Abstract
Background
Although Ras-association domain family of gene 2 (RASSF2) has been shown to undergo promoter methylation at high frequency in some cancer types and in brain metastases, its clinical utility as a useful prognostic molecular marker remains unclear in gastric cancer.
Methods
Prognostic significance of RASSF2 expression was retrospectively analysed by immunohistochemically in 105 patients with gastric cancer who underwent curative gastrectomy.
Results
Low RASSF2 expression was detected in 58 (55 %) patients, whereas 47 patients (45 %) had high RASSF2 expression. Lymph node involvement, pT stage, TNM stage, vascular invasion, perineural invasion and the presence of recurrence were found to be significantly related to RASSF2 expression levels. Low PRL-3 expression was closely correlated with lymph node metastasis (p = 0.001), advanced pT stage (p = 0.021), advanced TNM stage (p < 0.001), the presence of vascular invasion (p < 0.001), perineural invasion (p = 0.018) and high prevalence of recurrence (p = 0.003) compared with high RASSF2 expression. The median disease-free survival (DFS) time for patients with low RASSF2 expression was significantly worse than that of patients with high RASSF2 expression (10.2 vs. 50.6 months, p < 0.001). In addition, patients with high RASSF2 expression had the higher overall survival (OS) interval compared to patients with low RASSF2 expression (NR vs. 14.9 months, p < 0.001). In the multivariate analysis, the rate of RASSF2 expression levels was an independent prognostic factor, for DFS [p < 0.001, HR 0.12 (0.10–0.88)] and OS [p < 0.001, HR 0.10 (0.04–0.46)], as were pT stage and TNM stage, respectively.
Conclusions
RASSF2 may be an important molecular marker for carcinogenesis, prognosis and progression in gastric cancer, but the potential value of RASSF2 expression as a useful molecular marker in gastric cancer progression should be evaluated, comprehensively. It would be possible to develop treatments targeting RASSF2 and advance new treatment strategies for gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is one of the leading causes of global cancer mortality [1]. Most patients with gastric cancer are symptomatic and already have advanced incurable disease at the time of presentation. At diagnosis, approximately 50 % have disease that extends beyond locoregional confines, and only one-half of those who appear to have locoregional tumour involvement can undergo a potentially curative resection. The 5-year overall survival (OS) rate is approximate 25 % [2]. Although the detection of prognostic markers may enable us to evaluate the precise status of these diseases and allow a more effective management of the patient, information on the prognostic markers in GC is still limited.
HER2 evaluation becomes an important approach for predicting patient response to HER2-targeting agents. However, the significance of HER2 expression as a prognostic factor in gastric cancer remains much less clear. Recently, Sheng et al. evaluated HER2 expression as a prognostic factor in patients with gastric cancer, but they could not prove its prognostic significance [3]. Similarly, another study analysed HER2 expression in gastric cancer patients. It showed that OS of HER2-negative and -positive patients was not significantly different. However, in patients with stage III/IV, OS was worse in HER2-positive patients [4]. It is known that Ras signalling contributes to the activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) cascades that are involved in cancer progression [5]. The RASSF family of proteins consists of 10 members (RASSF1 to 10) with various isoforms, all of which share a region of homology, the Ras-association domain. The Ras-association (RalGDS/AF-6) domain family member 2 (RASSF2) protein belongs to the RASSF family that participates in Ras signalling [6, 7]. The Ras-association (RalGDS/AF-6) domain family member 2 (RASSF2) is a gene located at 20p13 with RASSF2 as the most important putative transcript [8]. It belongs to the RAS-association domain family of proteins, which has important functions in cell-cycle control, microtubule stabilization and motility [9]. The RASSF2 protein exhibits suppressor functions as it interacts with the proapoptotic MST kinases, which are known to activate the SAPK-JNK signalling pathway, leading to subsequent apoptosis [8, 10]. The inactivation of this gene by hypermethylation has been described in breast, colorectal, endometrial, gastric, lung, thyroid and cervical cancers [11–16]. As with other cancer types, a few trials have previously analysed the relation between the expression of RASSF2 and gastric cancer in the literature. Luo et al. [7] showed that in gastric cancer patients who had undergone curative gastrectomy, low RASSF2 expression was related with poor prognosis, and there was a significant association between the expression levels of RASSF2 and OS.
