Abstract
Purpose
MiRNA expression profiles previously showed the higher expression of microRNA(miR)-335 in bone marrow samples of pediatric acute myeloid leukemia (AML) patients than normal controls. Our aim was to investigate associations of miR-335 expression with tumor progression and prognosis in pediatric AML.
Methods
Real-time quantitative PCR was performed to detect the expression of miR-335 in bone marrow mononuclear cells and serum obtained from patients with pediatric AML and healthy controls.
Results
Expression levels of miR-335 in the bone marrow and serum of pediatric AML patients were both significantly higher than those in normal controls (both P < 0.001). Then, high serum miR-335 level occurred more frequently in French-American-British classification subtype M7 subtype than in other subtypes (P = 0.03). The expression of serum miR-335 in pediatric AML patients with unfavorable karyotypes was also significantly higher than those in intermediate and favorable groups (P = 0.008). Moreover, high serum miR-335 level was markedly associated with shorter relapse-free and overall survivals (both P < 0.001) of patients with pediatric AML. Furthermore, the multivariate analysis identified the serum miR-335 and cytogenetics risk as independent prognostic factors for both relapse-free and overall survivals. More importantly, the prognostic relevance of serum miR-335 expression was more obvious in the subgroup of patients with intermediate-risk cytogenetics.
Conclusion
Our data offer the convincing evidence for the first time that serum miR-335 level may be markedly and consistently increased in pediatric AML patients. Serum miR-335 may serve as a promising marker for monitoring the progression and predicting the clinical outcome of patients with this disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML), as a malignant disorder of the blood, is characterized by the uncontrolled proliferation of granulocytic, monocytic, megakaryocytic, or rarely, erythroid blast cells [1]. It is a clonal disease with a highly heterogeneous biology [2]. Cumulative studies have demonstrated that a number of different cytogenetic and molecular abnormalities may be involved into AML and these may lead to the significant alterations in regulatory processes controlling the growth and differentiation programs of the malignant cell. AML accounts for more than 30 % of the deaths from pediatric leukemia, although it makes up only 15–20 % of pediatric leukemia [3]. In the most successful studies, the 5-year disease-free survival in pediatric AML patients is approximately 50 % [4]. Dose-intensive treatment by induction chemotherapy and allogeneic stem-cell transplantation with a matched related donor has been considered as effective treatment for pediatric AML, however, the majority of patients without an appropriate related donor need receive continuous chemotherapy [5]. Of note, pediatric AML has different response to therapy and prognosis when compared to adult AML. Although clinical outcome can be partly predicted by age, cytogenetic findings, and serum lactate dehydrogenase at the time of diagnosis, the prognosis of an individual pediatric AML patient cannot yet be estimated accurately [6]. Therefore, it is of great clinical significance to identify novel and effective molecular markers which can improve the prognostic stratification in order to develop risk-based post-remission therapy.
MicroRNAs (miRNAs), as a class of small, noncoding RNA molecules of 18–25 nucleotides in length, has been recognized as important new regulators of approximately 30 % of all protein-coding RNAs [7]. miRNAs are highly conserved in the genomes of invertebrates, vertebrates and plants, and can posttranscriptionally regulate gene expression, predominantly through imperfect base pairing with the 3′-untranslated region of target mRNAs. Until now, there have been more than 500 miRNAs identified by various studies [8]. Some of them are expressed ubiquitously and some of them are tissue restricted. They play several critical roles in multiple biological processes including development, embryogenesis, proliferation, differentiation, organogenesis, and apoptosis. Dysregulation of miRNAs is also implicated in various diseases, including cancer [9]. Biological evidences have been demonstrated that some miRNAs may act as oncogenes by contributing to the transformed phenotype and suppressing tumor suppressor genes [10], and some miRNAs may act as tumor suppressors by allowing the expression of oncogenes [11], implying that the influence of miRNAs may be dependent on cancer type [12]. miRNAs have been presented as effective potential biomarkers for identifying the tissue origin in malignant tumors of unknown primary origin; miRNAs expression profiles can reflect the developmental lineage and differentiated state of the tumors [13]. These characteristics make miRNAs good candidate markers of diagnosis and prognosis for patients with cancer. Especially in the research of leukemia, recent studies have highlighted the potentials of aberrant expression patterns and functional abnormalities in various miRNAs as prognostic markers or therapeutic targets for AML [14–16].
