Abstract
Immunotherapy is rapidly transforming cancer care across a range of tumor types. Although Sipuleucel-T represented the first successful vaccine for the treatment of established cancer, other immunotherapeutic approaches for prostate cancer such as checkpoint inhibitors have been relatively disappointing to date. However, significant promise is on the horizon as there is a wide array trials evaluating immunotherapy in prostate cancer patients. These include both immune checkpoint inhibitors and antigen-specific approaches including vaccines, antibody-drug conjugates, and antitumor antibodies. Furthermore, a better understanding of the key mechanisms that promote the immunosuppressive microenvironment of prostate cancer is emerging. These insights may eventually make it possible to determine which patients will benefit from immunotherapy. This review will discuss the successes and failures of immunotherapy in prostate cancer. We will also present key lessons learned from completed trials and highlight important ongoing studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the most commonly diagnosed and the third deadliest cancer in men, with over 160,000 diagnoses and 26,000 deaths in 2017 [1]. Patients with localized disease are typically treated surgically or with radiation therapy (RT) [2]. However, 20–40% of patients undergoing a radical prostatectomy and 30–50% of patients receiving RT will have recurrence of disease [3]. Standard therapy for metastatic disease generally involves androgen ablation, either by bilateral orchiectomy or by chemical castration (LHR agonists/antagonists) [4]. Although androgen ablation is highly effective, it is associated with significant side effects and patients eventually develop castration-resistant prostate cancer (CRPC) [5]. Metastatic CRPC (mCRPC) currently has no curative treatment option and is associated with a poor prognosis. This challenging outlook for mCRPC patients has driven efforts to develop more effective therapy.
In recent years, the development of immune checkpoint blockade, tumor-targeted antibodies, and cancer vaccines has had a major impact on the treatment of solid tumors, sparking enthusiasm that some of these may be effective in prostate cancer. In 2010, Sipuleucel-T, a personalized cancer vaccine that targets prostatic acid phosphatase (PAP), became the first FDA-approved immunotherapy for mCRPC [6]. The success of Sipuleucel-T spurred a multitude of clinical trials of various vaccines targeting other prostate-associated antigens such as prostate-specific membrane antigen (PSMA) and prostate-specific antigen (PSA). The major immunotherapy approach currently in the clinic involves immune checkpoint inhibitors which have been approved for the treatment of a range of solid tumor malignancies [7]. Disappointingly, immune checkpoint inhibitors have not been as successful in patients with mCRPC (Table 1) [8, 9, 10••]. The only approved checkpoint inhibitor for treatment of mCRPC is pembrolizumab, which can be used in patients with tumors demonstrating microsatellite instability (MSI) [10••, 11]. Unfortunately, such patients are rare, comprising less than 5% of the total population of men with CPRC. A number of promising approaches utilizing novel vaccines, antibody-drug conjugates, bi-specific antibodies, and adoptive cellular therapy are currently in development to overcome the immunosuppressive prostate tumor microenvironment and improve outcomes for prostate cancer patients (Table 2). This review will explore the successes and failures of immunotherapy trials and highlight promising areas that could lead to more effective treatments for advanced prostate cancer.
Immune Checkpoint Blockade
One of the most exciting innovations in immunotherapy in the last several years has been the development of immune checkpoint inhibitors, which target inhibitory ligands on immune cells, promoting antitumor immunity (Fig. 1). Anti-cytotoxic T lymphocyte-associated protein 4 (CTLA-4) (ipilimumab) was the first successful immune checkpoint inhibitor, receiving FDA approval for the treatment of metastatic melanoma in 2011 [8]. Based on the success of ipilimumab in other diseases, there was initial excitement for this therapy in prostate cancer. A phase 1/2 trial evaluated ipilimumab as a monotherapy or in combination with RT [12]. A few patients at all dose levels of both arms showed antitumor activity in the form of PSA declines. There were 8 patients with PSA declines > 50% in the highest dose level of the combination arm (10 mg/kg) as well as a rare but exciting complete response (CR) that lasted over 11 months [12]. A phase 1 dose escalation trial, evaluating ipilimumab in combination with the granulocyte-macrophage colony-stimulating factor-transduced cell-based allogeneic prostate cancer vaccine (GVAX), also showed encouraging antitumor activity, with PSA declines > 50% in 25% of patients and was well tolerated [13]. This early clinical evidence of antitumor activity prompted further exploration of ipilimumab in phase 3 trials. In the first phase 3 trial, patients who had progressed after docetaxel chemotherapy were randomized to ipilimumab or placebo after receiving palliative radiation therapy to at least one metastatic site. Although progression-free survival was significantly longer in the ipilimumab group, the study showed narrowly missed its primary endpoint of overall survival (HR 0.85, P = .053) [14••]. An exploratory subgroup analyses showed that ipilimumab may be most active in patients with favorable prognostic features—specifically patients with normal alkaline phosphatase, normal hemoglobin, and no visceral metastases. These patients showed an increased overall survival with ipilimumab [14••]. In a second trial, ipilimumab monotherapy was compared to placebo in chemotherapy-naïve mCRPC patients. Disappointingly, that trial also failed to meet its OS endpoint. Interestingly, patients on the treatment arm experienced longer median progression-free survival compared to those on the placebo arm (5.6 months to 3.8 months) and were more likely to experience a PSA decline [15•].
