Abstract
Functional beverages play an essential role in our modern life and contribute to nutritional well-being. Current efforts to understand and develop functional beverages to promote health and wellness have been enhanced. The present study aimed to investigate the production of three fermented plants beverages (FPBs) from aromatic and medicinal plants and to evaluate the fermented product in terms of physio-biochemical composition, the aromatic compounds, antioxidant activity, and in vivo protective effects on hepatotoxicity and nephrotoxicity induced by carbon tetrachloride (CCl4). The results showed that the fermented beverage NurtBio B had the highest levels of polyphenols, flavonoids, and tannins; 242.3 ± 12.4 µg GAE/mL, 106.4 ± 7.3 µg RE/mL and 94.2 ± 5.1 µg CE/mL, respectively. The aromatic profiles of the fermented beverages showed thirty-one interesting volatile compounds detected by GC–MS headspace analyses such as benzaldehyde, Eucalyptol, Fenchone, 3-Octadecyne, Estragole, and Benzene propanoic acid 1-methylethyl ester. In addition, the fermentation process was significantly improved, indicating its great potential as a functional food with both strong antioxidant activity and good flavor. In vivo administration of CCl4 in mice induced hepatotoxicity and nephrotoxicity by a significant rise in the levels of serum liver and kidney biomarkers. The protective effects of the FPBs showed that they significantly restored the majority of these biological parameters to normal levels, along with increase antioxidant enzyme activities, as well as an improvement of histopathological changes, suggesting their protective effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, consumers are conscious of the relationship between diet and health. Therefore, the newly produced fermented food would not only satisfy hunger and meet nutritional values but also prevent chronic diseases and have a good impact on both physical and mental health on wellbeing. Fermentation technology is one of the oldest, simplest and most economical methods for producing and preserving food and beverages, as well as for improving the nutritional, sensory and shelf-life properties of the products [1]. In addition, fermentation process induced by microorganisms resulting in conversion of carbohydrates into alcohols or organic acids and enhance the extraction of the bioactive metabolites [2].
The demand for functional beverages products has risen and probiotics are used to create ready-to-drink beverages made from fruits and vegetables. Fermented foods with plant origin have been evaluated as vectors for administration of probiotic lactic acid bacterial cultures following the proficiency of the production of vegetable based fermented products via lactic acid bacteria [3].
In recent years, there has been an increased interest in the production of fermented beverages especially probiotics due to their health beneficial effects and nutritional properties. Fermented food and beverage contain abundant enzymes, vitamins, minerals, and secondary metabolites [4]. Aromatic and medicinal plants are very useful for selective bioprocesses because they already contain a variety of bioactive components, such as phenolic compounds, carotenoids, anthocyanins, and tocopherols [5]. In addition, the plants materials can be provided with an optimal environment for the growth of lactic acid bacteria (LAB) [6].
Several authors have successfully obtained fermented beverages with strong antioxidant activity from vegetables, fruits, or plants [7,8,9]. For example, Sauerkraut, olives, cucumbers, and kimchi, are world-famous fermented fruit and vegetable items that provide health benefits [10]. Fermentation has many advantages and features, making this approach useful for improving the organic and nutritional qualities of fermented food and beverage based fruits and vegetables [10]. In particular, LAB, mediated fermentation can decrease phytate and trypsin inhibitors and hydrolyze tannic acid [11, 12]. Moreover, during the fermentation process, many new compounds are synthesized, such as isoflavones, water-soluble vitamins, and vitamin K2 (menaquinone-7), which play a significant role in human health [13, 14]. Functional foods are considered to promote health-boosting effects in addition to their nutritional value, which are attributed to the content of biologically active components in adequate amounts [10].
