Abstract
Background
The aim of this study is to evaluate the epidemiology of and prognostic factors for appendiceal carcinomas (ACs).
Methods
All cases of ACs registered in the Surveillance, Epidemiology, and End Results (SEER) database from 1973 to 2014 were retrospectively identified in this study. Age-adjusted incidence and survival rates were calculated.
Results
We analyzed 7170 patients with ACs. We observed a significant increase in the reported annual age-adjusted incidence of ACs from 1973 (0.18/100,000) to 2014 (1.11/100,000). The elevation of the incidence was noted in all the histological types, stages, and grades. The most common histological type varied by race, with the appendiceal mucinous adenocarcinoma (AMA) being the most common in white, Asian/Pacific Islander, and American Indian/Alaskan Native patients, and the appendiceal adenocarcinoma (AA) being the most common in African American patients. In multivariate analysis of patients with all ACs, gender (P < 0.001), year of diagnosis (P < 0.001), age (P < 0.001), race (P < 0.001), tumor grade (P < 0.001), disease stage (P < 0.001), retrieved regional lymph nodes (P < 0.001), type of surgery performed (P = 0.002), and histologic subtype (P < 0.001) were predictors of outcome. Survival time for all ACs increased from the 1973–1993 period to the 1994–2014 period (HR 0.76; 95% CI, 0.69 to 0.85). Additionally, the 5-year survival rates were 88% for malignant carcinoid, 70% for goblet cell carcinoid, 51% for colonic type adenocarcinoma, 59% for mucinous adenocarcinoma, and 25% for signet ring cell type.
Conclusions
We observed increased reported incidence of ACs and increased survival durations over time, suggesting that clinicians pay more attention to ACs and mastering the characteristic of these tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Appendiceal carcinoma (AC) is a rare malignant tumor with aggressive potentials [1]. Morphologically, ACs can be very similar to colon adenocarcinoma. However, AC is more likely to exhibit a mucinous phenotype that causes pseudomyxoma peritonei [2, 3]. Often, the diagnosis of AC is unexpected; these rare neoplasms are seldom suspected before surgery and they are found in approximately 1% of all appendectomy specimens [3, 4]. Although rare, ACs are associated with considerable mortality, which is due in part to phenomenon of symptom hiding, resulting in the frequency of late stage at diagnosis. ACs exhibit a different spectrum of biologic behavior. This disease remains poorly understood and there is much controversy about the optimal treatment modalities [5]. In order to understand the characteristics of ACs, we need to discover commonalities from a large amount of public data. In this way, this study will help clinicians master the characteristic of these tumors so that patients can benefit from this research.
For rare tumors, population-based data sources, which are not affected by selection bias due to institutional referral patterns, can provide investigators a large amount of analyzable data to summarize such diseases. Sponsored by the National Cancer Institute (NCI), the Surveillance, Epidemiology, and End Results (SEER) program is a system of population-based cancer registries. Currently, the SEER program covers approximately 28% of the US population from geographically defined areas [6]. In addition to reporting national cancer statistics on morbidity and survival, the SEER registries can also serve as a terrace for studying cancer-related care and health differences. The purpose of this study, therefore, was to perform a population-based evaluation of the epidemiology of and prognostic factors for ACs.
Methods
Data source
SEER*Stat software, version 8.2.1 (Surveillance Research Program, National Cancer Institute), was used for data acquisition and incidence analysis. As a population-based cancer registry, SEER program has undergone 2 major expansions to include additional areas (SEER 13 in 1992 and SEER 18 in 2000), since its inception in 1973 (SEER 9 registry). SEER program currently includes 20 geographic areas with demographics representative of the entire US population [7]. The pertinent population data used in the SEER*Stat software are obtained from the US Census Bureau’s Population Estimates Program, in collaboration with the National Center for Health Statistics and with support from the NCI through an interagency agreement. Mortality data are obtained from the US National Center for Health Statistics. The SEER 9, 13, and 18 registries cover approximately 9.5%, 13.8%, and 28%, respectively, of the total US population [7]. The SEER program collects data periodically on patient demographics, tumor morphology, primary tumor site, disease stage at diagnosis, first course of treatment, and follow-up for vital status. Incidence rates were calculated by the incidence rate function provided by the SEER*Stat program.