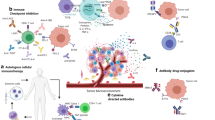
Immunotherapy approaches in prostate cancer. (a) Sipuleucel-T involves leukapheresis of immune cells followed by incubation with specific fusion protein (PA2024), which consists of prostatic acid phosphatase (PAP) coupled with granulocyte-macrophage colony-stimulating factor (GM-CSF). Cells are then re-infused allowing for APC maturation and activation of CD4+ and CD8+ T cells to recognize and kill PAP presenting tumor cells. (b) Bi-specific antibodies are engineered antibodies that contain two binding sites, one for CD3 receptors found on T cells and another for an antigen found on tumor cells. Several different constructs including bi-specific T cell engagers (BiTEs) and dual-affinity retargeting antibodies (DARTs) are in clinical development. (c) J591 is a humanized monoclonal antibody specific for prostate-specific membrane antigen (PSMA). The antibody can be labeled with lutetium-177, an isotope ideal for radiation therapy. The radioactive antibody targets PSMA presenting tumor cells and kills them upon radiation. (d) Chimeric antigen T cell receptors (CAR T) are receptors engineered to target antigens via an antibody-derived single-chain variable fragment, allowing the T cell to function independent of the major histocompatibility complex. Pictured is a second-generation CAR, which contains a costimulatory domain and CD3ζ signaling domain. (e) Checkpoint inhibitors are monoclonal antibodies which target immune checkpoints including programmed cell death 1 (PD-1) and cytotoxic T lymphocyte antigen 4 (CTLA-4) and prevent binding to PD-L1 and CD80 or CD86, respectively, which are expressed on tumor and other immune cells. This leads to an enhanced antitumor T cell response
A few small patient cohorts evaluated PD-1 blockade in men with metastatic prostate cancer. In the phase 1b trial of nivolumab, there were no objective responses seen in the 17 CRPC patients enrolled [9]. The two prostate tumor samples that were collected both tested negative for PD-L1 expression. Other studies also observed the paucity of PD-L1 expression on prostate tumors, highlighting another challenge in treating the disease [16]. By contrast, preliminary results of the mCRPC cohort of the KEYNOTE-028 study of pembrolizumab showed that 13% of patients with measurable disease had a partial response and 39% had stable disease [17]. In contrast to the phase I nivolumab trial, this study only included patients with PD-L1 expression in ≥ 1% of tumor or stroma cells. As is the case for many tumor types, it remains unclear if PD-L1 expression can predict response to checkpoint blockade in prostate cancer.
Whether particular subsets of prostate cancer patients might respond to immunotherapy remains unclear. In general, prostate tumors express relatively low levels of PD-L1 [16]. Interestingly, resistance to enzalutamide, an antiandrogen commonly used in the treatment of mCRPC, is associated with increased PD-L1 expression [18]. In a phase 2 trial in which the anti-PD-1 antibody pembrolizumab was added to enzalutamide in patients progressing on the enzalutamide, three of the first 10 patients treated experienced a rapid PSA drop to ≤ 0.2 ng/mL. Two of the patients who had a biochemical response had measurable disease upon study entry, and both of them experienced a partial response [19•]. One of the partial responders tested positive for microsatellite instability (MSI), which corroborates the results of earlier studies of MSI as a predictive biomarker of response to PD-1 blockade [20]. As mentioned above, although only 1 patient in the MSI-H basket trial of the anti-PD-1 agent pembrolizumab had prostate cancer, this drug is now approved across tumor types for patients with MSI-H tumors.
