Abstract
Purpose
We have shown that activation of the receptor tyrosine kinase (RTK)-RAS pathway in gastric adenocarcinoma (GA) promotes acquisition of cancer stem-like cell (CSC) phenotypes including metastasis and chemotherapy resistance. Here, we evaluated the prognostic value of the CSC marker CD44 and the RTK-RAS activation marker phosphorylated MEK (p-MEK) in patients with resectable GA.
Methods
CD44 and p-MEK were measured in tumors from GA patients who underwent curative-intent gastrectomy at Fujian Medical University Union Hospital (FMUUH, n = 134) and Memorial Sloan Kettering Cancer Center (MSKCC, n = 56). Overall survival (OS) was estimated by the Kaplan-Meier method, and multivariate analysis was performed by Cox proportional hazards regression modeling.
Results
Despite multiple significant differences in clinicopathologic characteristics between the FMUUH and MSKCC cohorts, high CD44 and high p-MEK expression were both independent negative prognostic factors for OS on univariate analysis in both cohorts (p < 0.05). Both factors were also significant on multivariate analysis when the cohorts were combined (p ≤ 0.003). On subgroup analysis, the 5-year OS of patients with both high CD44 and high p-MEK was 39.5–41.6% compared with 55.4–66.4% for patients with low CD44. High CD44 expression was associated with more advanced TNM stage in the FMUUH cohort and larger tumor size and undifferentiated histology in the MSKCC cohort. High p-MEK was associated with undifferentiated histology in the FMUUH cohort and larger tumor size in the MSKCC cohort.
Conclusions
Increased CD44 and p-MEK expression are predictive of worse OS in GA patients. Thus, targeting the RTK-RAS pathway may benefit patients with CD44-positive, RAS-activated GA by inhibiting metastasis and reversing chemotherapy resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer, the vast majority of which are gastric adenocarcinomas (GAs), is the fifth most common malignancy worldwide and is the third leading cause of cancer death.1,2 The disease continues to carry a poor prognosis, accounting for over 700,000 deaths per year. The majority of patients with gastric cancer present with advanced or metastatic disease.3 Though the response rate to multi-agent chemotherapy is 50% or greater, nearly all patients develop chemotherapy resistance, and median survival is extended to only 9–11 months.4,5
There is growing evidence to support the cancer stem cell (CSC) theory which postulates that a small population of cells within a solid tumor has “stem-like” characteristics including self-renewal and differentiation.6,7,8 These CSCs generally demonstrate resistance to chemotherapy or radiation.9,10 The cell surface protein CD44 is the only gastric CSC marker associated with tumor formation in immunodeficient mice and spheroid colony formation in vitro.11 CD44 is the major cell surface receptor for hyaluronic acid encoded on the short arm of chromosome 11 in humans.12 The extracellular domain of CD44 binds to hyaluronic acid, where their interaction promotes cell migration and maintains proliferation and differentiation of CSCs.13
Genes encoding the receptor tyrosine kinase (RTK)-RAS signaling pathway are altered in 60% of GAs.14 The RAS family of proteins (in humans, HRAS, KRAS, and NRAS) are small GTPases involved in cellular signal transduction supporting cell growth and survival.15KRAS is amplified or mutated in 17% of GAs.14 Upon stimulation by upstream receptors, KRAS switches from an inactive, GDP-bound form to an active, GTP-bound form. This conformational change leads to its binding with RAF. KRAS recruits RAF to the membrane where it promotes RAF dimerization and activation. Activated RAF phosphorylates and activates MEK, and activated MEK phosphorylates and activates ERK.
We have previously shown that oncogenic Kras can increase gastric tumorigenesis and metastasis in a genetically engineered mouse model.16 In GA driven by Cdh1 and Trp53 loss in gastric parietal cells, 69% of mice developed diffuse-type GA that metastasized to lymph nodes at 1 year.17 Combining that with oncogenic Kras (KrasG12D) increased the penetrance of GA development to 100% and reduced survival to 76 days. In these triple-conditional (Tcon) mice, both intestinal and diffuse primary tumors are observed throughout the stomach, as well as lymph node, lung, and liver metastases. When Tcon mice are treated with a MEK inhibitor starting at 4 weeks of age, median survival increases from 76 to 95 days.
