Abstract
Prostate cancer is the most common male malignancy in the Western world. Once it metastasizes, it is incurable. The current gold standard for metastatic disease is the combined docetaxel/prednisone regimen. Prostate cancer shows several characteristics that make it a suitable candidate for immunotherapy, as recently exemplified by the approval of sipuleucel-T, the first vaccine to treat any malignancy. Here, we review different tumor-associated antigen immunotherapy strategies currently being investigated, from a humanized radiolabeled monoclonal antibody (J-591) that targets radiation into tumor cells, moving on to vaccines and through to immunomodulator agents such as anti-CPLA-4 and anti-PD-1 monoclonal antibodies that activate T-cell responses via immune checkpoint inhibition. We explore different opinions on the best approach to integrate immunotherapy into existing standard therapies, such as androgen-deprivation therapy, radiotherapy or chemotherapy, and review different combination sequences, patient types and time points during the course of the disease to achieve a lasting immune response. We present data from recent phase III clinical trials that call for a change in trial endpoint design with immunotherapy agents, from the traditional tumor progression to overall survival and how such trials should include immune response measurements as secondary or intermediate endpoints to help identify patient clinical benefit in the earlier phases of treatment. Finally, we join in the recent questioning on the validity of RECIST criteria to measure response to immunotherapeutic agents, as initial increases in the size of tumors/lymph nodes, which are part of a normal immune response, could be categorized as disease progression under RECIST.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PC) is the most common male malignancy in the Western world; it is expected to affect 233,000 men and cause 29,500 deaths [1] in the United States (US) in 2014.
Although the majority of patients are diagnosed with localized disease, about a third will relapse after successful local therapy and others will present as locally advanced or metastatic disease upfront. Androgen-deprivation therapy (ADT) is the first-line gold standard in advanced PC [2–4]. However, despite initial response rates of 80–90 %, all patients will eventually progress and develop metastatic castration-resistant PC (mCRPC). Several compounds have demonstrated activity and improved overall survival (OS), gaining approval by regulatory authorities [5–15]. Among them, an autologous antigen presenting cell (APC)-based cancer vaccine, sipuleucel-T, was approved by the Food and Drug Administration (FDA) [16] in 2010 and by the European Medicine Agency (EMA) in 2014 for the treatment of patients with asymptomatic or minimally symptomatic mCRPC. Sipuleucel-T represents the first cell-based immunotherapy (IT) able to demonstrate an improvement in OS in cancer patients, opening a new treatment paradigm.
Different reasons make PC a suitable model for IT. Firstly, PC presents a variety of tumor-associated antigens (TAAs) such as prostate-specific antigen (PSA), prostatic acid phosphatase (PAP) and prostate-specific membrane antigen (PSMA), all of which have been shown to produce a clinical response through immunogenicity and also have been classified as self-antigens, with the advantage of being able to regulate the normal mechanisms that develop autoimmunity. Additionally, PC has a relatively slow growth rate that may allow the immune system (IS) the necessary time to produce a response and it seems to be more immunogenic than previously thought [17, 18]. Also, the prostate is a dispensable organ, so any autoimmunity generated would have little consequences. Multiple IT agents have recently been tested and will progressively join the clinic.
The general principles of immunology in cancer, with a focus on recently developed immune-based treatment strategies for mCRPC, as well as other relevant topics such as the integration of IT with other treatment strategies, response assessment and the identification of predictive biomarkers of response, are reviewed here.
Immunity and cancer
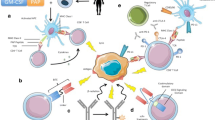
Innate immunity acts as a first line of defense upon foreign antigen (Ag) (from an infectious agent or a tumor cell) detection, involves neutrophils, macrophages and natural killer (NK) cells, and results in opsonization, phagocytosis and cytokine, chemokine and other proteolytic enzymes release. Adaptive immunity involves B and T-lymphocytes/cells, responsible for humoral [i.e., antibody (Ab) mediated] and cellular IRs, respectively [19, 20]. Early tumor cells are believed to be “attacked” by both innate and adaptive immune responses (IRs). Cells that escape these mechanisms move into an “equilibrium” phase that can last the host’s whole life and relies on the adaptive IS. However, tumor cells develop mechanisms that allow them to escape the host’s adaptive IS and to grow into clinically detectable malignancies.
B cells express B-cell receptors (BCR) on their surface and induce Ab production upon recognition of a foreign Ag. Some B cells will produce and release a specific Ab for a given Ag throughout the host’s life span (i.e., long-term immunity). Complex mechanisms avoid autoimmunity (prevention of auto-antigen recognition) by circulating B lymphocytes [21]. T cells originate in the bone marrow and migrate to the thymus, where, through a finely regulated gene re-arrangement process, will express a great variety of T-cell receptors (TCRs) on their surface. Once outside the thymus, T cells require two signals before they can recognize Ags specific to their TCRs. The first comes from the recognition of a human leukocyte antigen (HLA) receptor on the surface of an APC, a type of B cell. The second involves co-stimulatory molecules on the surface of APCs, known as B7 (CD80 and CD86) proteins [22] that recognize CD28 proteins on the surface of T cells. In parallel, a co-inhibitory signal mediated by cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed cell death-1 (PD-1) (also known as “checkpoints”) on the T cell and their respective ligands, will result in T-cell inhibition [23]. Other checkpoints, such as T-regulatory cells (Tregs), are activated by CTLA-4 [24]; hence, blocking CTLA-4 also leads to Treg inhibition. “Checkpoint” inhibition can be used as a strategy for activating T-cell-mediated IRs [25]. Activated T cells proliferate and generate two types of effector T cells: CD4+ T helper (Th) cells and CD8+ T cytotoxic (CTLs) cells. There are two types of CD4+ Th cells: Th1 cells are involved in cellular IRs and Th2 cells are involved in humoral IRs. CD8+ CTLs are mainly cytotoxic, recognize Ags bound to HLA receptors and destroy them either through the insertion of perforins in the target cell membrane (allowing the entrance of enzymes that kill the cell) or through binding the target cell Fas receptor (leading to intracellular caspase activation [19, 20] and death). Both CD4+ and CD8+ T cells have the ability to mount a rapid response upon subsequent exposure to the same Ag (i.e., immunological memory).