Aroma is vital for assessing the quality of fermented plant beverages and is heavily influenced by the type and amount of volatile compounds present. Studying these compounds during fermentation is actively researched to track their changes over time and their potential effects on distinct aroma characteristics and overall flavor. During fermented beverage storage under different conditions, the volatile composition can be changed due to the appearance of some volatiles that may change the fermented beverage aroma quality [15]. Therefore, the flavor of products is an important factor for consumers to adhere to long-term use. Some natural medicinal plant raw materials generally have bad flavors, such as grass and bitterness [16]. So, it is difficult for consumers to persist in consumption. Results of studies have shown that microbial fermentation can reduce the odor of plants such as grass [17, 18]. Therefore, the fermentation technology improving efficacy and flavor to develop functional beverages with both efficacy and good flavor, which could give pleasant-positive aroma attributes to the flavor of the beverages [9, 14].
Functional beverages-based plants that contain significant amounts of bioactive compounds can offer several health benefits. Numerous recent studies showed the significance of antioxidant compounds in scavenging free radicals in the human body and preventing diseases [19, 20]. Moreover, previous investigations have indicated the importance of fermented medicinal herbs as a substrate with a good source of natural antioxidants due to bioactive metabolites such as phenolic compounds, which have the potential to reduce the risk of oxidative stress-related diseases such as cancer, coronary heart disease, and atherosclerosis [9, 21, 22]. For instance, they can prevent some damaging physiological activities including metabolic and cardiovascular diseases [23]. A comprehensive inventory of microorganisms and their status as GRAS (generally recognized as safe) and/or QPS (qualified presumption of safety) organisms for the intended use is provided by Bourdichon and Casaregola [24].
Therapeutic claims for fermented beverages exist for non-communicable diseases (obesity, diabetes, cardiovascular disease, hypertension, metabolic syndrome), gut health (allergies, food intolerance, inflammatory diseases) and gastrointestinal tract cancer [25, 26].
Kidneys and liver play an important role in body functions, such as regulating water fluid levels, metabolism, biotransformation (glycolysis, lipids, and amino acids), and detoxification of exogenous chemicals which are toxic to human health [27]. Currently, many xenobiotics produced by environmental pollutants cause major health problems worldwide. Carbon tetrachloride (CCl4) has well-known dangerous effects; it can cause hepatotoxicity and nephrotoxicity [27]. Under the effect of CCl4, there is a decrease in hepatic content of cytochrome P450; complex of enzymes which are responsible for the oxidation of xenobiotic chemicals including drugs, pesticides, and carcinogens. In laboratory assays, CCl4 is widely used to induce liver damages [28]. The number of patients presenting with kidney and liver disorders is increasing at an alarming rate [29]. Nowadays, there are approximately over one million people worldwide who require dialysis or a functioning graft. Kidney and liver replacement have been the only therapy for end stage of renal failure, and dialysis has remained the only alternative when a kidney transplant is not possible [29].
The necessity for developing an antioxidant-based preventative and therapeutic approach as an alternative to conventional medications used to treat organ damage caused by xenobiotics is increasing. In view of these findings, the current investigation aimed at the production of three functional beverages from aromatic and medicinal plants and determine their antioxidant activity and protective effect on hepatotoxicity and nephrotoxicity induced by carbon tetrachloride in vivo. The physicochemical properties were characterized by pH, titration acidity, total sugar, total flavonoids, total tannins and total polyphenols. The antioxidant activity was evaluated by DPPH, ·and ABTS· scavenging activity and Reducing power (FRAP value). Volatile flavor compounds were determined by gas chromatography–mass spectrometry (GC–MS).