We also recently reported that KRAS activation in GA promotes epithelial-to-mesenchymal transition (EMT) and acquisition of cancer stem-like cell (CSC) phenotypes including metastasis and chemotherapy resistance.18 Inhibition of the RTK-RAS pathway using RNA interference or pharmacologic inhibition decreased spheroid formation (a marker of CSCs), expression of EMT-related proteins, migration, invasion, and chemotherapy resistance. KRAS inhibition in GA spheroid cells led to reduced growth of human gastric cancer cell flank xenografts, loss of the infiltrative tumor border, fewer lung metastases, and increased survival.
Here we evaluate the prognostic value of the GA CSC marker CD44 and the RTK-RAS activation marker phosphorylated MEK (p-MEK) in two cohorts of patients with resectable GA.
Methods
Patient Population
Tumor tissue analysis was performed on patients from Fujian Medical University Union Hospital (FMUUH, Fujian, China) and from Memorial Sloan Kettering Cancer Center (MSKCC, New York, USA). At FMUUH, 134 patients who underwent gastrectomy between 2013 and 2014 were identified for inclusion in this study, and at MSKCC, 56 patients who underwent gastrectomy between 2007 and 2011 were identified for inclusion in this study. All patients had adenocarcinoma arising in the stomach or gastroesophageal junction (GEJ) Siewert type II or III and underwent curative-intent gastrectomy. Patients who received neoadjuvant chemotherapy were excluded. All research protocols in the current study were approved by the Institutional Review Boards of both FMUUH and MSKCC. A portion of each patient’s tumor was fixed in 10% buffered formalin for 24 h and processed into paraffin blocks. Clinicopathologic data were collected from the two institutions’ prospective databases. Pathologic staging was determined according to the 8th edition American Joint Committee on Cancer (AJCC) staging system.19
Tissue Microarray and Immunohistochemistry
A representative core biopsy measuring 2 mm in diameter was obtained from each tumor paraffin block and deposited into a recipient tissue microarray (TMA) block using a precision tissue array instrument (from Shanghai Outdo Biotech Co. Ltd. at FMUUH; and from Beecher Instruments, Inc., Sun Prairie, WI, at MSKCC).20 Several serial sections 4 μm in thickness were cut from the TMA blocks, and one section from each was stained with hematoxylin-eosin and served as a reference. Immunohistochemistry (IHC) was performed using monoclonal antibodies against CD44 (eBioscience, BMS113, San Diego, CA) and phosphorylated (Ser217/221)-MEK1/2 (Cell Signaling, #9154, Danvers, MA) and the VECTASTAIN Elite ABC Kit per the manufacturer’s protocol (Vector Laboratories, Burlingame, CA).21 TMA sections were deparaffinized with xylene, rehydrated by alcohol gradient, and subjected to antigen retrieval using citrate buffer (pH 6.0) and microwave heating. Endogenous peroxidase activity was blocked, and the sections were incubated overnight at 4 °C with the primary anti-CD44 antibody or the primary anti-p-MEK antibody diluted 1:50 in CAS-Block blocking reagent (Invitrogen, Grand Island, NY). Staining was visualized using anti-IgG biotinylated secondary antibody, streptavidin-HRP (Vector Laboratories), and 3,3′-diaminobenzidine. The sections were counterstained with hematoxylin.
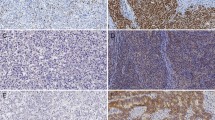
Immunostained tissue sections were examined and scored independently evaluated by two experienced pathologists (Q.Y. and S.Q.). The intensity of staining for CD44 and p-MEK was scored as 0 to 3 (Fig. 1a). The proportion of positively stained cells was scored as follows: ≤ 5% positive cells, 0; 6 to 25% positive cells, 1; 26 to 50% positive cells, 2; ≥ 51% positive cells, 3. To obtain an IHC score that considers the IHC signal intensity and the frequency of positive cells, the intensity score was multiplied by the percentage score. Composite scores less than 3 were defined as low expression and scores of 4 or higher, as high. Discordant results in terms of intensity of staining or proportion of positively stained cells were found in less than 10% of cases. Discordant results were resolved through re-analysis by both pathologists to arrive at a concordant result.