Passive immunotherapy
Passive IT uses antitumor agents generated in vitro, such as monoclonal antibodies (mAbs) or cytokines, with intrinsic immunological activity. To date, only a humanized radiolabeled mAb, J591, is used to target radiation directly to the tumor cells. A summary of phase I–II trials with J591 is presented in Table 1 [26–28].
Active immunotherapy
Active IT (immunization/vaccination) intends to generate an IR by the host by activating CTLs [23] against TAAs. Four types of vaccines, as well as immunomodulators (used to block immune “checkpoints”) are able activate CTL responses and are currently being investigated for the treatment of mCRPC. B-cell Ab responses have also been observed in mCRPC patients who respond to CTLA-4 blockade treatment [29].
Autologous vaccines
Sipuleucel-T is designed against PAP. The vaccine process includes the collection of the patient’s peripheral dendritic cells (DCs) (a type of APC) via leukapheresis and its incubation with a fusion protein (PA2024) composed of PAP (which targets the IR to PC cells) and granulocyte/macrophage-colony stimulating factor (GM-CSF) (which enhances the IR) [30, 31]. During a 36-h ex vivo incubation period, the patient’s DCs break PA2024 into small peptides, later displayed by the HLA receptors. The activated DCs are then re-infused into the patient with the goal of generating a PAP-specific IR and the ensuing antitumor effect. A sipuleucel-T treatment means repeating this whole process three times at 2-week intervals. The quality of the reinfusion mix is ensured if it contains more than 40 million CD54+ DCs, as this has shown prolonged survival rates [32]. This approach has the advantages of activating APCs away from an immunosuppressive environment and directing the IR through an Ag-targeting process.
Three phase III clinical trials evaluated the efficacy of sipuleucel-T in mCRPC (Table 2). The first two trials [33, 34] (reported as a single integrated analysis) randomized patients to sipuleucel-T (n = 147) or placebo (n = 78) and had time-to-progression (TTP) as primary endpoint. A statistically significant OS benefit was demonstrated for patients treated with sipuleucel-T compared to those treated with placebo, translating into a 33 % reduction in the mortality risk. The most common adverse events (AEs) associated with treatment consisted of mostly mild-to-moderate chills, pyrexia, headache, asthenia, dyspnea, vomiting and tremor. Similar results were achieved in the third trial (IMPACT) [16] (Table 2), which randomly assigned patients to either sipuleucel-T (341 patients) or placebo (171 patients) and had OS as its primary endpoint. A relative reduction of 22 % in the mortality risk for patients treated with sipuleucel-T as compared to the placebo group translated into a statistically significant improvement in median OS. Results were unaltered after adjustment for the use of docetaxel after the study treatment (HR: 0.78; 95 % CI: 0.62–0.98; P = 0.03). IRs were reported in patients who received sipuleucel-T [32]. The AEs more frequently reported included chills, fever, and headache. Significantly, none of the trials showed a statistically significant advantage in the risk of disease progression (PD) for sipuleucel-T. Based on its survival advantage, sipuleucel-T was approved by the FDA in 2010 for the treatment of asymptomatic or minimally symptomatic mCRPC.
Cell-based vaccines
GVAX was developed by using an androgen-sensitive and an mCRPC cancer cell line (LNCaP and PC-3, respectively), and expressing GM-CSF [35–37].
Two phase III randomized trials tested GVAX in mCRPC (VITAL-1 and VITAL-2 in Tables 2, 5, respectively). VITAL-1 compared GVAX to D/P (n = 626) in asymptomatic CRPC patients, VITAL-2 compared a combination of GVAX with docetaxel against the gold standard D/P (n = 408) in symptomatic CRPC patients. Neither trial was able to show an OS advantage for GVAX. Both trials were prematurely terminated, VITAL-1 due to efficacy concerns (a futility analysis reported a less than 30 % chance of meeting an improved survival) and VITAL-2 due to an increased mortality rate in the GVAX plus docetaxel arm (67 deaths) compared to the D/P arm (47 deaths) [38, 39]. Although still unexplained, the increased death rate did not seem to be related to increased toxicity. The lack of a placebo-control group in the single-agent study (VITAL-1) has been used as an argument against the design of this trial [40], suggesting that, unless a placebo-control arm is used, CT should be standardized across treatment arms. Failure to optimize the dosing and timing of docetaxel in the combination regimen in a prior phase II study is also challenging the quality of VITAL-2 [40].
Another cell-based vaccine, Onyvax, has been made from three allogeneic PC cell lines, selected to contain elements from the major sites of the disease, OnyCap23 (similar to bone metastases), LnCaP (similar to lymph node metastasis) and P4E6 (derived from a primary PC biopsy). A phase II trial testing this vaccine in 26 mCRPC patients resulted in significant, prolonged reduction in PSA velocity (PSAV), no significant toxicity and an extended time to PD when compared to other standard treatments at a similar stage of the disease [41] (Table 2). PSAV-responding patients showed Th1 cytokine release in response to vaccine lysate re-stimulation and the immunologic profile correlated with PSAV response.
Cell-based vaccine therapies like GVAX and Onyvax have the potential to target multiple Ags with a favorable safety profile.
DNA-based vaccines
This type of vaccine uses plasmid DNA, which is taken up by the host’s cells, which will subsequently express the proteins encoded within the plasmid and induce an IR.