AC classification
AC was identified by pathological diagnosis in SEER dataset. We used histologic codes from the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3), to identify patients with ACs. According to ICD-O-3 oncology codes, histological types of ACs are classified into five subtypes. These codes correspond to the following clinical/histologic diagnoses: malignant carcinoid tumors (8240, 8241, 8249), goblet cell carcinoid (8243, 8244, 8245, 8246, 8574, 8013), adenocarcinoma (8010, 8020, 8140, 8141, 8144, 8211, 8210, 8255, 8260, 8261, 8262, 8263, 8310, 8440, 8460, 8550, 8560), mucinous adenocarcinoma (8470, 8471, 8472, 8480, 8481), and signet ring cell adenocarcinoma (8490).
Data collection
We used SEER histologic grade information to classify cases into four grades: grade I, also called well differentiated; grade II, also called moderately differentiated; grade III, also called poorly differentiated; grade IV, also called undifferentiated or anaplastic. The SEER staging system was used for ACs staging analysis. ACs were classified as localized, regional, or distant. A localized AC was defined as an invasive neoplasm confined entirely to the organ of origin. A regional AC was defined as a neoplasm that (1) extended beyond the limits of the organ of origin directly into surrounding organs or tissue, (2) involved regional lymph nodes, or (3) fulfilled both of the aforementioned criteria. Finally, a distant AC was defined as a neoplasm that spreads to parts of the body remote from the primary tumor. The type of surgical resection was obtained by using classification codes. This was classified broadly into no surgery, local surgery (excision, destruction, curettage), partial colectomy (including appendectomy), right colectomy or subtotal colectomy, total proctocolectomy, and colectomy plus resection of contiguous organ. Information on each case included age at diagnosis, year of diagnosis, sex, race, grade, histology, surgery type, vital status, and stage. As several clinicopathological variables had missing data, and as simply excluding patients with missing data is inefficient and increases the risk of selection bias, we used multiple imputation before data analysis (using multivariate imputation by chained equations [8]). Rubin’s rules were used to pool results across imputation data sets [9].
Statistical analysis
Given that there are 3 SEER registry groupings, to maximize the representativeness of this study, we calculated the 1973–1991 incidences using SEER 9, the 1992–1999 incidences using SEER 13, and the 2000–2012 incidences using SEER 18 databases. Comparisons of patients, tumor characteristics, and disease extension were performed using the chi-square test. One-way analysis of variance was used for comparison of continuous variables between groups. We measured survival durations by the Kaplan-Meier method and compared by the log-rank test. The statistical independence between prognostic variables was assessed using the Cox proportional hazards model.
The alpha error was set at 0.05, and all P values indicate two-sided tests. All other statistical calculations were performed using SPSS (version 20.0; SPSS Inc., Chicago, IL). All statistical tests were two-sided, and P values < 0.05 were considered statistically significant.
Result
Patient characteristics
A total of 7170 patients with ACs identified in this study, of which 3832(53.4%) were women and 3338 (46.6%) were men. 83.2% of the patients were white, 10.2% were African American, 6.1% were Asian/Pacific Islander, and 0.5% were American Indian/Alaskan native. The median age at diagnosis was 59 years (interquartile range 48–71). ACs are commonly classified by histologic subtype as malignant carcinoid, goblet cell carcinoid, adenocarcinoma, mucinous adenocarcinoma, and signet ring cell adenocarcinoma. Of the 7170 cases, 642 (9.0%) were malignant carcinoid, 909 (12.7%) were goblet cell carcinoid, 2348 (32.7%) were adenocarcinoma, 2805 (39.1%) were mucinous adenocarcinoma, and the remaining 466 (6.5%) were signet ring cell carcinoma.