Immunohistochemical staining of tumors for CD44 and phosphorylated MEK (p-MEK). a Photos of GA tumors immunostained for CD44 and p-MEK. Examples of staining intensity from 0 to 3 are shown. CD44 staining was predominantly localized to the membrane, and p-MEK staining was predominantly localized to the cytoplasm. Scale bars represent 20 μm. b Proportion of tumors with low versus high staining for CD44 and p-MEK in the FMUUH and MSKCC cohorts
Follow-up
All patients were followed up post-gastrectomy every 3 months for the first 2 years and every 6 months for the next 3 years. Each follow-up visit included a physical examination and laboratory tests including serum CA19-9 and CEA. At every other visit, chest X-ray or chest CT and abdominal CT were performed. Patients who did not undergo total gastrectomy underwent an upper endoscopy each year or every other year. The median follow-up was 37.0 months (range, 2–61 months) for FMUUH patients and 46.0 months (range, 1–98 months) for MSKCC patients.
Statistical Analysis
Descriptive statistics (mean and standard deviation) were calculated using IBM SPSS version 22.0.0 (IBM Corporation, Armonk, NY). Differences in the distribution of patient-, tumor-, and treatment-specific factors between groups were evaluated using Fisher’s exact test or Pearson’s χ2 test for categorical variables and Student’s t test for continuous variables. Kaplan-Meier estimates were used to generate overall survival plots. The log-rank test was used to compare survival outcomes. Cox proportional hazards regression modeling was used in univariate and multivariate survival analyses. Significant differences were assumed at p values of less than 0.05 in a two-tailed test.
Results
Clinicopathologic Characteristics
Table 1 summarizes the demographic, treatment, and pathologic characteristics of the 134 FMUUH patients and 56 MSKCC patients. FMUUH patients were on average 5.6 years younger than MSKCC patients, and FMUUH patients were more frequently male (79.1% vs. 57.1%, p = 0.002). Mean BMI was higher in MSKCC patients (26.7 ± 5.1 kg/m2 vs. 22.2 ± 3.4 kg/m2, p < 0.001). All patients treated at FMUUH were Asian, whereas 78.6% of patients treated at MSKCC were Caucasian, 12.5% were Asian, and 8.9% were African-American.
More than half of MSKCC patients underwent distal gastrectomy (53.5%), while more than half of FMUUH patients underwent total gastrectomy (58.2%). Fewer lymph nodes were examined in MSKCC patients compared with FMUUH patients (21.7 ± 10.6 vs. 37.4 ± 15.0).
Upon pathologic review, FMUUH patients had a higher rate of undifferentiated tumors (65.7% vs. 44.6%), a lower rate of vascular invasion (33.6% vs. 69.6%), and a lower rate of perineural invasion (29.9% vs. 58.9%). FMUUH patients overall had more advanced tumors in terms of T status, N status, and TNM stage (all p < 0.05).
Tumor Expression of CD44 and p-MEK
Tumor samples were immunohistochemically stained and analyzed to quantify expression of CD44, a marker of GA CSCs, and phosphorylated MEK (p-MEK), a marker of RTK-RAS activation (Fig. 1a). Of the 134 FMUUH patients, 102 (76.1%) had tumors expressing high levels of CD44 (Fig. 1b), and 48 (35.8%) had high expression of p-MEK. Among the 56 MSKCC patients, 43 tumors (76.8%) had high CD44 expression, and 23 (41.1%) had high p-MEK expression. The proportions of patients with high versus low CD44 and p-MEK expression in the FMUUH and MSKCC cohorts did not significantly differ (all p > 0.05).
Relationships Between CD44 and p-MEK Expression and Clinicopathologic Characteristics
Clinicopathologic characteristics of patients categorized by CDK44 and p-MEK protein expression (within each cohort) are compared in Supplemental Table 1. Within FMUUH and MSKCC patient groups, those whose tumors exhibited low versus high CD44 and p-MEK expression did not significantly differ in gender distribution, age, tumor location, vascular invasion, or perineural invasion (all p > 0.05). High CD44 expression was associated with more advanced TNM stage in the FMUUH cohort and larger tumor size and undifferentiated type in the MSKCC cohort. High expression of p-MEK was associated with undifferentiated type in the FMUUH cohort and larger tumor size in the MSKCC cohort. Since these correlations are not consistent, the significant p values may be from multiple testing and may not be true correlations.