The first trial evaluating a DNA vaccine against CRPC was a phase I study with pVAX/PSA [42], targeting PSA. Vaccination with the plasmid vector pVAX/PSA plus GM-CSF and IL-2 as adjuvants, was tested at three different doses (n = 8). At the highest dose, the vaccine showed a PSA-specific cellular IR and a humoral IR. The vaccine had no AEs (Table 2). Other Ags targeted with this strategy include PSMA. A first phase I/II trial [43] (n = 26) could not conclude the effectiveness of the vaccine due to heterogeneity in the patient population and the concomitant use of hormone therapy in many patients. A second phase I trial targeting PSMA created a DNA vaccine encoding human PSMA and was followed by a DNA vaccine encoding mouse DNA (or vice versa) [44] under the hypothesis that a xenogeneic antigen was a more potent immunogen than self-Ags. T-cell responses to fibroblasts expressing PSMA were observed at the highest dose. In the next phase I/II trial, a DNA fusion vaccine encoded a domain from a fragment of the tetanus toxin (DOM) linked to a HLA-A2-binding epitope from PSMA [45]. The vaccine induced significant DOM-specific CD4+ and PSMA-specific CD8+ T-cell responses, PSA doubling time (PSA-DT) increased significantly over the 72-week follow-up, it was safe and well tolerated (Table 2). PAP has also been targeted in DNA vaccines. A phase I/IIa trial tested different doses of a DNA vaccine encoding human PAP plus GM-CSF (pTVG-HP) in patients with stage M0 PC [46] (patients with a diagnosis of PC and biochemical (serum PSA) recurrence after definitive surgery and/or radiation therapy with no evidence of suspected lymph node, bone, or visceral metastatic disease on bone scans or computed tomography scans). PAP-specific CD8+ T-cell responses were observed immediately after treatment and after 1-year patient follow-up [47] and PAP-specific CD4+ and/or CD8+ T-cell proliferation was also observed (Table 2). Humoral response was not detected and PSA-DT increased from 6.5 months pre-treatment to 8.5 months on-treatment and 9.3 months in the 1-year post-treatment period. A randomized phase II trial (NCT01341652) is currently evaluating the 2-year metastasis-free rate in patients receiving the vaccine and another trial (NCT00849121) is evaluating the safety of serial vaccinations and long-lived IRs and trying to find a better vaccination schedule. A cancer-testis Ag NY-ESO-1 has also been targeted with a DNA vaccine in a trial that included multiple solid tumor patients, including 10 patients with PC [48]. NY-ESO-1-specific CD4+ T-cell and CD8+ T-cell responses were reported after vaccination. However, responses were transient and disease progressed in most cases (despite a temporary increase in PSA-DT). In vitro depletion of Tregs restored detectable levels of Ag-specific effector T cells, an indication that Tregs down-regulate NY-ESO-1-specific T-cell responses [49].
Non-human DNA vaccines have already been approved in the US, preempting their potential in human IT. Among their advantages are the easier manufacturing, manipulation, storage and transport of the plasmid DNA as compared to peptides/proteins, bacterial and viral vectors, their cost-effectiveness and the fact that the bacterial backbone of the plasmid acts as an adjuvant. On the down side, they show weaker initial IRs than some of the alternatives already discussed, although this aspect can be overcome with repetitive immunizations [47] or with the alternative approach known as “altered peptide ligands (APLs)” consisting in the alteration of ligands in such a way that it results in an increased binding of the Ag to HLA receptors and an enhanced IR. One such case has been reported [50] (Table 2) in which two peptides within SSX2, a cancer-testis Ag expressed in 25 % of mCRPC lesions, were modified prior to insertion in a plasmid DNA vaccine in such a way that it increased their binding to HLA-A2, generated robust peptide-specific CD8+ T cells and produced Th1 cytokines specific for each epitope.
Viral vector-based vaccines
A gene is inserted into a recombinant virus vector, often a poxvirus (e.g., vaccinia, fowlpox). The Ags encoded in the viral vector (with or without co-stimulatory molecules) will then be lysed and taken up by APCs, which will present their peptides to CD4+ and CD8+ T cells. One of the intrinsic disadvantages of this type of vaccine is the development of host-induced Abs to the viral vector itself, which neutralizes the vaccine after several administrations and means most viral-based vaccines can be given only once [51]. A prime-boost approach has been used with ProstVac, a PSA-targeted poxviral vaccine, as an improved strategy that enhances the IR. It uses recombinant vaccinia for the prime vaccination and recombinant fowlpox for multiple booster injections that have been shown to induce non-neutralizing Abs in humans [52]. It also contains three T-cell co-stimulatory molecules (B7.1, ICAM-1 and LFA-3), designated as TRICOM [53]. Several studies have been conducted with ProstVac in mCRPC. A placebo-controlled phase II study [54] randomized (2:1) 125 patients to ProstVac plus GM-CSF or to control empty vectors plus saline injections. Median OS was significantly higher in the experimental arm, mortality at 3 years was significantly reduced with ProstVac and PD was similar in the two groups (Table 2). There were no detectable Ab responses to PSA and the vaccine was well tolerated. A global phase III study (NCT01322490) plans to recruit up to 1200 patients, who will be randomized to ProstVac, ProstVac plus GM-CSF or placebo and has OS as its primary endpoint.
Adenovirus type 5 (Ad5) vectors are also used for gen delivery and are useful adjuvants for the delivery of TAA-coding genes, due to their high affinity for DCs [55]. A phase I clinical trial tested Ad5-PSA in 32 mCRPC patients [56]. Antibodies against PSA were produced by 34 % of patients and anti-PSA T-cell responses were produced by 68 % of patients. PSA-DT was increased in 48 %, whereas 55 % survived longer than predicted (Table 2). A phase II study is in progress testing two vaccination protocols to determine if Ad5-PSA vaccines can yield therapeutic benefit. Patients with newly recurrent PC are being treated with Ad5-PSA/collagen matrix as a single intervention or following ADT (collagen matrixes have been shown pre-clinically to inhibit the production of anti-adenovirus Abs against the viral vector, resulting in more robust IRs than the use of Ad5-PSA alone) [57], while individuals with low CRPC disease burden are being treated with Ad5-PSA/collagen matrix alone. The development of anti-PSA IRs is the primary endpoint for patients with recurrent disease, while PSA-DT, time to progression and OS are the primary endpoints for CRPC patients with low disease burden. It has recently been reported [58] that 100 % of patients with recurrent disease and 67 % of patients with CRPC low disease burden have developed anti-PSA T-cell responses (Table 2).