Detailed demographic and tumor/therapy characteristics by histologic subtype in SEER database are presented in Table 1. As for the disease stage of the total 7170 cases, 2925 (40.8%) were localized, 1782 (24.9%) were regional, and 2463 (34.3%) were distant. AC patients with signet ring cell carcinoma were more likely to have poorly differentiated histology (78.8%) and they were more prone to distant metastasis (60.3%). Compared with adenocarcinomas, mucinous tumors were more likely to have metastatic disease and be well-differentiated tumors (Table 1). As for therapeutic information of AC patients, 225 (3.1%) did not undergo surgery, 371 (5.2%) undergone local surgery, 2334 (32.6%) undergone partial colectomy (including appendectomy), 2791 (38.9%) undergone right colectomy and subtotal colectomy, 52 (0.7%) were performed total colectomy, while 1397 (19.5%) were resected contiguous organ.
Incidence
Using population data registered in the SEER database, we calculated the incidence of ACs per 100,000 per year. Because the SEER 9, 13, and 18 are based on population data in different states, we computed the age-adjusted incidence for three periods: SEER 9, 1973 to 1991; SEER 13, 1992 to 1999; SEER 18, 2000 to 2014. We observed a significant increase in the reported annual age-adjusted incidence of ACs from 1973 (0.18/100,000) to 2014 (1.11/100,000). In contrast, the overall incidence of all malignant neoplasms in the USA has shown a trend of increasing first and then decreasing from 1973 to 2014 (Fig. 1). We also performed separate time-trend analysis of ACs by histologic subtype (Fig. 2a), tumor stage (Fig. 2b) and histologic grade (Fig. 2c). The elevation of the incidence was noted in all the histological types (P < 0.001), stages (P < 0.001), and grades (P < 0.001).
The diseases stage of patients with ACs varied significantly by sex (P < 0.001; Table 2). Female patients were more likely to have distant ACs than male patients. The disease stage also varied significantly by race (P < 0.001; Table 2). In particular, the distant ACs were more often among American Indian/Alaskan native patients (47%) than among other racial patients. We next analyzed the incidence of different histologic subtypes of ACs by sex and race. We observed significant difference between female and male patients (P < 0.001; Table 2). Female patients were more likely to have malignant carcinoid, mucinous adenocarcinoma, and signet ring cell carcinoma, whereas male patients were more likely to have goblet cell carcinoid and adenocarcinoma. The histologic subtypes also varied significantly by race (P < 0.001; Table 2). Asian/Pacific Islander were less likely to have signet ring cell carcinoma histologic subtypes of ACs than patients in the other racial groups. In contrast, mucinous adenocarcinoma of ACs occurred at a markedly higher frequency among American Indian/Alaskan native (49%) than among white (34%) patients, African American (31%), and Asian/ Pacific Islander (29%) (P < 0.001).
Age at diagnosis
We examined age at diagnosis of ACs by race, sex, and histologic subtype. Overall, American Indian/Alaskan Native patients were younger at diagnosis than other racial patients were (P < 0.001). We observed no difference in age at diagnosis by sex. The ages at diagnosis did varied significantly by histologic subtype (P < 0.001). Patients with malignant carcinoid tumors were younger at diagnosis than other racial patients were, while patients with adenocarcinoma were older at diagnosis. Details regarding age at diagnosis are presented in Table 3.
Tumor stage
We next examined disease stage at diagnosis of ACs by race, sex, and histologic subtype and observed a significant correlation between these factors and disease stage (P < 0.001). Details regarding disease stage at diagnosis are presented in Table 3.
We found that Asian/Pacific Islander were the most likely to present with advanced disease (P < 0.001). Female patients were more likely to have metastasis at presentation than male (P < 0.001). Mucinous adenocarcinoma and signet ring cell carcinoma were more likely to develop into distant stage than other histologic subtypes of ACs (P < 0.001).
