not improve CSS. This result might be explained by the fact that more elderly patients underwent an appendectomy and they had increased non-cancer-related mortality over time. However, the extent of surgical resection (appendectomy, partial colectomy or right hemicolectomy) did not affect cancer-related survival of stage I appendiceal adenocarcinoma patients. Taking into account low rate of lymph node metastasis, omission of standard right hemicolectomy might be safe and feasible for early-stage appendiceal adenocarcinoma with favourable biological features.
Lymph node metastasis is an important prognostic factor for appendiceal adenocarcinoma patients. The general consensus is that right hemicolectomy is the best surgical procedure for advanced stage patients. The current study supported a survival advantage of right hemicolectomy or partial colectomy rather than appendectomy for advanced appendiceal adenocarcinoma. However, right hemicolectomy did not show a better oncological outcome than partial colectomy in terms of cancer-related survival for stage II patients. Theoretically, right hemicolectomy could effectively dissect regional lymph nodes along with the ileocolic territory (including the root of the appendix and mesoappendix), providing a reliable N stage and potential survival benefit for appendiceal adenocarcinoma patients [8, 16]. Our data also indicated that right hemicolectomy had a larger number of lymph node yield than partial colectomy [16.0 (13.0, 22.0) vs 14.0 (9.0, 20.0), P < 0.001]. However, 67.1% (262/390) of patients who underwent partial colectomy achieve at least 12 lymph node harvest. Yada et al. analysed the relationship between arterial branching patterns and lymph node metastasis in cecum cancer patients, and found that positive nodes was mainly detected along the ileocolic artery [17]. They believed that ileocaecal resection might be sufficient to fulfil the demand of lymphadenectomy for cecum cancer. These findings might be applicable equally to appendiceal adenocarcinoma patients and thus explain why right hemicolectomy did not further improve oncological outcome. In the multivariate Cox regression analysis, ≥ 12 lymph nodes harvest was an independent predictor of better survival for appendiceal adenocarcinoma patients, highlighting the prognostic significance of adequate lymphadenectomy. Therefore, partial colectomy but less than hemicolectomy (e.g. colectomy or ileocecectomy) might be appropriate for advanced appendiceal adenocarcinoma if adequate lymphadenectomy is performed.
In routine clinical practice, appendiceal tumour usually is accidentally detected during appendectomy or in surgically resected samples. In this context, the pathological evaluation for biological characteristics of the lesion had an important guidance role in the selection of lymphadenectomy. In the current study, our data demonstrated that moderately/poorly differentiated adenocarcinoma, tumour size > 4 cm and pT3–T4 stage were independent risk factors for lymph node metastasis in appendiceal adenocarcinoma. For patients with these clinicopathologic features, adequate lymphadenectomy should be strongly recommended. In addition, we found that mucinous adenocarcinoma had a lower frequency of lymph node metastasis than other histological types. As previously reported, mucinous adenocarcinoma, which was characterised by abnormal mucin production, was the most common subtype of appendiceal adenocarcinoma [18, 19]. It is often thought that mucinous adenocarcinoma has less frequent lymph node involvement but higher risk of peritoneal dissemination [18,19,20]. Therefore, it is necessary to pay more attention to postoperative surveillance of this histological type.
In addition to lymph node metastasis and lymph node yield, pT3–T4 stage, low grade of histology and elevated CEA level were identified as independent prognostic factors for appendiceal adenocarcinoma patients. Surgical intervention alone may be difficult to obtain a satisfactory treatment outcome for patients with these aggressive biological features. With the development of multi-disciplinary treatment concept, adjuvant chemotherapy has become an indispensable part of clinical management for cancer patients. However, there is no specific consensus on the therapeutic role of adjuvant chemotherapy for appendiceal adenocarcinoma. By analysing data from the National Cancer Data Base (NCDB), recently, Asare et al. reported a survival benefit from adjuvant chemotherapy for appendiceal adenocarcinoma patients without distant metastasis irrespective of histological type [20]. This finding supported the use of adjuvant chemotherapy in non-metastatic appendiceal adenocarcinoma patients. However, whether the prognosis of appendiceal adenocarcinoma patients could be improved by adjuvant chemotherapy needs to be further investigated.
Several limitations of this study require further acknowledgement. First, the current study was limited by its retrospective design and inherent selection bias from the large population-based database. Second, a few clinicopathological variables, such as adjuvant treatment, resection margins and molecular features, were not available in the public SEER database. These important parameters could not be adjusted in the current analysis. In addition, up to 31.5% and 67.6% of patients had missing data on tumour size and serum CEA level, which was another major limitation of this analysis. Third, surgical code of SEER Programme database may be not fully accurate because it could not distinguish appendectomy from partial colectomy. It is also unclear whether an initial appendectomy has been performed for appendiceal adenocarcinoma patients who underwent right hemicolectomy.
In conclusion, the results of this large population-based analysis showed that right hemicolectomy may be not always necessary for all appendiceal adenocarcinoma patients. Appendectomy could be sufficient for therapeutic effect of stage I patients with favourable biological features. In contrast, the oncological outcome of an appendectomy was limited for stage II patients. Right hemicolectomy was not superior to partial colectomy for advanced stage patients, suggesting omission of standard hemicolectomy might be feasible. However, adequate lymphadenectomy should be strongly recommended.
Data availability
The supportive data of this manuscript are available from the public SEER database (https://seer.cancer.gov/).