The main advantage of viral vector-based vaccines lies on the fact that they retain their immunogenicity and lead to an increase in the TAA-specific T-cell IR, enhanced by the pro-inflammatory environment produced by the expression of viral proteins. Their main disadvantage is that most viral-based vaccines can be given only once to minimize Ab development to the viral vector.
Immunomodulator therapies
Co-inhibitory signaling result in T-cell inhibition and involves the following immune checkpoint molecules present on the T-cell surface: CD28, CTLA-4 and PD1. Immunomodulators are able to block co-inhibitory signals on the T cell, hence activating T-cell IRs.
Ipilimumab is a fully human monoclonal antibody that blocks CTLA-4, enhancing antitumor activity. Trials testing ipilimumab in PC are shown in Table 3. A phase I trial tested ipilimumab in mCRPC at increasing doses in combination with fixed doses of GM-CSF in order to see whether GM-CSF could enhance its antitumor efficacy. Three out of six patients treated at the highest dose had confirmed PSA declines of >50 %. Effector T-cell (CD25+ CD69+ CD8+) responses were of a higher magnitude at higher doses than with the same doses of either ipilimumab or GM-CSF alone [59]. A phase I/II trial is currently evaluating ipilimumab alone or in combination with radiotherapy (RT) in mCRPC [60] (Table 5). Results appeared to be in favor of the combination. Common AEs included fatigue, rash, pruritus, nausea, constipation and weight loss. Adrenal insufficiency, hepatitis and autoimmune colitis were some of the effects observed as a result of the activation of the IS. Two phase III trials are currently underway with ipilimumab in mCRPC. Patients with at least one bone metastasis from CRPC, were randomly assigned (1:1) in a phase II study to receive bone RT followed by either ipilimumab (10 mg/kg) or placebo every 3 weeks for up to four doses [61] after progressing to docetaxel. Non-progressing patients could continue to receive ipilimumab or placebo as maintenance therapy every 3 months until PD, unacceptable toxicity or death. Although OS (the primary endpoint) was not significantly different between the two arms, some signs of activity in favor of ipilimumab were observed. The most frequent grade 3–4 AEs included diarrhea (16 % in the ipilimumab group vs. 2 % in the placebo group), fatigue (11 vs. 9 %), anemia (10 vs. 11 %) and colitis (5 vs. 0 %). NCT01057810 is comparing ipilimumab with placebo in asymptomatic or minimally symptomatic CT-naïve mCRPC patients. Both have OS as their primary endpoint (Table 3).
PDL-1 is found on T-cells present in the prostate of men with mCRPC. Anti-PD-1 Abs block the PD1/PDL-1 interaction activating T-cell IRs. A phase I trial showed objective responses (complete or partial) in approximately 1:4–1:5 patients with other solid tumors with no significant AEs. No objective responses were observed in a group of 17 mCRPC patients [62]. To note, patients who did not respond had PDL-1-negative tumors.
Immunotherapy in neoadjuvancy in mCRPC
Neoadjuvancy aims at reducing the size of the tumor or the extent of the disease, increasing the probability of success of subsequent definitive procedures (surgical or RT) or decreasing the risk associated with such procedures when administered more extensively. Despite improvement in the reduction of prostate volumes and reduced serum PSA, published trials testing neoadjuvant ADT [63, 64], CT [65, 66] or targeted agents [67, 68] before radical prostatectomy (RP) surgery have not been able to prove a positive impact on OS, PFS or other clinically meaningful outcomes.
Given its novelty, clinical trials investigating neoadjuvant IT in mCRPC are sparse, highlighting the need to continue to investigate this aspect (Table 4). Early reports of a phase II trial currently taking place (NCT00715104) with neoadjuvant sipuleucel-T have reported the recruitment of effector CD3+ T cells into the tumor edge, supporting the proposed MOA for sipuleucel-T [69]. A neoadjuvant trial of GVAX pre-RP (NCT01696877) is currently randomizing patients with localized PC to ADT alone or low-dose cyclophosphamide (CP) followed by GVAX and ADT. Primary endpoints are intraprostatic CD8+ T-cell infiltration and safety and tolerability of the vaccine (Table 4). Neoadjuvant docetaxel/GVAX has been studied in locally advanced disease prior to RP [70]. Patients (n = 6) received four cycles of docetaxel and 2–3 days later, four courses of GVAX IT (in a prime-boost modality) preoperatively; six additional courses were given post-operatively. The primary endpoint of the trial was a pathologic state of pT0 (defined as no evidence of PC). Median change in PSA following neoadjuvant therapy was 1.47 ng/ml and four of the five patients completing RP showed a downgrade in their Gleason score. Undetectable PSA was achieved in three patients (2 months after RP) and in two patients (3 years after RP) (Table 4). No serious drug-related AEs were observed. A phase II study of neoadjuvant ipilimumab (NCT01194271) in combination with ADT prior to RP is currently ongoing. Primary endpoints include the measurement of the ratio effector T cell/Treg cell (in blood and tumor), CD4+ ICOS+ T cells (in blood and tumor), CD8+ ICOS+ T cells (in blood and tumor), NY-ESO-1 antibodies (in blood) and absolute lymphocyte count (in blood).