There are several prostate cancer trials underway using anti-PD-1 antibodies in combination with other agents (Table 2). The combination of CTLA-4 and PD-1 inhibition has demonstrated synergistic effects in preclinical prostate cancer models as well as impressive results in melanoma patients [21]. The first application of this combination treatment in prostate cancer was studied in patients with ARV7+ mCRPC. Early results showed that the combination therapy was well tolerated with 1 of 6 patients treated experiencing a PSA decline > 50% [22] (NCT02601014), and with patients with DNA repair deficient tumors being enriched among those responding to therapy. Administration of radiation has been shown to induce a pro-inflammatory microenvironment in prostate tumors, increasing the likelihood of a response to checkpoint blockade [23]. Although RT has been known to enhance antitumor immunity, it should be noted that recent mouse models have demonstrated that RT also increased tumor-infiltrating Tregs, which can have an immunosuppressive affect [24]. A pilot study treating patients with oligo-metastatic prostate cancer with cryosurgery and pembrolizumab has completed enrollment but results have not been reported (NCT02489357).
Antitumor Antibodies
Another emerging immunotherapy approach utilizes engineered antibodies to target specific antigens that are highly expressed on tumor cells, thereby inducing antibody-dependent cellular cytotoxicity (ADCC) [25]. The best-studied antitumor antibody in prostate cancer is J591, a monoclonal antibody that targets prostate-specific membrane antigen (PSMA). PSMA is commonly expressed in malignant prostate epithelial cells and vascular endothelial cells, making it a reasonable target [26]. J591 has been conjugated with various radioisotopes to facilitate directed killing of prostate tumor cells (Fig. 1) [25]. Early trials of J591 demonstrated good trafficking of the antibody to prostate cancer metastases in bone and soft tissue [27]. A phase 2 trial of J591 in combination with low-dose interleukin-2 (IL-2) was well tolerated. While 9 of 16 patients had a stable PSA (− 50% < change in PSA < 25%), no patients had a PSA decline of > 50% [28]. A phase 2 trial of J591 radiolabeled with lutetium-177 (177Lu-J591) showed more encouraging results, with 59.6% of patients experiencing a PSA decline after treatment. One of 12 patients with measurable disease had a partial response and 8 had stable disease [29].
Another strategy for antibody-directed T cell killing is the use of bi-specific antibodies in which the two arms of an IgG molecule are specific for different targets [30]. Many of these agents target the CD3 molecule expressed on T cells in conjunction with a second tumor-associated target in an effort to direct trafficking of T cells to tumors. A variety of different constructs are in clinical development including bi-specific T cell engagers (BiTEs) and dual-affinity retargeting antibodies (DARTs). BiTEs utilize two single-chain variable fragments (scFv), one specific for a tumor-associated antigen and the other specific for T cells (CD3), which enable its bi-specificity (Fig. 1) [30]. Studies have also shown that BiTEs can induce cytotoxic T cell activation without the costimulation of other pathways [31]. Blinatumumab, which targets CD3 and CD20, is the most extensively studied BiTE and has demonstrated a longer overall survival than standard of care chemotherapy in patients with relapsed of refractory B cell precursor acute lymphoblastic leukemia [32]. The only BiTE to be tested in prostate cancer is BAY2010112, which is specific for the CD3 receptor on T cells and PSMA. Promising preclinical studies of BAY2010112 in prostate cancer mouse models were characterized by rapid reductions in tumor size and complete remissions [33]. Daily administration of BAY2010112 was evaluated in a phase 1 trial, although results have yet to be made available (NCT01723475). Similar to BiTEs, dual-affinity retargeting molecules (DARTs) use bi-specificity to traffic T cells to tumor cells and facilitate immune-mediated tumor cell lysis. Like BITEs, DARTs have two antigen-binding sites, one that binds the CD3 complex commonly expressed on T cells and another that binds a target antigen. For prostate cancer, a DART targeting B7-H3 (CD276) is in development [34]. Zang et al. analyzed the expression of B7-H3 in over 800 prostate cancer patients who had undergone a radical prostatectomy over the course of 7 years. They found B7-H3 had aberrant expression in 93% of tumors, with a strong intensity found in 26%. Strong intensity was associated with several poor prognostic factors including greater likelihood of disease spread at time of surgery, higher chance of recurrence after surgery, and higher chance of cancer related death compared to none and moderate B7-H3 intensity [35]. Currently, a phase 1 trial for the drug MGD009, a B7-H3/CD3 DART, is enrolling for various cancer types including prostate cancer (NCT02628535) [36].
