Abstract
Aim
To investigate clinical significance of retinoic acid-induced protein 3 (RAI3) in hepatocellular carcinoma (HCC).
Methods
Expression of RAI3 at both mRNA and protein levels in tumor, para-tumor and normal liver tissues was detected in 106 HCC patients by real-time quantitative RT-PCR, Western blot and immunohistochemistry. Then, the correlation of RAI3 expression with clinicopathological characteristics and survivals of HCC patients was analyzed.
Results
Our data first found that RAI3 mRNA and protein expression were both significantly higher in HCC than in para-tumor (both P < 0.001) and normal liver tissues (both P < 0.001). The correlation analysis showed a positive correlation between RAI3 mRNA level and RAI3 protein level in HCC tissues (r = 0.8, P < 0.001). Immunohistochemistry data also revealed that overexpression of RAI3 was present in 73.6 % (78/106) of HCC tissues. In addition, high RAI3 protein expression was correlated with advanced TNM stage (P = 0.001), high serum AFP (P = 0.008), vascular invasion (P = 0.01) and tumor recurrence (P = 0.008). Moreover, HCC patients with overexpression of RAI3 had significantly shorter overall (P = 0.01) and disease-free survival (P = 0.01). Furthermore, multivariate analysis showed that overexpression of RAI3 was an independent prognostic factor for both overall (P = 0.02) and disease-free survival (P = 0.03) in HCC.
Conclusion
Our data for the first time provide a basis for the concept that overexpression of RAI3 may contribute to the malignant progression of HCC and predict poor prognosis for patients with this deadly disease after curative hepatectomy. RAI3 might be an important marker for tumor progression and prognosis, as well as a potential therapeutic target of HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary hepatic malignancy and ranks the fifth most prevalent malignant tumors worldwide [1]. Although the majority of cases are still found in Asian Pacific region, recent evidence has shown that the incidence and mortality rate of HCC are rising in the United States and in Western Europe [2]. HCC has been the third leading cause of cancer-related death worldwide. Although many risk factors, such as hepatitis B or C viral infections, alcohol consumption and genetic predisposition, have been identified, the exact molecular mechanisms and pathogenic processes underlying hepatocarcinogenesis have not been fully elucidated. Despite the advancement of diagnostic and therapeutic technology in HCC, the mortality of this tumor is very high, because most patients are in advanced stage at the time of diagnosis and cannot be treated with radical hepatectomy [3]. It is therefore critical to identify novel and effective molecular markers which could give some clues to understand the mechanism of HCC and develop novel diagnostic, therapeutic, and preventive strategies.
Retinoic acid-induced protein 3 (RAI3, also known as RAIG1 or GPRC5), as a member of G-protein-coupled receptors (GPCRs) which constitute one of the largest superfamilies of receptors, was originally identified in 1998 as a retinoic acid (RA)-responsive gene in the human oral squamous carcinoma cell line, UMSCC-22B, using differential display [4, 5]. It is characterized by seven transmembrane helices, three intracellular loops, three extracellular loops, an extracellular N-terminus, and an intracellular C-terminus [6]. Since the discovery of RAI3, another three members of GPCRs: RAIG2 (also known as GPRC5B), RAIG3 (also known as GPRC5C) and GPRC5D have been identified. These four proteins all have a unique tissue specificity of expression: RAI3 is expressed preferentially in lung tissue, RAIG2 is predominately localized in tissues of the central nervous system, RAIG3 expression is mainly found in a variety of tissues, and GPRC5D is observed principally in the skin. Recent studies have demonstrated that RAI3 functions as a potential tumor suppressor or oncogene in various human cancers [7]. For example, Fujimoto et al. [8] found that RAI3 expression was decreased in non-small-cell lung cancer and was associated with lung inflammation; Nagahata et al. [9] implied that the up-regulation of RAI3 may be a frequent feature of breast carcinogenesis; Cheng et al. [10] indicated that RAI3 was significantly elevated in gastric cancer tissues and might be a potential biomarkers for this cancer; Zougman et al. [11] identified RAI3 overexpression as a prognostic marker for high recurrence risk of colon cancer. More recently, Wang et al. [12] identified another RAI protein—RAI16 as a tumor marker for HCC. However, the roles of RAI3 in HCC carcinogenesis have not been elucidated.
To confirm the potential value of RAI3 in human HCC, we performed real-time quantitative RT-PCR (qRT-PCR) and Western blot to determine the expression of RAI3 in HCC samples, and evaluated their relationship to clinical features and prognosis of HCC patients.