Because there were small numbers of patients with stage I and II disease, stage I and II patients (total n = 77) were combined and compared with stage III patients (total n = 113). In combining cohorts, stage III patients had high CD44 expression in 93/113 (82%) of tumors compared with 52/77 (68%) in stage I and II patients (p = ns). Stage III patients had high p-MEK expression (43/113, 38%) in tumors compared with 27/77 (35%) in stage I and II patients (p = ns). Thus, high expression of CD44 and p-MEK appear to be independent of tumor stage.
CD44 and p-MEK Expression and Overall Survival
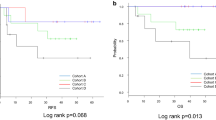
Estimated 5-year overall survival (OS) was 63.5% in FMUUH patients and 57.5% in MSKCC patients. High CD44 expression was associated with significantly worse overall survival in both cohorts (Fig. 2a, b). Five-year OS for high versus low CD44 expression was 54.3% versus 90.6% in FMUUH patients (p = 0.002) and 49.7% versus 92.3% in MSKCC patients (p = 0.044). High p-MEK expression also was associated with worse OS in both cohorts (Fig. 2c, d). Five-year overall survival for high versus low p-MEK expression was 47.2% versus 73.3% in FMUUH patients (p = 0.012) and 45.2% versus 65.1% in MSKCC patients (p = 0.032).
Overall survival stratified by CD44 and p-MEK expression. Kaplan-Meier curves comparing overall survival within the FMUUH (a) and MSKCC (b) cohorts between patients with low or high CD44 expression. Kaplan-Meier curves comparing overall survival within the FMUUH (c) and MSKCC (d) cohorts between patients with low or high p-MEK expression. Kaplan-Meier curves comparing overall survival within the FMUUH (e) and MSKCC (f) cohorts between patients with low CD44, high CD44/low p-MEK, or high CD44/high p-MEK expression
Among patients with low CD44 expression, the 5-year OS of patients with low versus high p-MEK expression did not significantly differ in either cohort (p > 0.05, Supplemental Fig. 1). Among patients with low CD44 expression, 5-year OS was 96.6–92.3%, regardless of p-MEK expression level. Among the patients with high CD44 expression, those with low p-MEK expression had an intermediate prognosis (5-year OS, 55.4–66.4%) and those with high p-MEK expression had the worst prognosis (5-year OS, 39.5–41.6%) (Fig. 2e, f). Similar results were found when the FMUUH and MSKCC cohorts were considered a single group (Fig. 3). Because of a small sample size of patients in stage I (21 patients in all patients), we combined stages I and II for survival analysis. The results showed that in stages I–II or III, the high expression of CD44 and p-MEK all have worse survival (Supplemental Fig. 2).
Factors Predictive of Survival
We next performed univariate and multivariate analyses of factors possibly associated with overall survival (Table 2). The following factors were significant negative prognostic factors on univariate analysis in the FMUUH cohort: advanced T status, advanced N status, high CD44 expression, and high p-MEK expression; all of these remained significant on multivariate analysis. In the MSKCC cohort, univariate analysis found that age, tumor size, vascular invasion, advanced T status, advanced N status, CD44 expression, and p-MEK expression were associated with worse overall survival. Multivariate analysis narrowed this list to tumor size, advanced N status, and high p-MEK expression. The same analysis among the combined cohort identified advanced N status and high CD44 and high p-MEK expression as negative prognostic factors (Supplemental Table 2).
Discussion
In this study, we evaluated expression of the CSC marker CD44 and the RTK-RAS activation marker p-MEK as prognostic factors for overall survival in two cohorts of patients undergoing curative-intent gastrectomy for GA, one from China and one from the USA, because some studies have found that there were several different clinicopathological characteristics between East and West.22,23 Although there were several differences in clinicopathologic variables and treatment between the two cohorts, CD44 and p-MEK expression predicted worse overall survival outcomes in both groups. Based on our prior studies,16,18 we suspect that the subgroup of patients with high tumor expression of CD44 and p-MEK have higher propensity for metastatic disease and are more resistant to adjuvant chemotherapy.