Determining the poor prognostic factors that may predict the tumour recurrence and prognosis of patients is an important for selection appropriate treatment protocols. Therefore, in the current study, we evaluated the prognostic significance of the expression of RASSF2 in patients with gastric cancer who underwent curative gastrectomy. Furthermore, the relationship between RASSF2 expression and clinicopathological factors and the effect of this expression on survivals were studied.
Materials and methods
This study consisted of 105 gastric carcinoma patients who had undergone a R0 curative gastrectomy, who were followed up and treated at the Department of Medical Oncology, Dr. Lutfi Kirdar Education and Research Hospital, between 2007 and 2013. Details concerning age, gender, resection type, tumour location, histopathology, pT stage, tumour size, histological grade, lymph node involvement, lymphatic vessel invasion, blood vessel invasion and perineural invasion, resection margins, adjuvant chemotherapy and radiation therapy, responses to treatment and survival were obtained from patients’ charts after written informed consent had been obtained from patients. The Local Ethics Committee of our hospital approved the study. Primary tumours were staged according to the seventh edition of American Joint Committee on Cancer (AJCC) TNM staging classification for gastric cancer [17] and the clinicopathologic findings were determined according to the Japanese Classification of Gastric Carcinoma (JCGC) [18]. The eligibility criteria included histologically confirmed R0 gastric resection, which was defined as no macroscopic or microscopic residual tumour and post-operative survival time >3 months. The patients with distant metastasis at diagnosis were excluded from the study.
After completion of treatments, follow-up schedule was started. Every 3 months for the two post-operative years, every 6 months up to 5 years and annually thereafter for at least 5 years during follow-up, medical histories and physical examinations were carried out. Complete blood counts and biochemistry panels, as well as tumour markers, carcinoembryonic antigen and carbohydrate antigen 19-9 were controlled every 3 months in the first and second years, and annually thereafter. Chest X-rays and abdominal CT scans were also performed every 3 months in the first year, every 6 months in the second post-operative year and annually thereafter for 5 years.
Histologic tumour specimens were re-evaluated by two pathologists, who was an expert in matters of gastric cancer and who had no knowledge of pathological findings after all patients or their relatives gave written consent.
Immunohistochemical staining of RASSF2 expression
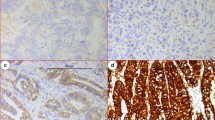
Firstly, paraffin blocks of gastric tumour tissue were cut into sections of 3 lm and deparaffinised. Hydrogen peroxide was applied after antigen retrieval solution to block the endogen peroxide activity. Next, sections were incubated with monoclonal mouse anti-human RASSF2 (LifeSpan Biosciences, USA) antibody in 1/200 dilution. After being applied to Bond primary and Bond polymer, the sections were kept in Bond DAB mix solution and haematoxylin was applied as contrast stain. The intensity of cellular staining and the proportion of stained tumour cells were scored separately. The scoring was designed according to the staining intensity of the tumour cells in sections: score 0, no stain; score 1, weak stain; score 2, medium stain and score 3, strong stain. The extent of positivity was scored according to the percentage of cells showing positive staining: 0, <5 %; 1, >5–25 %; 2, >25–50 %; 3, >50–75 %; 4, >75 % of the cells in the respective lesions. The final score was determined by multiplying the intensity of positivity and the extent of positivity scores, yielding a range from 0 to 12. The expression for RASSF2 was considered high expression when the scores were ≥5. The immunohistochemical staining intensity of RASSF2 for gastric cancer specimens is shown in Fig. 1.
Adjuvant and metastatic treatments
Totally 70 patients (67 %) had received adjuvant chemoradiotherapy with 5-Fluorouracil (5-FU) 425 mg/m2 per day, plus leucovorin 20 mg/m2 per day, for 5 days, followed by 4500 cGy of radiation at 180 cGy per day, given 5 days per week for 5 weeks, with modified doses of fluorouracil and leucovorin on the first four and the last 3 days of radiotherapy, within 4 weeks after surgery. However, only 51 patients (72 %) were able to complete their adjuvant treatment. During the follow-up, 51 (49 %) patients developed relapse. Regarding the localization of the relapse, 41 % of the patients had relapse in the liver, others in the peritoneum, para-aortic lymph nodes, lung, stomach, bone or ovaries. The majority of patients (n = 47, 92.1 %) who had relapse were treated with second-line chemotherapy including DCF (docetaxel-cisplatin-5-FU), FOLFOX (5FU-oxaliplatin-leucovorin calcium-5-FU infusion) and epirubicin-oxaliplatin-capecitabine (EOX). Four patients could not receive second-line treatment because of poor performance status. At the progressive disease, 24 patients were treated with third-line chemotherapy (paclitaxel, FOLFOX or XELOX and irinotecan).