Immunotherapy in combination treatments
ADT and immunotherapy
ADT has direct effects on the IS, such as the inhibition of immune tolerance (which commonly develops in PC due to the fact that many of the antigens on PC cells are also present on normal prostatic epithelium) to TAAs [71] and increased T-cell infiltration of the prostate [72]. Therefore, administration of ADT prior to a vaccine or other IT agent might offer a potential means of increasing the patient’s response to the treatment [72] (Table 5). A randomized phase II trial [73] evaluated sipuleucel-T when administered either 2 weeks before or 3 weeks after standard ADT. Ag-specific responses (the primary end point of the trial) were similar across arms; however, cytokine responses and CD8+ T-cell activation were higher when sipuleucel-T was administered after ADT, suggesting more robust IRs when the vaccine is administered after the ADT. Conflicting with this report are the results from a trial evaluating a combination treatment of ProstVac followed by nilutamide vs. nilutamide followed by ProstVac [74]. Although non-statistically significant, the results show a trend in OS in favor of the vaccine alone or the vaccine followed by nilutamide over nilutamide alone or followed by the vaccine. Given the conflicting results, more trials are necessary before any suggestions can be made as to where IT should fit with current standard ADT treatment. Another phase II trial (NCT00450463) is currently underway and testing the combination treatment ProstVac plus flutamide vs. flutamide alone. The primary endpoint is time-to-treatment failure. Ipilimumab is also being evaluated in combination with neoadjuvant ADT [75], as well as in hormone-naïve mPC and in mCRPC in two phase II trials (NCT01377389 and NCT01498978).
Chemotherapy and immunotherapy
Although the traditional view is that of CT being an immunosuppressive treatment, it is now thought that CT might actually stimulate the IS through Treg inhibition, activation of effector T cells and B cells and cytotoxicity (which results in higher processing and presentation of TAAs by APCs) [76]. Therefore, it is possible that neoadjuvant CT might help tumor shrinking prior to IT, as exemplified in the ongoing trial (NCT01696877) evaluating ADT alone vs. neoadjuvant low-dose CP followed by GVAX and ADT in localized PC patients (Table 5). The design was supported by pre-clinical work showing that low-dose CP can inhibit immune tolerance by increasing CD8+ T-cell infiltration into the prostate and inhibiting Tregs. A phase I trial (NCT00916123) is currently comparing the standard D/P CT in combination with increasing doses of the anti-PSMA mAb 177Lu-J591 in mCRPC patients. A phase II trial (NCT01145508) is currently ongoing in slowly progressing mCRPC that compares standard D/P CT with ProstVac (during 12 weeks) followed by the standard CT. The primary endpoint is OS.
Radiotherapy and immunotherapy
RT also has a cytotoxic effect that leads to both a higher processing and presentation of TAAs by APCs and an increase in the host’s IR. Efforts to combine RT with IT in order to improve the efficacy of IT treatments [77] are being done. A phase II trial carried out in 30 patients randomized to either RT alone or in combination with a poxviral PSA vaccine showed that 13 out of 17 patients receiving the combined treatment had a threefold increase in PSA-specific T cells (p < 0.0005) [78] (Table 5). A phase I/II [60] and more recently a phase III [79] trial testing ipilimumab plus RT versus ipilimumab alone in mCRPC showed clinical antitumor activity with disease control and manageable AEs in the combination arm.
Combination of immunotherapies
Given all the different MOAs discussed, it might be expected that the combination of two or more ITs would result in more robust IRs. A phase I trial tested ipilimumab in combination with GVAX [80], which appeared to be well tolerated and showed a decrease ≥50 % in PSA levels in 25 % of patients (Table 6). The safety and tolerability of a combination of ipilimumab and ProstVac are being analyzed in a phase I trial (NCT00113984), the safety and efficacy of IL-21 and anti-PD-1 are being tested in NCT01629758, the feasibility of treatment with sipuleucel-T with or without an anti-PD-1 mAb (CT-011) and low-dose CP in advanced CRPC is under review in clinical trial NCT01420965 and the mAb J591 is being investigated in combination with recombinant IL-2 in a phase II trial (NCT00040586).
The evaluation of response in immunotherapy
Measurable disease is infrequent in PC, yet Response Evaluation Criteria in Solid Tumors (RECIST) [81] continue to be the main guide to assess tumor response to therapeutic agents. The PC Clinical Trials Working Group (PC-CTWG) [82], which led the change from traditional trial objectives (early PSA decline and regression of target lesions) to time-to-event endpoints to ensure that a drug was not discontinued before it had had time to work, is now proposing two routes in drug evaluation, one for cytotoxic agents and a different route for non-cytotoxic drugs that work more on the basis of slow tumor growth. It advises to ignore early changes (within the first 12 weeks) in serum PSA, pain and bone scans, and recommends that disease assessments be performed at fixed intervals and at the time of study end. Moreover, it suggests that categorizations such as complete, partial or stable response be dropped in favor of “time-to-treatment failure” measures. Since the last PC-CTWG publication, three phase III clinical trials resulted in the approval of sipuleucel-T after showing a statistically significant OS benefit [16, 33, 34] despite showing no PFS improvement. These trials illustrate how OS, and not PFS, should be used as a more robust clinical trial endpoint in IT trials, as maximal antitumor IR may not occur until 12 weeks or longer after initiation of therapy [83]. OS benefit despite no PFS benefit in IT can be explained [84] because IT agents do not target the tumor itself, but the host’s IS and it takes weeks to months to mount a clinically significant IR after immunization. Using OS as a clinical trial endpoint would mean, however, that trials would take years to complete; introducing measures of IR as secondary/intermediate endpoints could help solve this issue. Biomarkers of an IR could identify patient benefit in the earlier treatment phases and guide decisions to continue/discontinue therapy. Most biomarkers used to date are based on measuring CTL responses to specific TAAs [85] (production of gamma IFN ex vivo in an ELISPOT assay). However, results vary from institution to institution and the test is not able to assess the expansion of a T-cell response to TAAs not present in the vaccine via the “antigen cascade” mechanism (presentation by APCs of TAAs derived from dying tumor cells). Therefore, intermediate endpoints of response to IT need to be defined further.