MiR-335 has been demonstrated to function either as a tumor suppressor or as an oncogene in various human cancer types, implying its crucial roles during tumor development, progression, metastasis and prognosis. MiRNA expression profiles of Zhang et al. [17] showed the higher expression of miR-335 in bone marrow samples of pediatric AML patients than normal controls. However, whether its aberrant expression is of clinical significance remains unclear. Thus, the aim of this study was to investigate the associations of miR-335 expression with tumor progression and prognosis in pediatric AML.
Materials and methods
Patients and tissue samples
The present study was approved by Huai’an First People’s Hospital Ethics Committee. Prior informed consent was obtained from the patients for the collection of specimens in accordance with the guidelines of Huai’an First People’s Hospital, China. All specimens were handled and made anonymous according to the ethical and legal standards.
A total of 106 patients with pediatric AML, including 58 boys and 48 girls, were collected from the Department of Pathology, Huai’an First People’s Hospital, China. All the patients were younger than 18 years of age (median 6 years). The diagnosis of AML was made according to a morphologic assessment of the Wright–Giemsa stained smears of the bone marrow aspirates along with special stains and immunophenotyping by flow cytometry. Laboratory investigation included conventional and molecular cytogenetic analyses. The median leukocyte count at diagnosis was 20,606/μL (range 420–352,906/μL). According to the French-American-British (FAB) classification, three patients had AML M0, 62 had M1/M2, 10 had M3, 21 had M4/M5 and 10 had M7. Among 26 patients with extramedullary disease, 21 patients had chloroma (scalp in 10 patients, orbit in 6 patients and skin in 5 patients), and five patients had a central nervous system involvement of leukemic cells. The clinical characteristic of 106 patients with AML was summarized in Table 1.
All patients with AML were treated with 10 days of induction chemotherapy, in which the dose of behenoyl 1-h-d-arabinofuranosylcytosine for the last 3 days was modified according to the bone marrow response on day 7. Discontinuation of the chemotherapy was allowed in patients who experienced sepsis with unstable vital signs before the completion of the induction regimen if at least 7 days of induction chemotherapy had been provided. If complete remission (CR) was not achieved after the primary induction chemotherapy regimen, an additional course of induction chemotherapy using high-dose 1-h-d-arabinofuranosylcytosine was given. Once CR had been achieved, patients with an appropriate stem-cell donor received consolidation chemotherapy until the hematopoietic stem-cell transplantation. An entire course of consolidation chemotherapy was given in patients without an appropriate stem-cell donor.
As normal controls, bone marrow was collected from 20 patients (12 boys and 8 girls; median age 9 years; range 3–18 years) with various diseases but with normal bone marrow morphology as demonstrated by cytological and histological analyses; Serum was collected from 50 healthy volunteers (30 boys and 20 girls; median age 12 years; range 5–18 years). Volunteers were all healthy, were not on medication, and had no signs or clinical symptoms of cancer, joint, liver, metabolic or endocrine diseases.
Real-time quantitative RT-PCR for miRNA
Real-time quantitative RT-PCR for miRNA was performed to detect miR-335 expression in bone marrow mononuclear cells and serum. Mononuclear cells were isolated by Ficoll Hypaque density gradient centrifugation of 2 ml bone marrow samples in EDTA from newly diagnosed patients and control samples. Total RNA in bone marrow mononuclear cells and serum were both extracted using a QIAamp RNA Blood kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. RNU6B were used as control genes to exclude any possible heterogeneous expression in the different AML subtypes. The miR-335 and RNU6B-specific cDNA were synthesized from total RNA using gene-specific primers according to the TaqMan MicroRNA assays protocol (Applied Biosystems, Foster City, CA, USA). The primer sequences were as following: for miR-335: forward 5′-GCG GTC AAG AGC AAT AAC GAA-3′, reverse 5′-GTG CAG GGT CCG AGG TAT TC-3′; for RNU6B: forward 5′-CGC TTC GGC AGC ACA TAT AC-3′, reverse 5′-TTC ACG AAT TTG CGT GTC AT-3′. Reverse transcription conditions were as described previously. Relative quantification of target miRNA expression was evaluated using the comparative cycle threshold (CT) method. The raw data were presented as the relative quantity of target miRNA, normalized with respect to RNU6B. Each sample was examined in triplicate. Mean normalized gene expression ± standard deviation (SD) was calculated from independent experiments.