Another exciting tumor targeting approach capitalizes on the affinity and specificity of antibodies to traffic cytotoxic chemotherapy agents to tumors. These antibody-drug conjugates (ADCs) may potentially avoid the harmful side effects that conventional chemotherapy agents have on healthy tissue [37]. The FDA approval of brentuximab, an ADC that delivers monomethyl auristatin E (MMAE) to CD-30 expressing cells in patients with Hodgkin’s lymphoma (HL), illustrates the clinical efficacy of these agents. In solid tumors, trastuzumab emtansine (TDM-1), an ADC that combines the targeting ability of the monoclonal antibody trastuzumab with the highly cytotoxic agent emtansine, was approved for treatment of HER2-positive metastatic breast cancer [38]. The relative specificity of ADC depends on the identification of tumor-specific targets that are not commonly expressed on other tissues. Prostate tumors are a good target for ADC because of the expression of PSMA and several other relatively prostate-specific targets. A phase 1 trial in taxane-refractory mCRPC patients of PSMA ADC (Progenics Pharmaceuticals), a fully human IgG1 antibody conjugated to the microtubule-disrupting agent MMAE which binds to PSMA-positive cells, showed antitumor activity, measured by decrease in PSA or circulating tumor cells (CTCs), in about 50% of patients treated with ≥ 1.8 mg/kg PSMA ADC [39]. The phase 2 trial demonstrated antitumor activity, PSA declines of ≥ 30 and ≥ 50% in 30 and 14% of patients respectively. For those with measurable disease, 61% had stable disease, 13% had partial responses, and 26% had progressive disease [40].
Cancer Vaccines
Cancer vaccines that prime the immune system to recognize tumor-associated antigens and elicit a T cell response have demonstrated some success in prostate cancer. Vaccines are generally comprised of an adjuvant that functions to activate APCs like dendritic cells (DC) and a target protein or peptide known to be relatively associated with the cancer [41]. After subcutaneous or intradermal injection, antigen loaded DC traffic to the draining lymph nodes where they present small peptide fragments of the target antigen to prime T cell recognition. After recognition of the MHC molecule/peptide complex, CD4+ T cells activate, which along with other costimulatory molecules leads to the maturation of CD8+ T cells. These mature CD8+ T cells can proliferate and lyse tumor cells presenting their target antigen. As above, prostate cancer is an appealing target for immunotherapy with vaccines because of its expression of specific tumor-associated antigens like PSA, PSMA, and prostatic acid phosphatase (PAP) [42].
Currently, the only therapeutic cancer vaccine approved by the FDA is Sipuleucel-T (Provenge™), which is used to treat mCRPC. This personalized immunotherapy product is generated when a patient’s immune cells are extracted and incubated with recombinant fusion protein PA2024, which links PAP to GM-CSF, thus activating PAP-specific T cells (Fig. 1). Activated antigen-presenting cells are then re-infused into the patient to elicit an antitumor immune response. Early clinical trials of Sipuleucel-T showed that treatment stimulated T cell proliferative responses and induced anti-PAP antibodies in approximately 50% of patients [43]. Three randomized phase 3 trials were conducted to test for an OS benefit in advanced mCRPC patients. The pivotal Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial demonstrated an increased overall survival of 4.1 months in Sipuleucel-T patients compared to placebo and led to FDA approval for the vaccine [6]. It should be noted that Sipuleucel-T treatment does not affect PSA, with only 2.6% of over 300 patients in the treatment arm experiencing a PSA decline ≥ 50% [6].
Emerging data indicate that therapeutic vaccines may be most effective in early-stage patients with less aggressive disease [44]. A phase 2 trial evaluated the effects of the sequence of therapy on patients with biochemically recurrent prostate cancer (BRPC) treated with Sipuleucel-T and androgen deprivation therapy (ADT). Approximately 60 patients with rapidly rising PSA levels were randomized either to receive Sipuleucel-T before ADT or to receive Sipuleucel-T after ADT. PAP024-specific T cell proliferation responses averaged across all timepoints were 2-fold higher in the arm that received the vaccine first [45••]. Overall, the treatment arm that received the vaccine first experienced a greater antitumor immune response. These results should be considered when evaluating the design of future clinical trials and indicate that the sequence of immunotherapy treatments can effect clinical activity.
Despite the effectiveness of Sipuleucel-T, other prostate cancer vaccines tested in phase 3 trials have been less promising (Table 1). Prostvac-VF, which utilizes a heterologous prime-boost strategy with vaccine virus (rV-PSA) and fowlpox virus (rF-PSA), was recently studied in a large phase 3 clinical trial. These vaccine vectors include the transgenes for TRICOM, which consists of the costimulatory molecules ICAM-1, B7.1, and LFA-3, and PSA. One of the challenges with poxvirus-based vaccines is their propensity to elicit strong antibody responses; if given repeatedly, the antibody response to viral proteins dampens the response to the encoded target antigen. The use of a heterologous fowlpox viral vector circumvents this challenge, allowing for repeated administration with increased T cell immunity. This heterologous prime-boost strategy was tested in a phase 2 trial of mCRPC patients; post hoc retrospective analyses showed an 8.5-month increase in OS with a 44% reduction in death rate [6, 46]. Recent results of the 1200-patient randomized phase III trial of Prostvac-VF in combination with GM-CSF (NCT01322490) showed that treatment did not improve overall survival. Those data led to the early discontinuation of the trial. Prostvac-VF is also being tested in other combinations involving either enzalutamide, ipilimumab, nivolumab, or docetaxel (NCT01867333, NCT02933255, NCT02506114, NCT02649855).