Survival
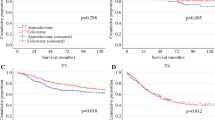
In the survival analysis, the median overall survival duration in the 7170 cases was 78 months. When we examined survival by histologic subtype (Fig. 3a), we noted that the median survival duration in patients with malignant carcinoid was better than others (396 months), while patients with signet ring cell carcinoma had worst median survival duration (29 months). We next analyzed survival by disease stage and histologic grade. We found that the median survival durations in patients with localized, regional, and distant ACs were 155, 86, and 34 months, respectively (Fig. 3b).
Then, we examined actuarial survival by disease stage and histologic subtype (Supplementary Table 1). Compared with other patients, those with signet ring cell carcinoma ACs had the worst outcomes. However, those with mucinous adenocarcinoma had better 3-year and 5-year survival rates, approaching that in malignant carcinoid tumors. We also found that histologic grade was a predictor of survival duration (Fig. 3C, P < 0.001). The median survival duration in patients with well-differentiated and moderately differentiated ACs was respectively 138 and 81 months. Patients with poorly differentiated and undifferentiated ACs had identical survival curves. The median survival duration in patients with poorly differentiated ACs was 26 months while that was 30 months in patients who had undifferentiated ACs.
Finally, we performed univariate and multivariate Cox proportional hazards analysis of ACs. We included potentially prognostic parameters such as disease stage, histology, age, sex, race, and period of diagnosis (1973 to 1993 and 1994 to 2014) in this model. We found that all the parameters that were significant in the univariate analyses were also significant in the multivariate analyses (Supplementary Table 2). In addition, we observed a dramatic improvement in survival duration over time (HR 0.77; 95% CI, 0.70 to 0.86; P < 0.001).
Discussion
In current study, we made the best of the vast amount of data from the SEER Program to reveal the largest series of ACs cases with a focus on epidemiology and prognostic factors. Our epidemiological study of AC is the largest retrospective study by far. Our analysis of population-based data on AC cases over the last 4 decades reveals 2 noteworthy findings: an increased incidence of ACs and increased survival durations over time. In this study, we took advantage of the data collected by the SEER program to evaluate the epidemiology of and prognostic factors for ACs. Similar to those of previous reports [7], we observed a significant increase in the reported annual age-adjusted incidence of ACs from 1973 (0.18/100,000) to 2014 (1.11/100,000). The elevation of the incidence was noted in all the histological types, stages, and grades. In contrast, the overall incidence of all malignant neoplasms in the USA has shown a trend of increasing first and then decreasing from 1973 to 2014. The widespread use of CT scanning has significantly increased since the late 1990s. According to the results from the National Hospital Ambulatory Medical Care Survey, the use of CT scans increased by 330% in emergency departments and CT scan use in patients with flank pain increased from 3.5% to more than 40% from 1996 to 2007, which may contribute to the apparent increase of ACs [10]. Also, wide spread use of colonoscopy likely caused the increase in reported incidence of ACs in part [11]. However, whether changes in environmental factors or dietary habits resulted in the elevation of the incidence of appendiceal cancer needs further investigation.
Using multivariate survival analysis, we found that gender, year of diagnosis, age, race, tumor grade, disease stage, retrieved regional lymph nodes, type of surgery performed, and histologic subtype were important predictors of outcome. We found the histologic subtype to be perhaps the most powerful predictor of outcome in patients with ACs. Using the histologic subtype as a prognostic marker, we were better able to separate outcomes into categories. In our analyses, we observed a statistically significant difference in survival duration among patients with different years of diagnosis. Patients who were diagnosed as ACs in the later period (1994–2014) had a dramatic improvement in survival duration than those diagnosed early (1973–1993). One possible explanation is that continuous progress in treatment of ACs has been achieved and clinicians pay more attention to ACs and mastering the characteristic of these tumors.