Statistical analysis
The software of SPSS version 16.0 for Windows (SPSS Inc, IL, USA) and SAS 9.1 (SAS Institute, Cary, NC) was used for statistical analysis. With regard to the correlation of leukemia clinical features with the serum level of miR-335 expression, inter-group comparisons were performed using one-way analysis of variance (ANOVA). When the equal variance test or normality test failed, the Kruskall–Wallis non-parametric test was applied. To address the problem of multiple comparisons, these tests (ANOVA, Kruskall–Wallis) were followed by a post hoc Bonferroni test. The correlation of miR-335 expression between bone marrow mononuclear cells and sera was determined by Spearman’s correlation analysis. The Kaplan–Meier survival curves were used to determine any significant relationship between the serum level of miR-335 expression and the status of the patients with respect to relapse-free survival (RFS) or overall survival (OS). Differences were considered statistically significant when P was less than 0.05.
Results
Upregulation of MiR-335 in patients with pediatric AML
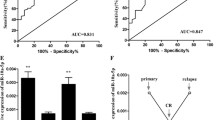
As shown in Fig. 1a, miR-335 expression in the bone marrow of patients with pediatric AML were significantly higher than those in normal controls (AML vs. normal: 4.40 ± 2.11 vs. 2.20 ± 0.70, P < 0.001). Similarly, the serum levels of miR-335 were also markedly upregulated in pediatric AML patients compared with healthy controls (AML vs. normal: 3.61 ± 1.73 vs. 1.82 ± 0.80, P < 0.001, Fig. 1b). Of note, the expression levels of miR-335 in the bone marrow of pediatric AML patients were significantly correlated with those in patients’ sera (Spearman’s correlation: r = 0.66, P = 0.01, Fig. 1c). Thus, we investigated the clinical significance of miR-335 in pediatric AML patients using its serum levels in the next sections.
MiR-335 expression in bone marrow and serum of patients with pediatric AML and normal controls were detected by real-time quantitative RT-PCR assay. a MiR-335 expression in the bone marrow of patients with pediatric AML were significantly higher than those in normal controls (AML vs. normal: 4.40 ± 2.11 vs. 2.20 ± 0.70, P < 0.001). b Serum miR-335 levels were markedly upregulated in patients with pediatric AML compared with healthy controls (AML vs. normal: 3.61 ± 1.73 vs. 1.82 ± 0.80, P < 0.001). c MiR-335 expression in the bone marrow of patients with pediatric AML were significantly correlated with those in patients’ sera (Spearman’s correlation: r = 0.66, P = 0.01)
High serum miR-335 associates with aggressive clinical characteristics of patients with pediatric AML
Then, we evaluated the associations of serum miR-335 levels with the clinical characteristics of patients with pediatric AML. The median value of serum miR-335 (3.59) expression was used as a cutoff point to divide 106 pediatric AML patients into miR-335-low (n = 50) and miR-335-high (n = 56) expression groups. As shown in Table 1, high serum miR-335 level occurred more frequently in FAB classification subtype M7 than in other subtypes (M0–M5, P = 0.03, Table 1). The serum miR-335 levels in pediatric AML patients with unfavorable karyotypes was also significantly higher than those in intermediate and favorable groups (P = 0.008, Table 1). There was no significant association of the serum miR-335 level with patients’ gender and age, leukocyte count, extramedullary disease and day 7 response to treatment (all P > 0.05, Table 1).
High serum miR-335 associates with unfavorable clinical outcome of patients with pediatric AML
All 106 patients with pediatric AML were receive follow-up analysis. The median follow-up duration was 35 months ranged from 10–86 months. Figure 2 shows the Kaplan–Meier curves for RFS and OS stratified according to serum miR-335 levels in patients with pediatric AML. Pediatric AML patients with high miR-335 expression had both shorter RFS and OS than those with low miR-335 expression (both P < 0.001, Fig. 2). In univariate analysis, the RFS in the patients with the FAB subtype M7 (P = 0.01, Table 2), unfavorable cytogenetic abnormalities (P < 0.001, Table 2), and high miR-335 expression (P < 0.001, Table 2) were all significantly shorter than those with controls. Parameters, such as patients’ gender and age, leukocyte counts, the presence of extramedullary disease, and day 7 response to treatment had no impact (all P > 0.05, data were not shown). On the other hand, the FAB classification subtype M7 (P = 0.008, Table 2), unfavorable cytogenetic abnormalities (P < 0.001, Table 2) and high miR-335 expression (P < 0.001, Table 2) were also associated with poor OS. Moreover, Cox proportional hazards multivariate analysis of the univariate predictors identified the cytogenetic abnormalities (P = 0.01 and 0.009, respectively) and the expression level of miR-335 (both P = 0.01) as independent prognostic factors for RFS and OS (Table 3).