Another vaccine construct that has been tested in phase 3 trials is GVAX, a vaccine composed of whole tumor cells that have been genetically modified to secrete GM-CSF. The tumor cells provide the antigens for the vaccine; GVAX uses two prostate cancer cell lines, LN-CaP and PC-3. The hormone sensitive LN-CaP and hormone refractory PC-3 cell lines are derived from lymph node and bone metastases respectively, which may provide an array of prostate cancer-associated antigens [47]. Two phase 2 trials demonstrated PSA responses and patients on higher dose levels exhibited development of vaccine antibodies. These promising results led to the launch of two phase 3 trials, VITAL-1 and VITAL-2. VITAL-2 compared GVAX plus docetaxel to docetaxel plus prednisone, but was terminated after data showed a disproportionate number of deaths in the GVAX arm compared to the standard treatment arm. The other trial, VITAL-1, was also terminated early after an early futility analysis revealed a low probability that the trial would meet its endpoint of improved survival [48].
Another vaccine strategy utilizes attenuated vaccines derived from Listeria monocytogenes (Lm). Lm is a gram-positive bacterium that can cause listeriosis. Lm enters antigen-presenting cells via phagocytosis, which induces a strong innate immune response [49]. The ideal vaccine would be able to harness the immunogenicity of listeria while preventing its pathogenic features from harming the recipient. Currently, there are two attenuated listeria vaccines in development for prostate cancer. One of these is ADXS031-142, an attenuated Lm vaccine that is genetically modified to knockout listeriolysin LLO with PSA as the target antigen. Listeriolysin LLO knockout decreases toxicity since listeriolysin LLO enables the bacteria to colonize the cytosol of the host cell and spread rapidly to other cells [50]. Murine studies showed good responses, as well as a reduction in the number of tumor-infiltrating Treg cells [51]. Murine studies also tested ADX031-142 in combination with RT compared to either treatment on their own. Those results showed additive effect of the vaccine and RT [52]. Currently, there is a trial ongoing that tests ADXS31-142 administered as a monotherapy and in combination with the PD-1 inhibitor pembrolizumab in mCRPC patients (NCT02325557).
A second listeria-based vaccine approach involves a live attenuated double-deleted (LADD) strain of the vaccine that is transduced with multiple prostate-associated antigens. Multiple preclinical studies led to the identification of several virulence genes. A mutant strain of Lm with the virulence factor actA deleted led to a 1000-fold attenuation compared to wild-type listeria, limiting the cell-to-cell spread of the bacteria while also maintaining its ability to induce effector and memory T cell responses [53]. The deletion of inlB was shown to interfere with listeria’s ability to infect nonphagocytic cells such as hepatocytes, resulting in a reduced toxicity when compared to the wild-type strain. The combination of these two deletions generated safe vaccine strain, as demonstrated by studies in pancreatic and lung cancer [54]. Additionally, the magnitude of the attenuation of this strain allows for a higher dose level, potentially increasing the likelihood of T cell recognition of the tumor-associated antigens [53, 55]. There is currently an ongoing phase 1 study testing this approach, utilizing a LADD vaccine strain transduced with multiple prostate-associated antigens (NCT02625857).
Adoptive Cellular Therapy
In recent years, adoptive cellular therapy, an approach in which patient’s T cells are removed and genetically engineered before reinfusion, has gained momentum. Several variations of ACT have been developed including tumor-infiltrating lymphocytes (TILs), engineered T cell receptors (TCRs), and chimeric antigen receptors (CARs) [56]. The most promising engineered T cells are CARs, which are engineered to target antigens via an antibody-derived single-chain variable fragment, allowing the T cell to function independent of the major histocompatibility complex [57,58,59]. Since the initial design, several “generations” of CAR T cells have been developed with significant modifications to the intracellular signaling domains. The first-generation CAR T cells included only a CD3ζ signaling domain which limited in vivo expansion and T cell proliferation leading to limited clinical activity [60]. Second- and third-generation CAR T cells include additional costimulatory domains that enhance CAR T cell expansion (Fig. 1) [57].