In essence, while RECIST guidelines (originally designed for cytotoxic agents) assumed that an early increase in tumor growth and/or the appearance of new lesions signaled PD and resulted in treatment discontinuation, these criteria may not be sufficient to fully characterize the outcomes of IT [86], where responses may occur after conventional PD. Therefore, “clinically insignificant” PD (e.g., small new lesions in the presence of other responsive lesions) and durable SD may both represent antitumor activity and therapy discontinuation may not be appropriate unless PD is confirmed (as is usually done for response). Recent attempts to create new immune-related response criteria (irRC) [86, 87] have been done exclusively on ipilimumab phase II clinical data. Therefore, it is not clear whether this system could be extended to other ITs, such as vaccines, and how it would fit with the OS endpoint proposal. Another aspect to be taken into account in IT response assessment is that statistical methods need to be modified, as hazard ratios (i.e., the difference in death rates between active treatment and control changes with time) in their traditional way have no meaning [88], since there is a delayed separation of the Kaplan–Meier survival curves between control and treatment arms [85].
Conclusions
Due to its particular nature, PC often shows resistance to cytotoxic drugs, hence new therapeutic approaches that do not rely on high cell proliferation, such as IT, are now a welcome reality. Different IT agents have shown to be well tolerated and less toxic than traditional CT, as well as extending patient survival significantly in some cases.
The combination of one or more IT strategies with other standard PC treatments such as ADT, RT or CT seems to be the most effective way of inducing a lasting IR. The nature and sequencing of these combinations, their integration with current standard treatments as well as the type of patient (low tumor burden vs. heavily pre-treated mCRPC) and the best time (neoadjuvancy vs. adjuvancy) during the patient’s clinical history remain to be characterized.
Given the time required for an IR to develop, and in view of the lack of association between OS and PFS, the former is now accepted as the more robust endpoint in IT trials. IR measurement should be introduced as a secondary endpoint to identify clinical benefit in the earlier phases of treatment. In addition to the CTL response measurement, humoral responses, Treg depletion, tissue-based biomarkers and recruitment of immune cells to the tumor microenvironment are suggested as predictive biomarkers.
The value of RECIST in IT is challenged, as lymph node or tumor mass size increase as a result of the IR could be categorized as PD. If IT is to play a significant role in the future of PC, new clinically validated, standardized response criteria must be developed.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29 (Epub 2014/01/09).
Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben-Josef E, Mendelson DS, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of clinical oncology practice guideline. J Clin Oncol. 2007;25(12):1596–605 (Epub 2007/04/04).
http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65(2):467–79 (Epub 2013/12/11).
Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–48 (Epub 2012/12/12).
Danila DC, Morris MJ, de Bono JS, Ryan CJ, Denmeade SR, Smith MR, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28(9):1496–501 (Epub 2010/02/18).
Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13(10):983–92 (Epub 2012/09/22).
Logothetis CJ, Basch E, Molina A, Fizazi K, North SA, Chi KN, et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol. 2012;13(12):1210–7 (Epub 2012/11/13).
Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 2010;375(9724):1437–46 (Epub 2010/04/20).
Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–90 (Epub 2009/04/11).
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–97 (Epub 2012/08/17).
Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–23 (Epub 2013/07/19).
de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–54 (Epub 2010/10/05).
Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–20 (Epub 2004/10/08).
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–12 (Epub 2004/10/08).
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–22 (Epub 2010/09/08).
Wang X, Yu J, Sreekumar A, Varambally S, Shen R, Giacherio D, et al. Autoantibody signatures in prostate cancer. N Engl J Med. 2005;353(12):1224–35 (Epub 2005/09/24).
Bradford TJ, Wang X, Chinnaiyan AM. Cancer immunomics: using autoantibody signatures in the early detection of prostate cancer. Urol Oncol. 2006;24(3):237–42 (Epub 2006/05/09).
Delves PJ, Roitt IM. The immune system. First of two parts. N Engl J Med. 2000;343(1):37–49 (Epub 2000/07/07).
Delves PJ, Roitt IM. The immune system. Second of two parts. N Engl J Med. 2000;343(2):108–17 (Epub 2000/07/13).
Nemazee D. Receptor editing in lymphocyte development and central tolerance. Nat Rev Immunol. 2006;6(10):728–40 (Epub 2006/09/26).
Linsley PS, Nadler SG. The clinical utility of inhibiting CD28-mediated costimulation. Immunol Rev. 2009;229(1):307–21 (Epub 2009/05/12).
Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339 (Epub 2006/05/30).
Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–5 (Epub 2008/10/11).
Lan KH, Liu YC, Shih YS, Tsaid CL, Yen SH, Lan KL. A DNA vaccine against cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) prevents tumor growth. Biochem Biophys Res Commun. 2013;440(2):222–8 (Epub 2013/09/18).
Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ, Bander NH. Phase I trial of yttrium-90-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for androgen-independent prostate cancer. J Clin Oncol. 2004;22(13):2522–31 (Epub 2004/06/03).
Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol. 2005;23(21):4591–601 (Epub 2005/04/20).
Tagawa ST, Milowsky MI, Morris M, Vallabhajosula S, Christos P, Akhtar NH, et al. Phase II study of lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin Cancer Res. 2013;19(18):5182–91 (Epub 2013/05/30).
Kwek SS, Dao V, Roy R, Hou Y, Alajajian D, Simko JP, et al. Diversity of antigen-specific responses induced in vivo with CTLA-4 blockade in prostate cancer patients. J Immunol. 2012;189(7):3759–66 (Epub 2012/09/08).
US Food and Drug Administration. FDA labelling information—Provenge. FDA website [online]. 2010. http://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM210031.pdf.
Beer TMS, Higano SF, Tejwani CS, Dorff S, Stankevich TB, Lowy E. Phase I trial of ipilimumab (IPI) alone and in combination with radiotherapy (XRT) in patients with metastatic castration resistant prostate cancer (mCRPC). J Clin Oncol 2008;26 (May 20 suppl; abstr 5004).
Sheikh NA, Petrylak D, Kantoff PW, Dela Rosa C, Stewart FP, Kuan LY, et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. 2013;62(1):137–47 (Epub 2012/08/07).
Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24(19):3089–94 (Epub 2006/07/01).
Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115(16):3670–9 (Epub 2009/06/19).
Shi Y, Liu CH, Roberts AI, Das J, Xu G, Ren G, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res. 2006;16(2):126–33 (Epub 2006/02/14).
Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90(8):3539–43 (Epub 1993/04/15).