More importantly, a separate univariate analysis of the intermediate cytogenetic risk group demonstrated that high miR-335 expression was significantly associated with both unfavorable RFS and OS (both P < 0.001, Fig. 3). But in the favorable and adverse cytogenetic risk groups, no association was found between miR-335 expression and outcome (P > 0.5 for all comparisons, data were not shown).
Kaplan–Meier curves of relapse-free survival (RFS, a) and overall survival (OS, b) of patients with pediatric AML in the intermediate cytogenetic risk group stratified by the serum miR-335 levels. A separate univariate analysis of the intermediate cytogenetic risk group demonstrated that both unfavorable RFS and OS were associated with high miR-335 expression (both P < 0.001)
Discussion
Serum miRNAs are emerging as promising biomarkers for the diagnosis and prognosis of human malignancies. In the current study, we firstly found the upregulation of miR-335 in the bone marrow and the serum from pediatric patients with newly diagnosed AML compared with normal controls. Then, the high serum miR-335 level was dramatically correlated with the aggressive clinical features of patients with pediatric AML. Moreover, both univariate and multivariate analyses revealed that the serum miR-335 expression was a predictor of poor RFS and OS, independent of the cytogenetic abnormalities. Furthermore, the prognostic value of serum miR-335 expression was especially more obvious in the subgroup of patients with intermediate-risk cytogenetics. These findings highlight the potential of miR-335 as a valuable marker for the progression and the prognosis in pediatric AML. This is the first report on the novel serum marker miR-335 in pediatric AML patients.
MiR-335, which is transcribed from the genomic region chromosome 7q32.2, has been observed to aberrantly expressed in malignant tumors and to play a crucial role in tumor initiation and tumor progression [18]. It functions either as oncogenes or tumor suppressor genes depending on various cancer types. For example, Yan et al. [19] reported that the gastric cancer patients with high level of miR-335 had a high frequency recurrence and poor survival compared with those with low level, and they also found that miR-335 were involved in regulating target genes in several oncogenic signal-pathways, such as p53, MAPK, TGF-β, Wnt, ERbB, mTOR, Toll-like receptor and focal adhesion. In contrast, miR-335 has been demonstrated to act as a tumor initiation and metastasis suppressor of breast cancer [20]; Cao et al. [21] observed that miR-335 expression level was reduced in epithelial ovarian cancer tissue samples, especially in omental metastases, and low miR-335 levels emerged as an independent prognostic factor for poor overall survival and relapse-free survival; Peng et al. [22] indicated that miR-335 was downregulated in a majority of patients primary gallbladder carcinoma and may be associated with the aggressive tumor behaviors; Wang et al. [23] also identified miR-335 as a tumor suppressor by targeting the ROCK1 gene and inhibiting osteosarcoma cells migration and invasion. Similar with the findings on gastric cancer, our data in the present study showed the elevated serum level of miR-335 expression in both bone marrow and serum of from pediatric AML patients. Since miRNAs are notably stable in blood and their expression patterns appear to be tissue-specific, serum miRNAs have been recognized as good candidates for noninvasive testing for cancer. In order to determine whether miR-335 can be used as a marker for pediatric AML, we also statistically analyzed the correlation of serum miR-335 with clinical characteristics and prognosis in patients with this disease. As the result, the increased serum miR-335 level was significantly associated with the FAB classification subtype M7 and the unfavorable cytogenetic risks. Both univariate and multivariate analyses further demonstrated that the pediatric AML patients with high serum miR-335 expression had shorter RFS and OS than those with low expression. More importantly, there may be a significant association between serum miR-335 level and the prognosis in patients of the intermediate cytogenetic risk group, but not in those of the favorable and unfavorable cytogenetic risk groups.