A phase IIa trial evaluated the efficacy of tisagenlecleucel, a CAR T cell that targets CD-19 in B cell cancers including patients with diffuse large B cell lymphoma (DLBCL) and follicular lymphoma. The ORR of the 28 patients treated was 64%, with complete remissions occurring in 43 and 71% of DLBCL patients and follicular lymphoma patients respectively [61]. Furthermore, these responses were durable, with over 57% of all patients treated maintaining progression-free survival at the median follow-up of 28.6 months. These impressive responses led to the approval of the CD19 CAR T cell for the treatment of children and young adults with acute lymphoblastic leukemia in 2017 (ALL). Axicabtagene ciloleucel, a second anti-CD-19 CAR T cell, was also recently approved for patients with large B cell lymphomas based on similarly impressive results of the ZUMA-1 trial, with ORR and CR response rates of 82 and 54% respectively in the 101 treated patients [62].
Given the success of CD19-targeted CAR T cells for hematologic malignancies, there is considerable interest in expanding this approach to solid tumors, including prostate cancer. One significant challenge of CAR T cell therapy is identifying a specific tumor-associated antigen. CAR T cells are extremely specific and can eradicate cells with even low target expression. Therefore, even minimal antigen expression in normal tissues can lead to significant toxicity. However, as previously discussed, prostate cancer has several relatively unique TAAs. There is currently a phase 1 trial of a PSMA-targeted CAR T in mCRPC patients ongoing (NCT01140373). Another CAR T cell that targets prostate stem cell antigen (PSCA) is in development and is expected to begin clinical trials in early 2018.
Conclusion
Despite significant advances in therapy over the last several decades, prostate cancer remains a disease for which there is no curative treatment option once metastatic. Although the recent developments of immune-based therapies have revolutionized cancer care, trials involving novel immunotherapeutic agents have delivered mixed results for prostate cancer. Checkpoint inhibitors have not been particularly successful in mCRPC to date. This is perhaps unsurprising given the immunosuppressive tumor microenvironment, low mutational burden, and low expression of PD-L1 commonly seen in prostate tumors. Combination therapies to improve upon this, either with multiple immunotherapies or with immunotherapy and chemotherapy/RT, are currently being evaluated. The optimal timing of immunotherapy in prostate cancer also remains unclear. On a more positive note, there are several TAAs commonly expressed in mCRPC, which could serve as potential targets for vaccines, antitumor antibodies, and ADCs. This notion has successfully been exploited by Sipuleucel-T, a cancer vaccine that targets PAP. Although much work remains to be done, the promise of prostate cancer immunotherapy remains.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major Importance
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
Walsh PC, DeWeese TL, Eisenberger MA. Clinical practice. Localized prostate cancer. N Engl J Med. 2007;357:2696–705.
Paller CJ, Antonarakis ES. Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clin Adv Hematol Oncol. 2013;11:14–23.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12.
Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20.
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22.
LaFleur MW, Muroyama Y, Drake CG, Sharpe AH. Inhibitors of the PD-1 pathway in tumor therapy. J Immunol. 2018;200:375–83.
Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res. 2011;17:6958–62.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54.
•• Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13. Pembrolizumab was found to be active in MSI-H tumors. This led to the approval of pembrolizumab for treatment of all MSI-H tumors, including prostate tumors. Although it is rare, < 5% of mCRPC patients, it marks the first approved checkpoint inhibitor treatment in prostate cancer.
Pritchard CC, Morrissey C, Kumar A, Zhang X, Smith C, Coleman I, et al. Complex MSH2 and MSH6 mutations in hypermutated microsatellite unstable advanced prostate cancer. Nat Commun. 2014;5:4988.
Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24:1813–21.
van den Eertwegh AJ, Versluis J, van den Berg HP, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:509–17.
•• Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh A, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–12. This is a phase 3 trial that evaluated ipilimumab monotherapy in patients with mCRPC who progressed after chemotherapy. There was no difference in overall survival with ipilimumab. However, subgroup analysis suggested patients with normal alkaline phospatase, normal hemoglobin, and no visceral metastases may be more responsive to ipilimumab.
• Beer TM, Kwon ED, Drake CG, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35:40–7. This is a phase 3 trial that evaluated ipilimumab monotherapy in patients with chemotherapy-naïve mCRPC. There was no significant difference in overall survival with ipilimumab.
Martin AM, Nirschl TR, Nirschl CJ, Francica BJ, Kochel CM, van Bokhoven A, et al. Paucity of PD-L1 expression in prostate cancer: innate and adaptive immune resistance. Prostate Cancer Prostatic Dis. 2015;18:325–32.