Materials and Methods
Plant Materials and Fermentation
The plant materials used for the formulation of each of the three FPBs are shown in Table 1. A voucher specimen from each of those species was kindly identified by Pr. Abderrazak Smaoui and deposited at the Laboratory of Aromatic and Medicinal Plants at the Center of Biotechnology of Borj-Cedria. In each of the three FPBs, the following species were used at the same rate (Table 1). The only difference is the addition of 150 g of Cucurbita maxima, Citrus sinensis and Daucus carota materials in NutrBio A, NutrBio B, and NutrBio C, respectively. Concerning the fermentation process, the selected plant materials were washed with distilled water and then submerged in 1 L bottles containing 30 g/l of organic sugarcane molasses dissolved in sterilized distillated water, the solution was sterilized by pasteurization (80 °C in 15 min) [9]. For the purpose of fermentation, a ready to use inoculum with activated suspension at average of 7 × 106 to 7 × 108 CFU at pH = 3.5 was added to each batch at the rate of 6% (v/v). The bottles were set to ferment without shaking or opening for 30 days at room temperature. Then, the three beverages were sterilized by filtration through 0.2 μm filters and preserved at + 4 °C until use.
Activation of Effective Microorganisms (EM-1 AMA)
The commercial consortiums start culture of EM-1 AMA offered by Effective Microorganisms Technology Tunisia (AMTT, a company in our Ecopark producing natural products based on GRAS microorganisms (lactic acid bacteria, acetic acid bacteria, yeasts) the only genuine Saion-EM producer and distributor of products from the mother of Saion-EM green technology owner (Sanko Sangyo Co. Ltd.) (www.saion-em.co.jp). The product was based on the original formulation developed in 1994 by [30] known as effective microorganisms (EM-1 AMA). It was inoculated inside an autoclaved 40% (v/v) molasses which was mixed with H2O. Activation of the EM-1 AMA was carried out by incubating the broth inside the incubator (Memmert, Germany) at 35 ± 1 °C. Activation was carried out for 7 days where microbial activity was assayed through changes in pH and sugar concentration. According to standard EM inoculation procedure, activated EM-1 AMA were introduced with 40% (v/v) molasses into the aquaculture wastewater to ensure sufficient carbon source.
Fermentative Parameters
Acidity was measured by titration using 0.1 M NaOH solution and phenolphthalein as indicator. The pH was measured using a calibrated Hanna pH meter. Total sugars were assessed by an ATC refractometer (Brix 0–32%). The determination of reducing sugars was assessed using the DNS method as described by [31]. The results were expressed as the means of three replicates.
Phytochemical Composition
Total Polyphenol Contents
The determinations of the total phenolic contents were carried out according to the Folin-Ciocalteu method as described by [32]. A volume of 0.5 mL of Folin–Ciocalteu reagent and 1.25 mL of Na2CO3 (7% w/v) was added to 0.125 mL of each fermented beverage. The absorbance of each sample was measured by spectrophotometer UV—Visible Agilent Cary 60. at 765 nm after incubation of the tubes for 90 min in the dark. Total polyphenols content, expressed as µg Gallic acid equivalents per mL of fermented beverage (mg GAE/mL).
Total Flavonoids Content
The determinations of the total flavonoid’s contents were carried out according to the method reported by [33]. The analyses were performed in triplicate. Total flavonoids, expressed in mg of quercetin equivalent per mL of fermented beverage (mg QE/mL), were estimated with respect to the quercetin standard curve (concentration range: 100 -750 µg/mL).
Condensed Tannin Contents
The determination of the condensed tannins contents was carried out according to the method reported by [34]. The analyses were performed in triplicate. The quantity of the condensed tannins, expressed as mg catechin equivalent per mL of fermented beverage (mg CE/mL), was determined using a catechin calibration curve (100 – 750 µg/mL).
Headspace Solid-Phase Microextraction of Volatile Compounds (HS SPME GC–MS)
The volatile compounds were analyzed by Headspace Solid-Phase Microextraction (HS-SPME) coupled with Gas Chromatography Mass Spectrometry (GC–MS) as previously described [35]. The released ions will be classified according to their mass/charge ratio (m/z). The analysis is carried out by a chromatograph coupled to an Agilent (Agilent Technologies, Palo Alto, CA, USA) mass spectrometer (5975C inert XL MSD) and electron impact ionization (70 eV). An HP-5MS capillary column (30 m × 0.25 mm, 0.25 μm film thickness) coated with 5% phenyl methyl silicone and 95% dimethylpolysiloxane (Agilent Technologies, Palo Alto, CA, USA) was used. The volatile compounds were identified by matching their mass spectra with those in the NIST1.l library of MS spectra. The Kovats retention index (RI) was calculated with a homologous series of n-alkanes (C6-C28) under the same conditions applied for the sample analyses. The volatile compounds with odor activity value > 1 were considered to contribute to the aroma of the fermented beverages [36].