In conclusion, our data offer the convincing evidence for the first time that the serum miR-335 level may be markedly and consistently increased in pediatric AML patients. Serum miR-335 may serve as a promising marker for monitoring the progression and predicting the clinical outcome of patients with this disease. However, there are two limitations in this study. At first, the intermediate cytogenetic risk group in our cohort is small in this moderate-sized pediatric sample. Secondly, the exact molecular mechanisms on the involvement of miR-335 dysregulation in pediatric AMLs need further more comprehensive investigations.
References
Russell NH. Biology of acute leukaemia. Lancet. 1997;349:118–22.
Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7.
Estey EH. Acute myeloid leukemia: 2013 update on risk-stratification and management. Am J Hematol. 2013;88:318–27.
Ganetsky A. The role of decitabine for the treatment of acute myeloid leukemia. Ann Pharmacother. 2012;46:1511–7.
Puumala SE, Ross JA, Aplenc R, Spector LG. Epidemiology of childhood acute myeloid leukemia. Pediatr Blood Cancer. 2013;60:728–33.
Danen-van Oorschot AA, Kuipers JE. Differentially expressed miRNAs in cytogenetic and molecular subtypes of pediatric acute myeloid leukemia. Pediatr Blood Cancer. 2012;58:715–21.
Liu X, Liu L, Xu Q. MicroRNA as a novel drug target for cancer therapy. Expert Opin Biol Ther. 2012;12:573–80.
Esteller M. Non coding RNAs in human disease. Nat Rev Genet. 2011;12:861–74.
van Kouwenhove M, Kedde M, Agami R. MicroRNA regulation by RNA binding proteins and its implications for cancer. Nat Rev Cancer. 2011;11:644–56.
Jansson MD, Lund AH. MicroRNA and cancer. Mol Oncol. 2012;6:590–610.
Nana-Sinkam SP, Croce CM. Clinical applications for microRNAs in cancer. Clin Pharmacol Ther. 2013;93:98–104.
Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69.
Medina PP, Slack FJ. microRNAs and cancer: an overview. Cell Cycle. 2008;7:2485–92.
Zhu C, Wang Y, Kuai W. Prognostic value of miR-29a expression in pediatric acute myeloid leukemia. Clin Biochem. 2013;46:49–53.
Zhi F, Cao X, Xie X. Identification of circulating microRNAs as potential biomarkers for detecting acute myeloid leukemia. PLoS One. 2013;8:e56718.
Croce CM. MicroRNA dysregulation in acute myeloid leukemia. J Clin Oncol. 2013;31:2065–6.
Zhang H, Luo XQ, Zhang P. MicroRNA patterns associated with clinical prognostic parameters and CNS relapse prediction in pediatric acute leukemia. PLoS One. 2009;4:e7826.
Png KJ, Yoshida M, Zhang XH. MicroRNA-335 inhibits tumor reinitiation and is silenced through genetic and epigenetic mechanisms in human breast cancer. Genes Dev. 2011;25:226–31.
Yan Z, Xiong Y, Xu W. Identification of hsa-miR-335 as a prognostic signature in gastric cancer. PLoS One. 2012;7:e40037.
Heyn H, Engelmann M, Schreek S. MicroRNA miR-335 is crucial for the BRCA1 regulatory cascade in breast cancer development. Int J Cancer. 2011;129:2797–806.
Cao J, Cai J, Huang D. miR-335 Represents an independent prognostic marker in epithelial ovarian cancer. Am J Clin Pathol. 2014;141:437–42.
Peng HH, Zhang YD, Gong LS. Increased expression of microRNA-335 predicts a favorable prognosis in primary gallbladder carcinoma. Onco Targets Ther. 2013;6:1625–30.
Wang Y, Zhao W, Fu Q. miR-335 suppresses migration and invasion by targeting ROCK1 in osteosarcoma cells. Mol Cell Biochem. 2013;384:105–11.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
X. Lin and Z. Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lin, X., Wang, Z., Zhang, R. et al. High serum microRNA-335 level predicts aggressive tumor progression and unfavorable prognosis in pediatric acute myeloid leukemia. Clin Transl Oncol 17, 358–364 (2015). https://doi.org/10.1007/s12094-014-1237-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-014-1237-z