Hansen A, Massard C, Ott PA, Haas N, Lopez J, Ejadi S, et al. Pembrolizumab for patients with advanced prostate adenocarcinoma: preliminary results from the KEYNOTE-028 study. Ann Oncol. 2016;27:725PD-PD.
Bishop JL, Sio A, Angeles A, Roberts ME, Azad AA, Chi KN, et al. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget. 2015;6:234–42.
• Graff JN, Alumkal JJ, Drake CG, Thomas GV, Redmond WL, Farhad M, et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. 2016;7:52810–7. Patients with mCRPC who progressed on enzalutamide were treated with pembrolizumab and surprisingly showed antitumor activity. PD-1 immune checkpoint inhibitors have had little success in mCRPC patients, so these results were unexpected and warrant further investigation.
Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20.
Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33.
Boudadi K, Suzman DL, Luber B, et al. Phase 2 biomarker-driven study of ipilimumab plus nivolumab (Ipi/Nivo) for ARV7-positive metastatic castrate-resistant prostate cancer (mCRPC). J Clin Oncol. 2017;35:5035.
Dallos MC, Drake CG. Blocking PD-1/PD-L1 in genitourinary malignancies: to immunity and beyond. Cancer J. 2018;24:20–30.
Muroyama Y, Nirschl TR, Kochel CM, Lopez-Bujanda Z, Theodros D, Mao W, et al. Stereotactic radiotherapy increases functionally suppressive regulatory T cells in the tumor microenvironment. Cancer Immunol Res. 2017;5:992–1004.
Jeske SJ, Milowsky MI, Smith CR, Smith KA, Bander NH, Nanus DM. Phase II trial of the anti-prostate specific membrane antigen (PSMA) monoclonal antibody (mAb) J591 plus low-dose interleukin-2 (IL-2) in patients (pts) with recurrent prostate cancer (PC). J Clin Oncol. 2007;25:15558.
Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59:3192–8.
Nanus DM, Milowsky MI, Kostakoglu L, et al. Clinical use of monoclonal antibody HuJ591 therapy: targeting prostate specific membrane antigen. J Urol. 2003;170:S84–8. discussion S8–9
Bander NH, Nanus DM, Milowsky MI, et al. Phase II trial of 177Lutetium radiolabeled anti-prostate-specific membrane antigen (PSMA) monoclonal antibody J591 (177Lu- J591) in patients (pts) with metastatic androgen-independent prostate cancer (AIPC). J Clin Oncol. 2007;25:15523.
Tagawa ST, Milowsky MI, Morris M, Vallabhajosula S, Christos P, Akhtar NH, et al. Phase II study of Lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin Cancer Res. 2013;19:5182–91.
Huehls AM, Coupet TA, Sentman CL. Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell Biol. 2015;93:290–6.
Torisu-Itakura H, Schoellhammer HF, Sim MS, Irie RF, Hausmann S, Raum T, et al. Redirected lysis of human melanoma cells by a MCSP/CD3-bispecific BiTE antibody that engages patient-derived T cells. J Immunother. 2011;34:597–605.
Kantarjian H, Stein A, Gokbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376:836–47.
Friedrich M, Raum T, Lutterbuese R, Voelkel M, Deegen P, Rau D, et al. Regression of human prostate cancer xenografts in mice by AMG 212/BAY2010112, a novel PSMA/CD3-Bispecific BiTE antibody cross-reactive with non-human primate antigens. Mol Cancer Ther. 2012;11:2664–73.
Moore PA, Zhang W, Rainey GJ, Burke S, Li H, Huang L, et al. Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood. 2011;117:4542–51.
Zang X, Thompson RH, Al-Ahmadie HA, et al. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci U S A. 2007;104:19458–63.
Tolcher AW, Alley EW, Chichili G, et al. Phase 1, first-in-human, open label, dose escalation ctudy of MGD009, a humanized B7-H3 x CD3 dual-affinity re-targeting (DART) protein in patients with B7-H3-expressing neoplasms or B7-H3 expressing tumor vasculature. J Clin Oncol. 2016;34:TPS3105-TPS.
Peters C, Brown S. Antibody-drug conjugates as novel anti-cancer chemotherapeutics. Biosci Rep. 2015;35:e00225.
Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91.
Petrylak DP, Kantoff PW, Mega AE, Vogelzang NJ, Stephenson J, Fleming MT, et al. Prostate-specific membrane antigen antibody drug conjugate (PSMA ADC): a phase I trial in metastatic castration-resistant prostate cancer (mCRPC) previously treated with a taxane. J Clin Oncol. 2013;31:119.