Materials and methods
Patients and tissue samples
The study was approved by the Research Ethics Committee of Fourth Military Medical University and the 302nd Hospital of PLA, China. Informed consent was obtained from all of the patients. All specimens were handled and made anonymous according to the ethical and legal standards.
Fresh tissue samples of tumor, para-tumor (defined as ≤2.0 cm distance from tumor edge) and normal (defined as >2.0 cm distance from tumor edge) liver tissues were collected from 106 HCC patients who underwent curative hepatectomy between April 2001 and May 2009 at Xijing Hospital, the 302nd Hospital of PLA and Tangdu Hospital. The criteria for curative hepatectomy were defined as complete resection of the tumor without macroscopic evidence of residual tumor. All patients were confirmed by histological diagnosis. None of the patients received preoperative anticancer treatment. One hundred and six patients with HCC included 78 male and 28 female, with a median age of 52 years old (mean ± SD 52.1 ± 14.8, range 31–72). Preoperative liver function was evaluated by the Child–Pugh score system. Tumor stage was determined according to the tumor-node-metastasis (TNM) classification system of the International Union against Cancer (2002). Tumor differentiation was graded by the Edmondson–Steiner classification system. The clinicopathological features of the patients are summarized in Table 1. The fresh tissue samples were immediately immersed in RNAlater (Ambion, Inc., USA) after surgical resection, stored at 4 °C overnight to allow thorough penetration of the tissue and then frozen at −80 °C until use.
Follow-up usually included serum α-fetoprotein (AFP) level, abdominal ultrasonography, and chest radiography every 1–3 months after curative hepatectomy, and was completed in December 2012. The follow-up time for these patients ranged from 2 to 65 months with a median follow-up time of 26 months. When tumor recurrence was suspected, computed tomography scan or/and magnetic resonance imaging scan was performed to confirm the diagnosis. Overall survival was defined as the interval between the date of surgery and the date of death. Disease-free survival was defined as the interval between the date of surgery and the date when recurrence was diagnosed or to the date of the last follow-up.
Real-time quantitative RT-PCR
Total RNA was extracted from frozen tissues using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instruction. The integrity of all tested total RNA samples was verified using a Bioanalyzer 2100 (Agilent Technologies). Reverse transcription (RT) was performed with 2 μg of total RNA. cDNA was synthesized from the RNA by M-MLV Reverse Transcriptase (Promega, USA) with random primers (Promega, USA) in a 20 μl volume. Real-time PCR amplifications were performed using ABI PRISM 7900HT instruments (Applied Biosystems) in a total volume of 10 μl with the following amplification steps: an initial denaturation step at 94 °C for 80 min, which was followed by 40 cycles of denaturation at 95 °C for 15 s and elongation at 60 °C for 1 min. Primers for RAI3 and GAPDH were listed in Table 2. The relative expression level of the RAI3 in HCC was normalized against the endogenous GAPDH using the comparative threshold cycle (2−ΔΔCt) method.
Western blot analysis
Fresh tissues from HCC patients were homogenated in a RIPA lysis buffer and centrifuged at 20,000×g for 60 min at 4 °C to pellet any precipitate. The protein concentration of the lysate was determined by the BCA assay. For Western blot analysis, 20 μg of the protein extracts were loaded onto 12 % SDS-PAGE and transferred to PVDF membranes (GE healthcare, USA). After being blocked with 5 % milk for 1 h at room temperature, the membranes were then incubated overnight at 4 °C with anti-RAI3 (1:150) (#ab71121, Abcam, Cambridge, MA, USA) or anti-GAPDH (1:1,000) (Santa Cruz Biotechnology, USA) antibodies. After being washed three times, the protein bands were developed by ECL. To confirm equal loading, GAPDH antibody was used as a control.
Immunohistochemistry analysis
Immunohistochemical staining was carried out following the protocol of our previous study [13]. The primary antibody against RAI3 (#ab71121, Abcam, Cambridge, MA, USA, dilution 1:500) was used. Secondary antibody for the detection of primary antibody: anti-rabbit IgG (Sigma, St. Louis, MO, USA). The negative controls were processed in a similar manner with PBS instead of primary antibody. Further, positive RAI3 expression confirmed by Western blotting was used as positive controls for immunostaining.
Following a hematoxylin counterstaining, immunostaining was scored by two independent experienced pathologists, who were blinded to the clinicopathological parameters and clinical outcomes of the patients. The scores of the two pathologists were compared and any discrepant scores were trained by re-examining the stainings by both pathologists to achieve a consensus score. The number of positive-staining cells showing immunoreactivity on the membrane for RAI3 in ten representative microscopic fields was counted and the percentage of positive cells was calculated. The percentage scoring of immunoreactive tumor cells was as follows: 0 (0 %), 1 (1–10 %), 2 (11–50 %) and 3 (>50 %). The staining intensity was visually scored and stratified as follows: 0 (negative), 1 (weak), 2 (moderate) and 3 (strong). A final score was obtained for each case by multiplying the percentage and the intensity score. Therefore, tumors with a multiplied score exceeding 4 (median of total scores for RAI3) were deemed to be high expressions of RAI3; all other scores were considered to be low expressions of RAI3.