Simons JW, Sacks N. Granulocyte-macrophage colony-stimulating factor-transduced allogeneic cancer cellular immunotherapy: the GVAX vaccine for prostate cancer. Urol Oncol. 2006;24(5):419–24 (Epub 2006/09/12).
Higano C, Saad F, Somer B, Curti B, Petrylak DP, Drake CG, et al. A phase III trial of GVAX immunotherapy for prostate cancer vs. docetaxel plus prednisone in asymptomatic castration-resistant prostate cancer (CRPC). Genitourinary Cancer Symposium: Proc Am Soc Clin Oncol. 2009 abstract # LBA150.
Small ED, Gerritsen WR. A phase III trial of GVAX immunotherapy for prostate cancer in combination with docetaxel versus docetaxel plus prednisone in symptomatic, castration-resistant prostate cancer (CRPC). Orlando. In: American Society of Clinical Oncology–Genitourinary Cancers Symposium; 2009. p. 26–8.
Drake CG. Immunotherapy for prostate cancer: walk, don’t run. J Clin Oncol. 2009;27(25):4035–7 (Epub 2009/07/29).
Michael A, Ball G, Quatan N, Wushishi F, Russell N, Whelan J, et al. Delayed disease progression after allogeneic cell vaccination in hormone-resistant prostate cancer and correlation with immunologic variables. Clin Cancer Res. 2005;11(12):4469–78 (Epub 2005/06/17).
Pavlenko M, Roos AK, Lundqvist A, Palmborg A, Miller AM, Ozenci V, et al. A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer. Br J Cancer. 2004;91(4):688–94 (Epub 2004/07/29).
Mincheff M, Tchakarov S, Zoubak S, Loukinov D, Botev C, Altankova I, et al. Naked DNA and adenoviral immunizations for immunotherapy of prostate cancer: a phase I/II clinical trial. Eur Urol. 2000;38(2):208–17 (Epub 2000/07/15).
Gregor PW, Pedraza A, Orlandi F. A xenogeneic PSMA DNA vaccine for patients with non-castrate metastatic (NCMPC) and castrate metastatic prostate cancer (CMPC)—a phase I trial of proof of principle. J Clin Oncol. 2007;25:3073S.
Chudley L, McCann K, Mander A, Tjelle T, Campos-Perez J, Godeseth R, et al. DNA fusion-gene vaccination in patients with prostate cancer induces high-frequency CD8 (+) T-cell responses and increases PSA doubling time. Cancer Immunol Immunother. 2012;61(11):2161–70 (Epub 2012/06/26).
McNeel DG, Dunphy EJ, Davies JG, Frye TP, Johnson LE, Staab MJ, et al. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J Clin Oncol. 2009;27(25):4047–54 (Epub 2009/07/29).
Becker JT, Olson BM, Johnson LE, Davies JG, Dunphy EJ, McNeel DG. DNA vaccine encoding prostatic acid phosphatase (PAP) elicits long-term T-cell responses in patients with recurrent prostate cancer. J Immunother. 2010;33(6):639–47 (Epub 2010/06/17).
Gnjatic S, Altorki NK, Tang DN, Tu SM, Kundra V, Ritter G, et al. NY-ESO-1 DNA vaccine induces T-cell responses that are suppressed by regulatory T cells. Clin Cancer Res. 2009;15(6):2130–9 (Epub 2009/03/12).
Hirayama M, Nishikawa H, Nagata Y, Tsuji T, Kato T, Kageyama S, et al. Overcoming regulatory T-cell suppression by a lyophilized preparation of Streptococcus pyogenes. Eur J Immunol. 2013;43(4):989–1000 (Epub 2013/02/26).
Smith HA, Rekoske BT, McNeel DG. DNA vaccines encoding altered peptide ligands for SSX2 enhance epitope-specific CD8+ T-cell immune responses. Vaccine. 2014;32(15):1707–15 (Epub 2014/02/05).
Larocca C, Schlom J. Viral vector-based therapeutic cancer vaccines. Cancer J. 2011;17(5):359–71 (Epub 2011/09/29).
Arlen PM, Dahut WL, Gulley JL. Immunotherapy for prostate cancer: what’s the future? Hematol Oncol Clin North Am. 2006;20(4):965–83, xi. (Epub 2006/07/25).
Arlen PM, Skarupa L, Pazdur M, Seetharam M, Tsang KY, Grosenbach DW, et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol. 2007;178(4 Pt 1):1515–20 (Epub 2007/08/21).
Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(7):1099–105 (Epub 2010/01/27).
Miller G, Lahrs S, Pillarisetty VG, Shah AB, DeMatteo RP. Adenovirus infection enhances dendritic cell immunostimulatory properties and induces natural killer and T-cell-mediated tumor protection. Cancer Res. 2002;62(18):5260–6 (Epub 2002/09/18).
Lubaroff DM, Konety BR, Link B, Gerstbrein J, Madsen T, Shannon M, et al. Phase I clinical trial of an adenovirus/prostate-specific antigen vaccine for prostate cancer: safety and immunologic results. Clin Cancer Res. 2009;15(23):7375–80 (Epub 2009/11/19).
Siemens DR, Elzey BD, Lubaroff DM, Bohlken C, Jensen RJ, Swanson AK, et al. Cutting edge: restoration of the ability to generate CTL in mice immune to adenovirus by delivery of virus in a collagen-based matrix. J Immunol. 2001;166(2):731–5 (Epub 2001/01/06).
Lubaroff DM, Williams RD, Vaena D, Joudi F, Brown J, Smith M. An ongoing phase II trial of an adenovirus/PSA vaccine for prostate cancer. 103rd Annual Meeting of the American Association for Cancer research. Chicago IL: Cancer research, 2012.
Fong L, Kwek SS, O’Brien S, Kavanagh B, McNeel DG, Weinberg V, et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69(2):609–15 (Epub 2009/01/17).
Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24(7):1813–21 (Epub 2013/03/29).
Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–12 (Epub 2014/05/17).
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54 (Epub 2012/06/05).