References
Kelly KJ. Management of Appendix Cancer. Clin Colon Rectal Surg. 2015;28(4):247–55.
Köhler F, Matthes N, Rosenfeldt M, Kunzmann V, Germer CT, Wiegering A. Neoplasms of the Appendix. Dtsch Arztebl Int. 2023 Sep 04 (Forthcoming). https://doi.org/10.3238/arztebl.m2023.0136
Connor SJ, Hanna GB, Frizelle FA. Appendiceal tumors: retrospective clinicopathologic analysis of appendiceal tumors from 7,970 appendectomies. Dis Colon Rectum. 1998;41(1):75–80.
Murphy EM, Farquharson SM, Moran BJ. Management of an unexpected appendiceal neoplasm. Br J Surg. 2006;93(7):783–92.
Benedix F, Reimer A, Gastinger I, Mroczkowski P, Lippert H, Kube R, et al. Primary appendiceal carcinoma–epidemiology, surgery and survival: results of a German multi-center study. Eur J Surg Oncol. 2010;36(8):763–71.
Marmor S, Portschy PR, Tuttle TM, Virnig BA. The rise in appendiceal cancer incidence: 2000–2009. J Gastrointest Surg. 2015;19(4):743–50.
Whitfield CG, Amin SN, Garner JP. Surgical management of primary appendiceal malignancy. Colorectal Dis. 2012;14(12):1507–11.
Gahagan JV, Whealon MD, Phelan MJ, Mills S, Pigazzi A, Stamos MJ, et al. Lymph node positivity in appendiceal adenocarcinoma: should size matter. J Am Coll Surg. 2017;225(1):69–75.
González-Moreno S, Sugarbaker PH. Right hemicolectomy does not confer a survival advantage in patients with mucinous carcinoma of the appendix and peritoneal seeding. Br J Surg. 2004;91(3):304–11.
Turaga KK, Pappas S, Gamblin TC. Right hemicolectomy for mucinous adenocarcinoma of the appendix: just right or too much. Ann Surg Oncol. 2013;20(4):1063–7.
Nasseri YY, Zhu R, Sutanto C, Wai C, Cohen JS, Ellenhorn J, et al. Role of right hemicolectomy in patients with low-grade appendiceal mucinous adenocarcinoma. Am J Surg. 2019;218(6):1239–43.
Hata K, Tanaka N, Nomura Y, Wada I, Nagawa H. Early appendiceal adenocarcinoma. A review of the literature with special reference to optimal surgical procedures. J Gastroenterol. 2002;37(3):210–4.
AlMasri SS, Hammad AY, Singhi AD, Paniccia A, Zureikat AH, Celebrezze JP Jr, et al. Appendectomy Is oncologically equivalent to right hemicolectomy for well-differentiated T1 appendiceal adenocarcinoma. Dis Colon Rectum. 2023;66(1):67–74.
Straker RJ 3rd, Grinberg SZ, Sharon CE, Shannon AB, Fraker DL, Shanmugan S, et al. Pathologic factors associated with low risk of lymph node metastasis in nonmucinous adenocarcinoma of the appendix. Ann Surg Oncol. 2022;29(4):2334–43.
Shannon AB, Goldberg D, Song Y, Paulson EC, Roses RE, Fraker DL, et al. Predictors of lymph node metastases in patients with mucinous appendiceal adenocarcinoma. J Surg Oncol. 2020;122(3):399–406.
Fleischmann I, Warschkow R, Beutner U, Marti L, Schmied BM, Steffen T. Improved survival after retrieval of 12 or more regional lymph nodes in appendiceal cancer. Eur J Surg Oncol. 2017;43(10):1876–85.
Yada H, Sawai K, Taniguchi H, Hoshima M, Katoh M, Takahashi T. Analysis of vascular anatomy and lymph node metastases warrants radical segmental bowel resection for colon cancer. World J Surg. 1997;21(1):109–15.
Mo S, Zhou Z, Ying Z, Dai W, Xiang W, Han L, et al. Epidemiology of and prognostic factors for appendiceal carcinomas: a retrospective, population-based study. Int J Colorectal Dis. 2019;34(11):1915–24.
Day RW, Chang YH, Stucky CC, Gray R, Pockaj B, Wasif N. A predictive model for nodal metastases in patients with appendiceal cancers. Ann Surg. 2021;274(1):155–61.
Enblad M, Graf W, Birgisson H. Risk factors for appendiceal and colorectal peritoneal metastases. Eur J Surg Oncol. 2018;44(7):997–1005.
Funding
This work was supported by the Tianjin Health Science and Technology Project (NO.ZC20213).
Author information
Authors and Affiliations
Contributions
(I) Study conception and design: ZBC and MKW; (II) Data collection: ZBC and WJC; (III) Data analysis and interpretation: ZBC, MZC, WJC and WWQ; (IV). Manuscript drafting: ZBC; (V) Manuscript editing and preparation: ZBC and MZC; (VI) Manuscript review and supervision: YT and MKW. All authors read and approved the final edition of this manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and informed consent
Ethical approval and informed consent were waived since the data of all patients were obtained from a publicly available database.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, B., Ma, Z., Wang, J. et al. Which is the appropriate surgical procedure for appendiceal adenocarcinoma: appendectomy, partial colectomy or right hemicolectomy?. Clin Transl Oncol 26, 297–307 (2024). https://doi.org/10.1007/s12094-023-03259-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03259-6