We previously found that CD44 expression distinguishes a subset of GA cells with CSC and malignant transformation properties that can be blocked by Hedgehog pathway inhibition.24 CD44 is the principal cell surface receptor for hyaluronic acid, a major component of extracellular matrix,12 and plays important roles in cell-matrix interactions, motility, matrix degradation, proliferation, and survival. Several studies have shown an association between expression of a specific CD44 isoform, variant 6, and lymph node metastasis and worse prognosis in GA.25,26 We used CD44 antibodies that recognize all CD44 isoforms, and did not analyze expression of specific CD44 isoforms. Our finding that high CD44 expression is associated with reduced overall survival confirms those of other studies.13,27,28 Other studies have shown that more than half of analyzed GAs have some CD44 expression.29,30 Additionally, CD44 expression in this study did not correlate with more advanced TNM stage, which differs from the review by Wang et al.31
While the relationship between RTK-RAS activation and CSC function has not been extensively studied in GA, there have been some studies in other gastrointestinal tumors. In colorectal cancer, Blaj et al. found that high MAPK activity promotes EMT and marks a progenitor cell subpopulation that served as the predominant source of growing flank xenografts.32 Also in colorectal cancer, Moon et al. showed that in cells carrying mutated APC, oncogenic KRAS increases expression of CSC markers (CD44, CD133, and CD166), spheroid formation, and the size of tumor xenografts. In pancreatic CSCs, inhibition of KRAS led to downregulation of JNK signaling and loss of self-renewal and tumor-initiating capacity.33
MEK overexpression has been identified in a number of other malignancies including renal cancer, breast cancer, and prostate cancer and has been linked to tumor progression and metastasis.34,35,36 While studies of the role of MEK in gastric cancer tumorigenesis are limited, one study by Liang et al. linked high expression to GA progression in patients. Among 42 patients, increased MEK expression was significantly associated with lymph node metastasis.37
Our results, by clinically linking high MEK activation to worse outcomes, suggest that RTK-RAS inhibition may benefit a subgroup of patients with GA. Targeted therapies are urgently needed to overcome the challenge of chemotherapy resistance. Appropriately selecting patients for targeted therapies is crucial to demonstrating their efficacy, as seen with trastuzumab plus chemotherapy in patients whose GA overexpresses human epidermal growth receptor 2 (HER-2), which prolonged survival from 11 to 14 months,38 compared with the lack of benefit of combining cytotoxic chemotherapy with agents targeting the vascular endothelial growth factor A (VEGF-A) or epidermal growth factor (EGF) pathways in unselected patients.39,40,41 RTK-RAS pathway inhibition may only be effective for the subset of GAs with high CD44 expression and high RTK-RAS activity. Our finding that patients with increased tumor levels of CD44 and increased p-MEK expression had significantly worse overall survival after resection of their tumors suggests that this may be a subgroup in which MEK inhibition would be most beneficial. Supporting this conclusion, in our genetically engineered mouse model of GA driven in part by mutant KRAS, MEK inhibition using PD0325901 starting at 4 weeks of age increased median survival times by 25% (Yoon CH et al., Mol Cancer Res in press). Several MEK inhibitors including trametinib, cobimetinib, and binimetinib are currently FDA-approved for use in patients with BRAF-mutated melanoma, which should facilitate initiation of a clinical trial in GA.42,43
There are several limitations to this study. First, the relatively limited number of patients in this study makes our findings less definitive than those of a larger-scale investigation. Second, the fact that all patients were treated at only two institutions means that these results require validation in other cohorts. Third, patients receiving neoadjuvant treatment were excluded, raising the potential for selection bias (they were excluded because such treatment can affect CD44 and MEK expression). Lastly, we did not examine correlations between CD44 and MEK expression and response to therapy among patients who developed recurrence and received treatment in the metastatic setting.
In summary, this study shows that high tumor expression of CD44 and p-MEK are associated with significantly worse overall survival in patients with GA. Together with our previous findings, we suspect that targeting the RTK-RAS-MEK pathway may be a means to inhibit metastases and reverse chemotherapy resistance in the subset of patients with high CD44 expression and RTK-RAS activation.
References
Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev. Jan 2016;25(1):16-27.
Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. Jan 21 2006;12(3):354-362.
Pozzo C, Barone C. Is there an optimal chemotherapy regimen for the treatment of advanced gastric cancer that will provide a platform for the introduction of new biological agents? The oncologist. Jul 2008;13(7):794-806.
Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. Jan 3 2008;358(1):36-46.
Cervantes A, Roda D, Tarazona N, Rosello S, Perez-Fidalgo JA. Current questions for the treatment of advanced gastric cancer. Cancer Treat Rev. Feb 2013;39(1):60-67.
Clevers H. The cancer stem cell: premises, promises and challenges. Nature medicine. 2011;17(3):313.