Yee DS, Lowrance WT, Eastham JA, Maschino AC, Cronin AM, Rabbani F. Long-term follow-up of 3-month neoadjuvant hormone therapy before radical prostatectomy in a randomized trial. BJU Int. 2010;105(2):185–90 (Epub 2009/07/15).
Gravina GL, Festuccia C, Galatioto GP, Muzi P, Angelucci A, Ronchi P, et al. Surgical and biologic outcomes after neoadjuvant bicalutamide treatment in prostate cancer. Urology. 2007;70(4):728–33 (Epub 2007/11/10).
Womble PR, VanVeldhuizen PJ, Nisbet AA, Reed GA, Thrasher JB, Holzbeierlein JM. A phase II clinical trial of neoadjuvant ketoconazole and docetaxel chemotherapy before radical prostatectomy in high risk patients. J Urol. 2011;186(3):882–7 (Epub 2011/07/28).
Narita S, Tsuchiya N, Kumazawa T, Maita S, Numakura K, Obara T, et al. Short-term clinicopathological outcome of neoadjuvant chemohormonal therapy comprising complete androgen blockade, followed by treatment with docetaxel and estramustine phosphate before radical prostatectomy in Japanese patients with high-risk localized prostate cancer. World J Surg Oncol. 2012;10:1 (Epub 2012/01/05).
Ross RW, Galsky MD, Febbo P, Barry M, Richie JP, Xie W, et al. Phase 2 study of neoadjuvant docetaxel plus bevacizumab in patients with high-risk localized prostate cancer: a prostate cancer clinical trials consortium trial. Cancer. 2012;118(19):4777–84 (Epub 2012/01/28).
Mathew P, Pisters LL, Wood CG, Papadopoulos JN, Williams DL, Thall PF, et al. Neoadjuvant platelet derived growth factor receptor inhibitor therapy combined with docetaxel and androgen ablation for high risk localized prostate cancer. J Urol. 2009;181(1):81–7 (Discussion 7). (Epub 2008/11/18).
Fong L, Weinberg VK, Chan SE, Corman JM, Amling CL, Stephenson RA. Neoadjuvant sipuleucel-T in localized prostate cancer: effects of immune cells within the prostate tumor microenvironment. J Clin Oncol. 2012;30 [suppl; abstr 2564].
Vuky J, Corman JM, Porter C, Olgac S, Auerbach E, Dahl K. Phase II trial of neoadjuvant docetaxel and CG1940/CG8711 followed by radical prostatectomy in patients with high-risk clinically localized prostate cancer. Oncologist. 2013;18(6):687–8 (Epub 2013/06/07).
Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7(3):239–49 (Epub 2005/03/16).
Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci USA. 2001;98(25):14565–70 (Epub 2001/12/06).
Antonarakis ES, Kibel A, Tyler RC, McCoy C, Wang Y, Sheikh NA, et al. Randomized phase II trial evaluating the optimal sequencing of sipuleucel-T and androgen-deprivation therapy (ADT) in patients (pts) with biochemically recurrent prostate cancer (BRPC) [abstract]. J Clin Oncol 31, (suppl 6; abstr 34).
Madan RA, Gulley JL, Schlom J, Steinberg SM, Liewehr DJ, Dahut WL, et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008;14(14):4526–31 (Epub 2008/07/17).
Granberg C, Thompson RH, Quevedo JF. Down-staging of locally-advanced prostate cancer with anti-CTLA-4 monoclonal antibody prior to radical prostatectomy. J Clin Oncol. 2009;27:16103 (abstr).
Ma Y, Kepp O, Ghiringhelli F, Apetoh L, Aymeric L, Locher C, et al. Chemotherapy and radiotherapy: cryptic anticancer vaccines. Semin Immunol. 2010;22(3):113–24 (Epub 2010/04/21).
Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105(4):256–65 (Epub 2013/01/08).
Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11(9):3353–62 (Epub 2005/05/04).
Gerritsen WR, Kwon ED, Fizazi K, Bossi A, Van den Eertwegh A, Logothetis C. CA184-043: A randomized, multicenter, double-blind phase 3 trial comparing overall survival (OS) in patients (pts) with post-docetaxel castration-resistant prostate cancer (CRPC) and bone metastases treated with ipilimumab (ipi) vs. placebo (pbo), each following single-dose radiotherapy (RT) [abstract]. European Cancer Congress, abstr 2850.
van den Eertwegh AJ, Versluis J, van den Berg HP, Santegoets SJ, van Moorselaar RJ, van der Sluis TM, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(5):509–17 (Epub 2012/02/14).
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47 (Epub 2008/12/23).
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group. J Clin Oncol. 2008;26(7):1148–59 (Epub 2008/03/04).
Small EJ, Fratesi P, Reese DM, Strang G, Laus R, Peshwa MV, et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18(23):3894–903 (Epub 2000/12/01).
Gulley JL, Madan RA, Heery CR. Therapeutic vaccines and immunotherapy in castration-resistant prostate cancer: current progress and clinical applications. Am Soc Clin Oncol Educ Book. 2013. (Epub 2013/05/30).
Bilusic M, Gulley JL. Endpoints, patient selection, and biomarkers in the design of clinical trials for cancer vaccines. Cancer Immunol Immunother. 2012;61(1):109–17 (Epub 2011/11/29).
Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–20 (Epub 2009/11/26).
Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19(14):3936–43 (Epub 2013/06/08).
Hoos A. Evolution of end points for cancer immunotherapy trials. Ann Oncol. 2012;23(Suppl 8):viii47–52. doi:10.1093/annonc/mds263.
Conflict of interest
EM Fernández-García, FE Vera-Badillo, B Perez-Valderrama, A Soto Matos-Pita and I Duran have no conflicts of interest related to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernández-García, E.M., Vera-Badillo, F.E., Perez-Valderrama, B. et al. Immunotherapy in prostate cancer: review of the current evidence. Clin Transl Oncol 17, 339–357 (2015). https://doi.org/10.1007/s12094-014-1259-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-014-1259-6