Statistical methods
Statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA) software. The Chi squared test and Fisher’s exact test were used to analyse the relationships between the relationships between clinicopathological factors and RASSF2 expression. Survival analysis and curve were established according to the Kaplan–Meier method and compared by the log-rank test. Disease-free survival (DFS) was defined as the time from curative surgery to disease progression or recurrence, or to the date of death or lost in follow-up. OS was described as the time interval from diagnosis to the date of the patient’s death or lost in follow-up. Firstly, univariate analyses were performed to evaluate the significance of RASSF2 expression and other clinicopathological features as prognostic factors. Thereafter, the multivariate analysis with the Cox proportional hazards model was made in order to further analyse the RASSF2 expression and all of the significant prognostic factors which were found in the univariate analysis. The confidence intervals (CI) of 95 % were defined to indicate the relationship between overall survival and each independent factor. All tests were done using two-sided and p values <0.05 were accepted statistically significant.
Results
Sixty-eight patients (64.8 %) were male and 37 (35.2 %) were female. The median age was 62 ranging from 29 to 79 years and fifty-six patients were older than 60 years (53.3 %). Fifty-six percent of the patients underwent subtotal gastrectomy and 43.8 % underwent total gastrectomy. The majority of the patients (57.1 %) had tumours located in the lower part, 21 % in the middle part and 20 % in the upper part of the stomach. Histopathological analysis revealed that 49.5 % had pure adenocarcinoma histology, and more than half (n = 63, 60 %) had poorly or undifferentiated tumours. Based on the lymph node involvement, 77 % of the patients were classified as node-positive (24 % had N1, 22 % had N2 and 31 % had N3 lymph node involvement) and the remaining 23 % of the patients were node-negative. Seven patients (6.6 %) were categorized as stage I, 34 (32.4 %) as stage II and 64 (61 %) as stage III according to the TNM staging.
IHC analysis from 105 gastric tumour samples revealed that low RASSF2 expression was determined in 58 (55 %) patients, whereas 47 patients (45 %) had high RASSF2 expression. Lymph node involvement, pT stage, TNM stage, vascular invasion, perineural invasion and the presence of recurrence were found to be significantly related to RASSF2 expression levels. Low RASSF2 expression was closely correlated with lymph node metastasis (p = 0.001), advanced pT stage (p = 0.021), advanced TNM stage (p < 0.001), the presence of vascular invasion (p < 0.001) and perineural invasion (p = 0.018) compared with high RASSF2 expression. The prevalence of recurrence for patients with low RASSF2 expression was significantly higher than that for patients with high RASSF2 expression (p = 0.003). The relationships between clinicopathological factors and RASSF2 expression levels are listed in Table 1.
The median follow-up time was 37 ranging from 19 to 89 months. The median DFS interval of patients with high RASSF2 expression was significantly better than that of patients with low RASSF2 expression (50.6 vs. 10.2 months, p < 0.001, Fig. 2). Furthermore, patients with high RASSF2 expression had the higher OS interval compared to patients with low RASSF2 expression (NR vs. 14.9 months, p < 0.001). OS curves according to the RASSF2 expression levels are indicated in Fig. 3. The univariate analysis showed that RASSF2 expression levels, lymph node involvement, pT stage, TNM stage, vascular and perineural invasion were found to be statistically significant prognostic factors for DFS. In addition, RASSF2 expression levels, lymph node involvement, pT stage, TNM stage, vascular and perineural invasion were important prognostic indicators for OS in the univariate analysis. The results of univariate analysis according to DFS and OS are summarized in Tables 2 and 3.