Garcia-Heredia JM, Lucena-Cacace A, Verdugo-Sivianes EM, Pérez M, Carnero A. The cargo protein MAP17 (PDZK1IP1) regulates the cancer stem cell pool activating the Notch pathway by abducting NUMB. Clinical Cancer Research. 2017.
Jiang Y, Yang S, Li P, et al. The promotion of the transformation of quiescent gastric cancer stem cells by IL-17 and the underlying mechanisms. Oncogene. 2017;36(9):1256.
Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell stem cell. 2014;14(3):275-291.
Zabala M, Lobo N, Qian D, et al. Overview: cancer stem cell self-renewal. Cancer Stem Cells: Academic Press; 2016:25-58.
Takaishi S, Okumura T, Tu S, et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 5/2009 2009;27(5):1006–1020.
Jang BI, Li Y, Graham DY, Cen P. The Role of CD44 in the Pathogenesis, Diagnosis, and Therapy of Gastric Cancer. Gut Liver. 12/2011 2011;5(4):397–405.
Yan Y, Zuo X, Wei D. Concise review: emerging role of CD44 in cancer stem cells: a promising biomarker and therapeutic target. Stem cells translational medicine. 2015;4(9):1033-1043.
Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 9/11/2014 2014;513(7517):202–209.
Samatar AA, Poulikakos PI. Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discov. Dec 2014;13(12):928-942.
Till JE, Yoon C, Kim BJ, et al. Oncogenic KRAS and p53 Loss Drive Gastric Tumorigenesis in Mice That Can Be Attenuated by E-Cadherin Expression. Cancer Res. Oct 01 2017;77(19):5349–5359.
Shimada S, Mimata A, Sekine M, et al. Synergistic tumour suppressor activity of E-cadherin and p53 in a conditional mouse model for metastatic diffuse-type gastric cancer. Gut. Mar 2012;61(3):344-353.
Yoon C, Till J, Cho SJ, et al. KRAS Activation in Gastric Adenocarcinoma Stimulates Epithelial-to-Mesenchymal Transition to Cancer Stem-Like Cells and Promotes Metastasis. Mol Cancer Res. Sep 2019;17(9):1945-1957.
Amin M.B.; Edge S.B.; Greene F.L.; et al e. AJCC Cancer Staging Manual. 8th ed. New York: Springer. 2017:203–220.
Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. Jul 1998;4(7):844–847.
Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochemical Journal. 2000;351(2):289–305.
Akgul O, Ocak S, Gundogdu SB, Yalaza M, Guldogan CE, Tez M. Comparison of East and West Survival Nomograms in Turkish Gastric Cancer Patients Who Underwent Radical Surgery. Scand J Surg. Dec 2018;107(4):308–314.
van Grieken NC, Aoyama T, Chambers PA, et al. KRAS and BRAF mutations are rare and related to DNA mismatch repair deficiency in gastric cancer from the East and the West: results from a large international multicentre study. Br J Cancer. Apr 16 2013;108(7):1495–1501.
Yoon C, Park dJ, Schmidt B, et al. CD44 expression denotes a subpopulation of gastric cancer cells in which Hedgehog signaling promotes chemotherapy resistance. Clin. Cancer Res. 8/1/2014 2014;20(15):3974–3988.
Liu YJ, Yan PS, Li J, Jia JF. Expression and significance of CD44s, CD44v6, and nm23 mRNA in human cancer. World J. Gastroenterol. 11/14/2005 2005;11(42):6601–6606.
Okayama H, Kumamoto K, Saitou K, et al. CD44v6, MMP-7 and nuclear Cdx2 are significant biomarkers for prediction of lymph node metastasis in primary gastric cancer. Oncology reports. 2009;22(4):745–755.
Cao X, Cao D, Jin M, et al. CD44 but not CD24 expression is related to poor prognosis in non-cardia adenocarcinoma of the stomach. BMC gastroenterology. 2014;14(1):157.
Ibrahim HM, AbdElbary AM, Mohamed SY, Elwan A, Abdelhamid MI, Ibrahim A. Prognostic Value of Cyclin D1 and CD44 Expression in Gastric Adenocarcinoma. Journal of gastrointestinal cancer. 2018:1–10.