Petrylak DP, Vogelzang NJ, Chatta GS, Fleming MT, Smith DC, Appleman LJ, et al. A phase 2 study of prostate specific membrane antigen antibody drug conjugate (PSMA ADC) in patients (pts) with progressive metastatic castration-resistant prostate cancer (mCRPC) following abiraterone and/or enzalutamide (abi/enz). J Clin Oncol. 2015;33:144.
Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol. 2014;11:24–37.
Westdorp H, Skold AE, Snijer BA, et al. Immunotherapy for prostate cancer: lessons from responses to tumor-associated antigens. Front Immunol. 2014;5:191.
Small EJ, Fratesi P, Reese DM, Strang G, Laus R, Peshwa MV, et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18:3894–903.
Gulley JL, Madan RA, Schlom J. Impact of tumour volume on the potential efficacy of therapeutic vaccines. Curr Oncol. 2011;18:e150–7.
•• Antonarakis ES, Kibel AS, Yu EY, Karsh LI, Elfiky A, Shore ND, et al. Sequencing of Sipuleucel-T and androgen deprivation therapy in men with hormone-sensitive biochemically recurrent prostate cancer: a phase II randomized trial. Clin Cancer Res. 2017;23:2451–9. This phase 2 trial evaluated sequencing of Sipuleucel-T and ADT in patients with biochemically recurrent prostate cancer. The study showed that Sipuleucel-T treatment before ADT appears to induce a greater immune response than the reverse. These results indicate that further research into the sequencing of combination treatments is warranted.
Kantoff PW, Gulley JL, Pico-Navarro C. Revised overall survival analysis of a phase II, randomized, double-blind, controlled study of PROSTVAC in men with metastatic castration-resistant prostate cancer. J Clin Oncol. 2017;35:124–5.
Simons JW, Sacks N. Granulocyte-macrophage colony-stimulating factor-transduced allogeneic cancer cellular immunotherapy: the GVAX vaccine for prostate cancer. Urol Oncol. 2006;24:419–24.
Sonpavde G, Slawin KM, Spencer DM, Levitt JM. Emerging vaccine therapy approaches for prostate cancer. Rev Urol. 2010;12:25–34.
Paterson Y, Maciag PC. Listeria-based vaccines for cancer treatment. Curr Opin Mol Ther. 2005;7:454–60.
Gedde MM, Higgins DE, Tilney LG, Portnoy DA. Role of listeriolysin O in cell-to-cell spread of Listeria monocytogenes. Infect Immun. 2000;68:999–1003.
Shahabi V, Reyes-Reyes M, Wallecha A, Rivera S, Paterson Y, Maciag P. Development of a Listeria monocytogenes based vaccine against prostate cancer. Cancer Immunol Immunother. 2008;57:1301–13.
Hannan R, Zhang H, Wallecha A, Singh R, Liu L, Cohen P, et al. Combined immunotherapy with Listeria monocytogenes-based PSA vaccine and radiation therapy leads to a therapeutic response in a murine model of prostate cancer. Cancer Immunol Immunother. 2012;61:2227–38.
Brockstedt DG, Giedlin MA, Leong ML, Bahjat KS, Gao Y, Luckett W, et al. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc Natl Acad Sci U S A. 2004;101:13832–7.
Le DT, Wang-Gillam A, Picozzi V, et al. Safety and survival with GVAX pancreas prime and Listeria monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33:1325–33.
Le DT, Dubenksy TW Jr, Brockstedt DG. Clinical development of Listeria monocytogenes-based immunotherapies. Semin Oncol. 2012;39:311–22.
Perica K, Varela JC, Oelke M, Schneck J. Adoptive T cell immunotherapy for cancer. Rambam Maimonides Med J. 2015;6:e0004.
Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood. 2014;123:2625–35.
Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989;86:10024–8.
Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64:891–901.
Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–23.
Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–54.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Michael C. Comiskey declares that he has no conflict of interest.
Matthew C. Dallos declares that he has no conflict of interest.
Charles G. Drake has received research support through grants from Janssen, Bristol-Myers Squibb, and Aduro Biotech; has compensation from Agenus, Dendreon, ImmunExcite, Janssen, Lilly, Merck, Pierre Fabre, and Roche/Genentech for service as a consultant; is a stockholder of Compugen, Potenza, Tizona, and Kleo; and has patents licensed to Janssen, AZ Immune, and Bristol-Myers Squibb.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Genitourinary Cancers
Rights and permissions
About this article
Cite this article
Comiskey, M.C., Dallos, M.C. & Drake, C.G. Immunotherapy in Prostate Cancer: Teaching an Old Dog New Tricks. Curr Oncol Rep 20, 75 (2018). https://doi.org/10.1007/s11912-018-0712-z
Published:
DOI: https://doi.org/10.1007/s11912-018-0712-z