RASSF2 expression was closely associated with DFS and OS of patients with radically resected gastric cancer in the univariate analysis. Therefore, a multivariate analysis with the Cox proportional hazards model was performed in order to further evaluate the prognostic significance of RASSF2 expression and all of the significant prognostic factors that were found in the univariate analysis. Multivariate analysis indicate that the rate of RASSF2 expression levels [p < 0.001, HR 0.12 (0.10–0.88)] was an independent prognostic factor, as were pT stage [p = 0.014, HR 1.39 (0.58–3.32)] for DFS. Moreover, it was found that the rate of RASSF2 expression levels [p < 0.001, HR 0.10 (0.04–0.46)] was an independent prognostic factor, as were TNM stage [p = 0.04, HR 1.94 (0.96–3.91)] for OS. Table 4 shows the results of multivariate analysis of DFS and OS.
Discussion
In the present study, low RASSF-2 expression was significantly correlated with lymph node involvement, pT stage, TNM stage, vascular invasion, perineural invasion and the presence of recurrence. In the multivariate analysis, we found that RASSF2 expression level was an independent prognostic factor for both DFS and OS, in addition to the already known important clinicopathological prognostic indicators such as pT stage and TNM stage, respectively, in patients with gastric cancer who had undergone radical surgery.
Gastric cancer is a disease driven by progressive genetic and epigenetic aberrations [19]. The role of epigenetics in the pathogenesis of cancer has come to the forefront over the last decade. It is now well established that epigenetic events, such as DNA methylation, can be driver events in the pathogenesis of gastric cancer, and that these epigenetic events co-operate with gene mutations in the progression of normal gastric mucosa to cancer, with more genes in the gastric cancer genome affected by altered DNA methylation than by gene mutations [19, 20]. These alterations in DNA methylation contribute to the molecular heterogeneity of gastric cancers, as illustrated by the identification of molecular subtype of gastric cancers that can be identified by their unique methylated gene signatures.
Previously, RASSF2 has been identified as a member of the recently discovered RASSF family of Ras effectors/tumour suppressors and demonstrated that it binds directly to K-Ras via the Ras effector domain [10, 12, 21]. K-Ras is a member of a family of oncoproteins that play an important role in many biological processes [5, 22, 23]. These abilities are mediated by the ability of RAS proteins to interact with a wide range of effector proteins [24]; for example via signalling through phosphatidylinositol 3-kinase and AKT, RAS proteins can induce cell division and oncogenesis, whereas interactions with RASSF2 can mediate apoptosis and cell-cycle arrest [10, 23]. It had previously demonstrated that RASSF2A was frequently methylated in colorectal tumours and adenomas and that methylation was tumour specific [12]. Furthermore, two reports [25, 26] have demonstrated that methylation of RASSF2A corresponds to loss of expression in primary tumours as well as tumour cell lines. Afterwards, epigenetic inactivation of RASSF2 was found in gastric cancer [27], oral squamous cell carcinoma [28], head and neck squamous cell carcinoma [29], hepatocellular [30] and thyroid cancers [15].
In the literature, only one clinical trial had addressed the relation between gastric cancer and RASSF2 expression levels. Previous retrospective study performed by Luo et al. [7], in 276 patients, who underwent curative gastrectomy and lymph node dissection for gastric cancer, showed that patient age, histological differentiation, depth of tumour invasion, regional lymph node and distant metastasis and TNM stage were found to be significantly related to RASSF2 levels. In addition, positive expression levels of RASSF2 were correlated with poor prognosis, and the levels of RASSF2 expression were significantly related to OS [7]. In our study, we detected that RASSF2 expression levels were significantly associated with lymph node involvement, pT stage, TNM stage, vascular invasion, perineural invasion and the presence of recurrence were found to be significantly related to RASSF2 expression levels. Thus, our results were compatible with their findings [7].
In our study, the median DFS time was found to be significantly higher for patients with high RASSF2 expression compared to patients with low RASSF2 levels. In addition, the multivariate analysis for DFS demonstrated that RASSF2 expression levels were independent prognostic factors. Likewise, the median OS time was found to be significantly higher for patients with high RASSF2 expression compared to patients with low RASSF2 expression. The multivariate analysis for OS demonstrated that RASSF2 expression levels were independent prognostic factors. Similarly, Luo et al. reported that the median OS interval was significantly better for patients with high RASSF2 expression, and RASSF2 expression was identified to be an independent prognostic indicator. Hence, our results were compatible with the literature [7].