Statistical analysis
SPSS13.0 software for Windows (SPSS Inc, USA) was used for statistical analysis. Continuous variables were expressed as \( \overline{X} \pm s \). Paired student t test was used to compare the expression levels of RAI3 between tumor and para-tumor, tumor and normal liver tissues. The Spearman rank correlation coefficient was also used to assess the significance of the correlation between mRNA and protein levels of RAI3 expression. The χ 2 test was performed to analyze the correlation between RAI3 protein expression and clinicopathological parameters. The Kaplan–Meier method (the log-rank test) was used for survival curves. Cox regression model with stepwise manner (forward, likelihood ratio) was utilized to perform a multivariate analysis. The P values of <0.05 were considered to be statistically significant.
Results
Overexpression of RAI3 at mRNA and protein levels in HCC
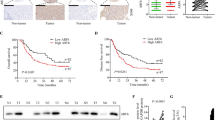
The expression of RAI3 at mRNA and protein levels were detected and analyzed in all fresh tissues from 106 HCC patients. The qRT-PCR results showed that the expression level of RAI3 mRNA were significantly higher in tumor tissue than those in para-tumor tissue and normal liver tissue (both P < 0.001; Fig. 1a; Table 3). The differences between para-tumor and normal liver tissues had no statistical significance. In addition, the Western blot analysis of RAI3 protein also showed that the expression of RAI3 protein were significantly higher in tumor tissue than those in para-tumor tissue (P < 0.001; Table 3) and in normal liver tissue (P < 0.001; Fig. 1b; Table 3). In order to detect the subcellular localization of RAI3 protein in HCC tissues, immunohistochemistry assay was performed in all 106 HCC tissues and the corresponding para-tumor and normal liver tissues. Results revealed that RAI3 expression was mainly present in the cytoplasm of cancer cells (Fig. 2). The overexpression of RAI3 was present in 73.6 % (78/106) of HCC tissues.
Expression of RAI3 at mRNA (a) and protein (b) levels in tumor (HCC), para-tumor (para) and normal liver (N) tissues. a Representative real-time RT-PCR results of two cases (left) and the relative quantitation of RAI3 mRNA expression (right); b representative Western blot results of two cases (left) and the relative quantitation of RAI3 protein expression (right)
Representative immunohistochemical staining of RAI3 in HCC, para-tumor and normal liver tissues. a Overexpression of RAI3 in HCC tissues, RAI3 immunostaining was predominantly located in cytoplasm of tumor cells (×400); b low expression of RAI3 in para-tumor tissues (×400); c negative expression of RAI3 in normal liver tissues (×400)
The Spearman rank correlation analysis showed that the expression level of RAI3 mRNA was closely correlated with that of RAI3 protein (Spearman’s r = 0.8, P < 0.001). Therefore, we chose the expression level of RAI3 protein to analyze its relationship with clinicopathological characteristics and prognosis in HCC patients.
Association of RAI3 expression with the clinicopathologic characteristics of HCC
In order to analyze the association of RAI3 expression with various clinicopathologic characteristics of HCC patients, we divided 106 HCC patients into four groups according to the expression levels of RAI3 protein. HCC patients expressing RAI3 protein at levels less than the median expression level (1.1) were assigned to the low RAI3 expression group (mean expression value 0.9, n = 48), and those samples with expression equal or above the median value were assigned to the high RAI3 expression group (mean expression value 1.3, n = 58). As shown in Table 1, the overexpression of RAI3 protein was significantly correlated with advanced TNM stage (P = 0.001), high serum AFP (P = 0.008), vascular invasion (P = 0.01) and tumor recurrence (P = 0.008). There was no significant difference in age, sex, etiology, background liver pathology, Child–Pugh, tumor size, tumor differentiation between the patients’ groups.
Association of RAI3 expression with prognosis in patients with HCC
The association of RAI3 expression in HCC with the survival of all 106 HCC patients was analyzed with Kaplan–Meier survival analysis. Patients with overexpression of RAI3 were likely to be with significantly shorter overall survival (P = 0.01; Fig. 3a) and disease-free survival (P = 0.01; Fig. 3b).