Distinguishing between the behavior of mucinous adenocarcinoma subtypes and adenocarcinoma subtypes of ACs has been difficult, and this study lends credence to the fact that the two histological types differ in their characteristics. In our study, compared with adenocarcinoma subtypes, mucinous adenocarcinoma cases were more likely to have metastatic disease and be well-differentiated tumors. However, those with mucinous adenocarcinoma had better survival rate, approaching that in malignant carcinoid tumors. As for therapeutic modalities, similar to signet ring cell carcinoma cases, mucinous adenocarcinoma subtypes were more likely to undergo colectomy plus resection of contiguous organ. Nevertheless, signet ring cell carcinoma of ACs were more likely to be poorly differentiated and undifferentiated tumors, while mucinous adenocarcinoma subtypes were more prone to be well-differentiated tumors. Again, this is consistent with tumors of relatively good tumor biology with higher survival compared with metastatic colorectal adenocarcinoma. These findings further support the unique biological behavior of mucinous adenocarcinomas of ACs.
At present, right colectomy is the major curative treatment for ACs and it should be performed for all non-carcinoid invasive tumors and AC tumors > 2 cm according to current guidelines. However, the results of a German multi-center study [12] suggested that the high rate of right hemicolectomy in patients with small carcinoid tumors needs to be critically discussed. Other studies [13, 14] showed that meaningful long-term survival could be achieved in patients with high-grade appendiceal carcinoma and extensive peritoneal carcinomatosis by cytoreductive surgery (CRS) followed by hyperthermic intraperitoneal chemotherapy (HIPEC). In the late 1980s, results of CRS plus HIPEC were reported with several retrospective studies suggesting survival benefits for this therapeutic model [15]. The major advancement in the management of advanced stage mucinous adenocarcinomas of ACs was the recognition of the importance of complete CRS plus HIPEC. This has led to the adoption of this approach as the standard therapy for advanced mucinous adenocarcinomas of ACs disease. Several published researches have suggested the effectiveness of this treatment in improving long-term survival rates [16].
We acknowledge that there are still many shortcomings in our study. The population-based data used for this study were collected from many sites around the USA and include diagnoses from hundreds of pathologists. Because of the way in which the data were collected, there are no effective means of establishing the reliability of the tissue diagnoses [5]. Some of the data submitted to the SEER program may be incorrect, which could affect the reliability of our results. Besides, in the absence of a clear and unified identification method, classifying ACs as benign or malignant may be difficult. Thus, whereas SEER registry data provide information about malignant ACs, the extent to which these data underestimate the frequency of benign-appearing ACs is unknown. Other limitations are related to temporal bias, limitation of the data relating to the nonsurgical therapies, and missing data and some useful information, such as specific details regarding the circumstances under which the disease was diagnosed in SEER database. Because AC is a rare disease, there still exist bias errors in the incidence and treatment-related prognosis of subgroup stratified analyses due to the small amount of data, such as ethnic-stratified analyses. Despite these shortcomings, our findings from this large-scale population-based analysis confirm many of the findings already reported in the literature about ACs. Even though our conclusions are limited by the observational nature of this study and the limitations of SEER database mentioned above, these data may serve as a basis for comparison for future studies.
Conclusion
In conclusion, we observed increased reported incidence of ACs and increased survival durations over time, indicating that clinicians are more concerned with ACs and grasp the characteristics of these tumors.