Ghaffarzadehgan K, Jafarzadeh M, Raziee HR, et al. Expression of cell adhesion molecule CD44 in gastric adenocarcinoma and its prognostic importance. World journal of gastroenterology: WJG. 2008;14(41):6376.
Nosrati A, Naghshvar F, Khanari S. Cancer stem cell markers CD44, CD133 in primary gastric adenocarcinoma. International journal of molecular and cellular medicine. 2014;3(4):279.
Wang T, Ong C, Shi J, et al. Sequential expression of putative stem cell markers in gastric carcinogenesis. British journal of cancer. 2011;105(5):658.
Blaj C, Schmidt EM, Lamprecht S, et al. Oncogenic Effects of High MAPK Activity in Colorectal Cancer Mark Progenitor Cells and Persist Irrespective of RAS Mutations. Cancer Res. Apr 1 2017;77(7):1763–1774.
Okada M, Shibuya K, Sato A, et al. Targeting the K-Ras--JNK axis eliminates cancer stem-like cells and prevents pancreatic tumor formation. Oncotarget. Jul 15 2014;5(13):5100–5112.
Oka H, Chatani Y, Hoshino R, et al. Constitutive activation of mitogen-activated protein (MAP) kinases in human renal cell carcinoma. Cancer research. 1995;55(18):4182–4187.
Fujii S, Tokita K, Wada N, et al. MEK–ERK pathway regulates EZH2 overexpression in association with aggressive breast cancer subtypes. Oncogene. 2011;30(39):4118.
Moriceau G, Hugo W, Hong A, et al. Tunable-combinatorial mechanisms of acquired resistance limit the efficacy of BRAF/MEK cotargeting but result in melanoma drug addiction. Cancer cell. 2015;27(2):240–256.
Liang B, Wang S, Zhu X-G, Yu Y-X, Cui Z-R, Yu Y-Z. Increased expression of mitogen-activated protein kinase and its upstream regulating signal in human gastric cancer. World journal of gastroenterology: WJG. 2005;11(5):623.
Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. Aug 28 2010;376(9742):687–697.
Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. May 2013;14(6):490–499.
Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. Oct 20 2011;29(30):3968–3976.
Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. May 2013;14(6):481–489.
Zhao Y, Adjei AA. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist. Jun 2015;20(6):660–673.
Yoon C, Till JE, Cho S-J, et al. KRAS activation in gastric adenocarcinoma stimulates epithelial-to-mesenchymal transition to cancer stem-like cells and promotes metastasis. Clinical Cancer Research (in press). 2019.
Acknowledgments
We thank the MSKCC senior editor Jessica Moore for reviewing this manuscript. We also thank Rashmi Kamath, Priyanka Kasbekar, and Faiza Anwar for their work on the management of the Memorial Sloan Kettering Cancer Center gastric database.
Funding
This study was funded by the National Natural Science Foundation of China (No. 81871899); Construction Project of Fujian Province Minimally Invasive Medical Center, China (No. [2017]171); Project supported by the Science Foundation of the Fujian Province, China (No. 2018 J01307); Startup Fund for Scientific Research, Fujian Medical University, China (No. 2016QH024); National Institutes of Health (P30 CA008748); and the DeGregorio Family Foundation Grant.
Author information
Authors and Affiliations
Contributions
Lin JX, Huang CM, and Yoon SS conceived the study, analyzed the data, and drafted the manuscript; Yoon C, Li P, and Yu Q helped collect data and design the study. Qiu SL and Zheng CH helped revise the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of Interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the FMUUH and MSKCC research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Synopsis
Receptor tyrosine kinase (RTK)-RAS signaling in gastric adenocarcinoma (GA) promotes acquisition of cancer stem-like cell (CSC) phenotypes including metastasis and chemotherapy resistance. Here we find that high tumor expression of the CSC marker CD44 and the RTK-RAS activation marker phosphorylated MEK are independent predictors of worse overall survival. Thus, the RTK-RAS pathway may be a therapeutic target in CD44-positive GA to inhibit metastasis and reverse chemotherapy resistance.
Rights and permissions
About this article
Cite this article
Lin, Jx., Yoon, C., Li, P. et al. Increased CD44 Expression and MEK Activity Predict Worse Prognosis in Gastric Adenocarcinoma Patients Undergoing Gastrectomy. J Gastrointest Surg 25, 1147–1155 (2021). https://doi.org/10.1007/s11605-020-04616-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-020-04616-4