Gharanei et al. analysed the methylation status of RASSF1A and RASSF2 in a cohort of Ewing sarcomas (ES) and determined any association with clinical outcome. They showed that overexpression of either RASSF1A or RASSF2 reduced colony formation ability of ES cells. Moreover, RASSF2 methylation was correlated with poor OS, finally, concluded that both RASSF1A and RASSF2 are novel epigenetically inactivated tumour suppressor genes in patients with ES, and RASSF2 methylation may have prognostic implications for this cohort [31]. Recently, in a study performed by Mezzanotte et al., the authors reported that promoter methylation leading to reduced expression of RASSF6 may play an important role in melanoma development and may contribute to brain metastases [32]. We also determined that low RASSF2 expression was associated with poor prognosis. Therefore, our results were similar to previous findings [31, 32].
The major limitation of our study was the retrospective nature. The other limitations of this study were relatively the small sample size and short follow-up interval. These factors might have influenced our results. Although our results should be confirmed using prospective studies with larger sample sizes, we believe that our study is noteworthy and our results contribute to the knowledge of prognosis and progression in gastric cancer.
In conclusion, our study indicates that low RASSF2 expression was associated with a poor prognosis for patients with gastric cancer who underwent curative surgery. RASSF2 may be an important molecular marker for carcinogenesis, prognosis and progression in gastric cancer. To validate the findings of this study, further research is needed, preferably by prospective studies that analyse the correlation between RASSF2 expression and treatment response including more patients. Thus, it would be possible to develop treatments targeting RASSF2 and advance new treatment strategies for gastric cancer.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30.
Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477.
Kataoka Y, Okabe H, Yoshizawa A, Minamiguchi S, Yoshimura K, Haga H, et al. HER2 expression and its clinicopathological features in resectable gastric cancer. Gastric Cancer. 2013;16:84–93.
Sheng WQ, Huang D, Ying JM, Lu N, Wu HM, Liu YH, et al. HER2 status in gastric cancers: a retrospective analysis from four Chinese representative clinical centers and assessment of its prognostic significance. Ann Oncol. 2013;24:2360–4.
Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3:459–65.
Liu C, Pan Y, Wang X, Lu J, Huang B, Li X. Activation of RASSF2A by p300 induces late apoptosis through histone hyperacetylation. Cell Biol Int. 2010;34:1133–9.
Luo D, Ye T, Li TQ, Tang P, Min SD, Zhao GF, et al. Ectopic expression of RASSF2 and its prognostic role for gastric adenocarcinoma patients. Exp Ther Med. 2012;3:391–6.
Cooper WN, Hesson LB, Matallanas D, Dallol A, von Kriegsheim A, Ward R, et al. RASSF2 associates with and stabilizes the proapoptotic kinase MST2. Oncogene. 2009;28:2988–98.
Richter AM, Pfeifer GP, Dammann RH. The RASSF proteins in cancer; from epigenetic silencing to functional characterization. Biochim Biophys Acta. 2009;1796:114–28.
Vos MD, Ellis CA, Elam C, Ulku AS, Taylor BS, Clark GJ. RASSF2 is a novel K-Ras-specific effector and potential tumor suppressor. J Biol Chem. 2003;2(78):28045–51.
Cooper WN, Dickinson RE, Dallol A, Grigorieva EV, Pavlova TV, Hesson LB, et al. Epigenetic regulation of the ras effector/tumour suppressor RASSF2 in breast and lung cancer. Oncogene. 2008;27:1805–11.
Hesson LB, Wilson R, Morton D, Adams C, Walker M, Maher ER, et al. CpG island promoter hypermethylation of a novel Ras-effector gene RASSF2A is an early event in colon carcinogenesis and correlates inversely with K-ras mutations. Oncogene. 2005;24:3987–94.
Liao X, Siu MK, Chan KY, Wong ES, Ngan HY, Chan QK, et al. Hypermethylation of RAS effector related genes and DNA methyltransferase 1 expression in endometrial carcinogenesis. Int J Cancer. 2008;123:296–302.
Maruyama R, Akino K, Toyota M, Suzuki H, Imai T, Ohe-Toyota M, et al. Cytoplasmic RASSF2A is a proapoptotic mediator whose expression is epigenetically silenced in gastric cancer. Carcinogenesis. 2008;29:1312–8.