References
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. 68(1):7–30
Khan F, Vogel RI, Diep GK, Tuttle TM, Lou E (2016) Prognostic factors for survival in advanced appendiceal cancers. Cancer Biomark 17(4):457–462
Smeenk RM, van Velthuysen ML, Verwaal VJ, Zoetmulder FA (2008) Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. Eur J Surg Oncol 34(2):196–201
Deans GT, Spence RA (1995) Neoplastic lesions of the appendix. Br J Surg 82(3):299–306
McCusker ME, Cote TR, Clegg LX, Sobin LH (2002) Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973-1998. Cancer. 94(12):3307–3312
Noone AM, Lund JL, Mariotto A, Cronin K, McNeel T, Deapen D, Warren JL (2016) Comparison of SEER treatment data with Medicare claims. Med Care 54(9):e55–e64
Marmor S, Portschy PR, Tuttle TM, Virnig BA (2015) The rise in appendiceal cancer incidence: 2000-2009. J Gastrointest Surg 19(4):743–750
Buuren SV, Groothuis-Oudshoorn K (2011) Mice: multivariate imputation by chained equations in R. J Stat Softw 45:1–67
Marshall A, Altman DG, Holder RL, Royston P (2009) Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol 9:57
Kocher KE, Meurer WJ, Fazel R, Scott PA, Krumholz HM, Nallamothu BK (2011) National trends in use of computed tomography in the emergency department. Ann Emerg Med 58(5):452–62 e3
Phillips KA, Liang SY, Ladabaum U, Haas J, Kerlikowske K, Lieberman D, Hiatt R, Nagamine M, Van Bebber SL (2007) Trends in colonoscopy for colorectal cancer screening. Med Care 45(2):160–167
Benedix F, Reimer A, Gastinger I, Mroczkowski P, Lippert H, Kube R, Study Group Colon/Rectum Carcinoma Primary T (2010) Primary appendiceal carcinoma--epidemiology, surgery and survival: results of a German multi-center study. Eur J Surg Oncol 36(8):763–771
Piso P, Bektas H, Werner U, Schlitt HJ, Kubicka S, Bornscheuer A, Manns M, Klempnauer J (2001) Improved prognosis following peritonectomy procedures and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from appendiceal carcinoma. Eur J Surg Oncol 27(3):286–290
El Halabi H, Gushchin V, Francis J, Athas N, Macdonald R, Nieroda C, Studeman K, Sardi A (2012) The role of cytoreductive surgery and heated intraperitoneal chemotherapy (CRS/HIPEC) in patients with high-grade appendiceal carcinoma and extensive peritoneal carcinomatosis. Ann Surg Oncol 19(1):110–114
Sugarbaker PH, Graves T, DeBruijn EA, Cunliffe WJ, Mullins RE, Hull WE, Oliff L, Schlag P (1990) Early postoperative intraperitoneal chemotherapy as an adjuvant therapy to surgery for peritoneal carcinomatosis from gastrointestinal cancer: pharmacological studies. Cancer Res 50(18):5790–5794
Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, Baratti D, Deraco M, Elias D, Sardi A, Liauw W, Yan TD, Barrios P, Gomez Portilla A, de Hingh IH, Ceelen WP, Pelz JO, Piso P, Gonzalez-Moreno S, Van Der Speeten K, Morris DL (2012) Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 30(20):2449–2456
Acknowledgments
The authors would like to thank the Surveillance, Epidemiology, and End Results (SEER) database for the support.
Funding
This study was supported by the National Key R&D Program of China (No. 2016YFC0905300 and 2016YFC0905301), the Grant of Science and Technology Commission of Shanghai Municipality (No. 16401970502), the Grant of National Natural Science Foundation of China (No. 81572351), the Shanghai Shenkang Program (No. SHDC12014206), the National Science Foundation of China (No. 81702353), and Shanghai Municipal Natural Science Foundation (17ZR1406400).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Ethical Committee and Institutional Review Board of the Fudan University Shanghai Cancer Center reviewed and approved this study protocol.
Competing interests
The authors declare that they have no competing interests.
Disclaimer
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mo, S., Zhou, Z., Ying, Z. et al. Epidemiology of and prognostic factors for appendiceal carcinomas: a retrospective, population-based study. Int J Colorectal Dis 34, 1915–1924 (2019). https://doi.org/10.1007/s00384-019-03387-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-019-03387-y