Then, we evaluated the expression of RAI3, and other clinicopathologic characteristics on prognosis of HCC using univariate analyses. Results as shown in Table 4 indicated that the expression of RAI3 (both P = 0.01), TNM stage (both P = 0.001), serum AFP level (P = 0.01 and 0.02, respectively) and tumor recurrence (P = 0.02 and 0.04, respectively), were significantly associated with overall survival and disease-free survival of HCC patients.
Furthermore, the expression of RAI3, and those clinicopathologic characteristics significant in univariate analysis (TNM stage, serum AFP level and tumor recurrence) were further evaluated in multivariate analysis. Results as shown in Table 5 suggested that the expression of RAI3 (for overall survival: HR 9.1, 95 % CI 1.0–18.8, P = 0.02; for disease-free survival: HR 8.5, 95 % CI 0.8–17.2, P = 0.03) was an independent predictor for overall survival and disease-free survival of HCC patients.
Discussion
HCC is resistant to conventional chemotherapy and is rarely amenable to radiotherapy, leaving this disease with no effective therapeutic options and a very poor prognosis. Thus, it is necessary to discover biological markers useful for HCC diagnosis and prognostic prediction to provide scientific guidance to clinical management. In the present study, we examined RAI3 expression profiles and its correlations with clinicopathologic parameters and prognosis in HCC. Our data revealed that both mRNA and protein levels of RAI3 were significantly higher in HCC tissues than in the corresponding para-tumor tissues and normal liver tissues, suggesting that the overexpression of RAI3 may play an important role in tumorigenic process of HCC. To our knowledge, this is the first study to analyze RAI3 expression in HCC at both mRNA and protein levels.
RAI3 is an orphan GPCR homologous to glutamate receptors and is localized at the plasma membrane and in intracellular vesicles [14]. RAI3 mRNA is expressed at high levels in normal lung and at low levels in other normal tissues such as liver, kidney, placenta, pancreas, colon, testis and ovary [15]. Although RAI3 is thought to regulate cell proliferation, to date the exact molecular function is unknown and is a matter of some controversy. Recent studies have indicated that RAI3 acts as a potential tumor-suppressor gene because of its presumed regulation by retinoids, which are derivatives of vitamin A and are potent anti-tumor agents by inducing cell differentiation [16]. Retinoids play important roles in binding and activating specific retinoic acid and retinoic X receptors that, following activation by ligand binding, localize to the nucleus where they regulate gene transcription [17]. Retinoids also influence tumorigenesis and exert significant therapeutic and preventative effects on tumors [18]. In a further study, RAI3 has been demonstrated to be a growth-promoting gene that may be involved in cell cycle regulation with a peak expression in G1 phase, as analyzed in the human cervical epithelial tumor cell line [19, 20]. The growth-promoting effect of RAI3 is further underlined by the finding that RAI3 is a p53-transcriptional target gene [21]. These prompted us to examine the involvement of RAI3 in HCC.
Our observation showed that the increased expression of RAI3 in HCC was positively correlated with advanced TNM stage, high serum AFP, vascular invasion and tumor recurrence. Moreover, patients with high RAI3 expression had significantly worse overall and disease-free survival when compared with patients with low expression of RAI3. Multivariate analysis demonstrated that among the factors analyzed, RAI3 expression was an independent prognostic factor for overall and disease-free survival in patients with HCC. Meanwhile, TNM stage, serum AFP and tumor recurrence were all independent prognostic factors for overall survival; while TNM stage and serum AFP were independent prognostic factors for disease-free survival. These results clearly demonstrated that RAI3 overexpression is associated with poor progression and unfavorable clinical outcome of HCC. RAI3 expression at both mRNA and protein levels could be used as an additional tool in identifying those patients at risk of HCC progression.
In conclusion, our data for the first time provide a basis for the concept that overexpression of RAI3 may contribute to the malignant progression of HCC and predict poor prognosis for patients with this deadly disease after curative hepatectomy. RAI3 might be an important marker for tumor progression and prognosis, as well as a potential therapeutic target of HCC. The current results are based on a cohort of Chinese HCC patients. Therefore, other HCC cohorts should be used for further confirmation.
References
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics. CA Cancer J Clin. 2005;55:74–108.
Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36.
El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76.
Jörissen H, Bektas N, Dahl E, Hartmann A, ten Haaf A, Di Fiore S, et al. Production and characterisation of monoclonal antibodies against RAI3 and its expression in human breast cancer. BMC Cancer. 2009;9:200.
Chen Y, Deng J, Fujimoto J, Kadara H, Men T, Lotan D, et al. Gprc5a deletion enhances the transformed phenotype in normal and malignant lung epithelial cells by eliciting persistent Stat3 signaling induced by autocrine leukemia inhibitory factor. Cancer Res. 2010;70:8917–26.