Schagdarsurengin U, Richter AM, Hornung J, Lange C, Steinmann K, Dammann RH. Frequent epigenetic inactivation of RASSF2 in thyroid cancer and functional consequences. Mol Cancer. 2010;9:264.
Zhang X, Ma Y, Wu Y, Lin L, Ma X, Zhang Y. Aberrant promoter methylation and silencing of RASSF2A gene in cervical cancer. J Obstet Gynaecol Res. 2014;40:1375–81.
Edge SB, Byrd DR, Compton CC. American Joint Committee on Cancer Staging Manual. 7th ed. New York: Springer; 2010. p. 117.
Association Japanese Gastric Cancer. Japanese classification of gastric carcinoma. 2nd English ed. Gastric Cancer. 1998;1:10–24.
Qu Y, Dang S, Hou P. Gene methylation in gastric cancer. Clin Chim Acta. 2013;424:53–65.
Calcagno DQ, de Arrodo Cardoso Smith M, Burbano RR. Cancer type-specific epigenetic changes: gastric cancer. Methods Mol Biol. 2015;1238:79–101.
Agathanggelou A, Cooper WN, Latif F. Role of the ras-association domain family 1 tumor suppressor gene in human cancers. Cancer Res. 2005;65:3497–508.
Schubbert S, Bollag G, Shannon K. Deregulated Ras signaling in developmental disorders: new tricks for an old dog. Curr Opin Genet Dev. 2007;17:15–22.
Cooper WN, Dickinson RE, Dallol A, Grigorieva EV, Pavlova TV, Hesson LB, et al. Nuclear localisation and epigenetic inactivation of the ras effector/tumour suppressor RASSF2A in multiple human cancers. Oncogene. 2008;27:1805–11.
Cox AD, Der CJ. The dark side of Ras: regulation of apoptosis. Oncogene. 2003;22:8999–9006.
Park HW, Kang HC, Kim IJ, Jang SG, Kim K, Yoon HJ, et al. Correlation between hypermethylation of the RASSF2A promoter and K-ras/BRAF mutations in microsatellite-stable colorectal cancers. Int J Cancer. 2007;120:7–12.
Zhang Z, Sun D, Van do N, Tang A, Hu L, Huang G. Inactivation of RASSF2A by promoter methylation correlates with lymph node metastasis in nasopharyngeal carcinoma. Int J Cancer. 2007;120:32–8.
Endoh M, Tamura G, Honda T, Homma N, Terashima M, Nishizuka S, et al. RASSF2, a potential tumour suppressor, is silenced by CpG island hypermethylation in gastric cancer. Br J Cancer. 2005;93:1395–9.
Imai T, Toyota M, Suzuki H, Akino K, Ogi K, Sogabe Y, et al. Epigenetic inactivation of RASSF2 in oral squamous cell carcinoma. Cancer Sci. 2008;99:958–66.
Steinmann K, Sandner A, Schagdarsurengin U, Dammann RH. Frequent promoter hypermethylation of tumor-related genes in head and neck squamous cell carcinoma. Oncol Rep. 2009;22:1519–26.
Ren J, He W, Zhang R, Li Z, Cao W, Yao J, et al. RASSF2A promoter methylation in hepatitis B virus-related hepatocellular carcinogenesis and its correlation with elevated serum alpha-fetoprotein level. J Huazhong Univ Sci Technol Med Sci. 2009;29:309–12.
Gharanei S, Brini AT, Vaiyapuri S, Alholle A, Dallol A, Arrigoni E, et al. RASSF2 methylation is a strong prognostic marker in younger age patients with Ewing sarcoma. Epigenetics. 2013;8:893–8.
Mezzanotte JJ, Hill V, Schmidt ML, Shinawi T, Tommasi S, Krex D, et al. RASSF6 exhibits promoter hypermethylation in metastatic melanoma and inhibits invasion in melanoma cells. Epigenetics. 2014;9:1496–503.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Aydin, D., Bilici, A., Kayahan, S. et al. Prognostic importance of RASSF2 expression in patients with gastric cancer who had undergone radical gastrectomy. Clin Transl Oncol 18, 608–616 (2016). https://doi.org/10.1007/s12094-015-1405-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-015-1405-9