Robbins MJ, Michalovich D, Hill J, Calver AR, Medhurst AD, Gloger I, et al. Molecular cloning and characterization of two novel retinoic acid-inducible orphan G-protein-coupled receptors (GPRC5B and GPRC5C). Genomics. 2000;67:8–18.
Tao Q, Cheng Y, Clifford J, Lotan R. Characterization of the murine orphan G-protein-coupled receptor gene Rai3 and its regulation by retinoic acid. Genomics. 2004;83:270–80.
Fujimoto J, Kadara H, Garcia MM, Kabbout M, Behrens C, Liu DD, et al. G-protein coupled receptor family C, group 5, member A (GPRC5A) expression is decreased in the adjacent field and normal bronchial epithelia of patients with chronic obstructive pulmonary disease and non-small-cell lung cancer. J Thorac Oncol. 2012;7:1747–54.
Nagahata T, Sato T, Tomura A, Onda M, Nishikawa K, Emi M. Identification of RAI3 as a therapeutic target for breast cancer. Endocr Relat Cancer. 2005;12:65–73.
Cheng L, Yang S, Yang Y, Zhang W, Xiao H, Gao H, et al. Global gene expression and functional network analysis of gastric cancer identify extended pathway maps and GPRC5A as a potential biomarker. Cancer Lett. 2012;326:105–13.
Zougman A, Hutchins GG, Cairns DA, Verghese E, Perry SL, Jayne DG, et al. Retinoic acid-induced protein 3: identification and characterisation of a novel prognostic colon cancer biomarker. Eur J Cancer. 2013;49:531–9.
Wang W, Zhao LJ, Yang Y, Wang RY, Ren H, Zhao P, et al. Retinoic acid induced 16 enhances tumorigenesis and serves as a novel tumor marker for hepatocellular carcinoma. Carcinogenesis. 2012;33:2578–85.
Guo XD, Xiong L, Zou L, Zhao JM. Upregulation of bone morphogenetic protein 4 is associated with poor prognosis in patients with hepatocellular carcinoma. Pathol Oncol Res. 2012;18:635–40.
Hirano M, Zang L, Oka T, Ito Y, Shimada Y, Nishimura Y, et al. Novel reciprocal regulation of cAMP signaling and apoptosis by orphan G-protein-coupled receptor GPRC5A gene expression. Biochem Biophys Res Commun. 2006;351:185–91.
Acquafreda T, Soprano KJ, Soprano DR. GPRC5A: a potential tumor suppressor and oncogene. Cancer Biol Ther. 2009;8:963–5.
Connolly R, Nguyen NK, Sukumar S. Molecular Pathways: Current Role and Future Directions of the Retinoic Acid Pathway In Cancer Prevention and Treatment. Clin Cancer Res. 2013 (in press).
Shim E, Yeum KJ, Tang G, Ahn SH, Hwang J, Lee-Kim YC. Retinoids, carotenoids, and tocopherols in breast adipose tissue and serum of benign breast disease and breast cancer patients. Nutr Cancer. 2012;64:956–63.
Garattini E, Paroni G, Terao M. Retinoids and breast cancer: new clues to increase their activity and selectivity. Breast Cancer Res. 2012;14:111.
Kadara H, Fujimoto J, Men T, Ye X, Lotan D, Lee JS, et al. A Gprc5a tumor suppressor loss of expression signature is conserved, prevalent, and associated with survival in human lung adenocarcinomas. Neoplasia. 2010;12:499–505.
Deng J, Fujimoto J, Ye XF, Men TY, Van Pelt CS, Chen YL, et al. Knockout of the tumor suppressor gene Gprc5a in mice leads to NF-kappaB activation in airway epithelium and promotes lung inflammation and tumorigenesis. Cancer Prev Res (Phila). 2010;3:424–37.
Wu Q, Ding W, Mirza A, Van Arsdale T, Wei I, Bishop WR, et al. Integrative genomics revealed RAI3 is a cell growth-promoting gene and a novel P53 transcriptional target. J Biol Chem. 2005;280:12935–43.
Author information
Authors and Affiliations
Corresponding authors
Additional information
J. Zheng and X. Guo equally contributed to this study.
Rights and permissions
About this article
Cite this article
Zheng, J., Guo, X., Gao, X. et al. Overexpression of retinoic acid-induced protein 3 predicts poor prognosis for hepatocellular carcinoma. Clin Transl Oncol 16, 57–63 (2014). https://doi.org/10.1007/s12094-013-1040-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-013-1040-2