In Vitro Antioxidant Activity Determination
The antioxidant activities of different FPBs were determined by four different but complementary tests: α-diphenyl-β-picrylhydrazyl (DPPH) free radical scavenging method and ABTS·+ radical scavenging assay according to the methods described by [32]. Furthermore, Ferric reducing power assay (FRAP) was assessed following the procedure described by [34] and reported by Prieto [37]. The appearance of the blue green color was measured at 700 nm on UV–Vis spectrophotometer Agilent Cary 60. The results were expressed as the means of three replicates.
In Vivo Experiments
Experimental Animals
This study was conducted with permission from the Experimental Animal Commodities of Soukra and obtained according to the ethical approval from the Bio-Medical Ethics Committee of the Pasteur Institute of Tunis, Tunisia, 2019/2/I/ LR16IPT09/V2. Swiss albino mice about 22–25 g body weight (bw) were used. All animal experiments were conducted with permission from the Tunisian Code of Practice for the Care and Use of Animals for Scientific Purposes and also according to the European convention for the protection of vertebrate animals used for experimental and other scientific purposes. Animals were kept for 2 weeks to be acclimatized prior to the investigation. Throughout the experimentation period, animals were given a standard pellet diet and water ad libitum.
Acute Toxicity Study
The control group received distilled water orally while the other groups received different doses of FPBs (5, 10, 50, and100 mg/kg of bw) and monitored for toxic symptoms and death rate during 12 h and 72 h.
Ccl4-Induced Hepato and Nephro-Toxicity in Mice
Experimental Design
The animals were divided into five groups, with six mice in each group. Oral Treatments were carried out as follows: (1) The first group named Control (saline); (2) the second group named Tox (saline and CCl4) received normal saline (10 mL/kg bw) each Meanwhile; (3) the third group named A (NutriBio A, and CCl4); (4) fourth group named B (NutriBio B, CCl4); and (5) fifth group named B (NutriBio C, CCl4), groups were administered with 5 mg/kg bw of NurtiBio A, NutriBio B and NutriBio C, respectively. All treatments (saline and FPBs) were given for 15 consecutive days. On day 15, animals in groups 2, 3, 4, and 5 were intraperitoneally injected with CCl4 in corn oil (1:10, v/v) [38]. Animals in group 1 were used as CCl4-negative control. 24 h after CCl4 injection, animals were scarified; serum, kidneys, and liver were pooled and conserved at -80 °C until use.
Blood Biochemical Analysis
After treatment, the animals were weighed and sacrificed by decapitation in order to minimize the handling stress, and the trunk blood was collected, immediately. The serum was prepared by centrifugation (1500 rpm, 15 min, and 4 °C). Serum was used to perform the biochemical assays for Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), Alkaline Phosphatase (ALP), Blood Urea Nitrogen (BUN), total protein (TP), Glucose (GLU), Total Bilirubin (T-BIL), Creatinine (Crea) and total cholesterol (T-CHO) was determined using biochemistry analyzer at the Biochemistry laboratory in La Rabta Hospital, Tunis.
Measurement of MDA, SOD, CAT, and GPx in Liver and Kidney Homogenates
A total of 0.5 g of each tissue (kidney, liver) was homogenized in ninefold (w/v) cold normal saline and centrifuged at 2000×g for 10 min. The obtained supernatants were used to quantify the MDA, SOD, CAT and GPx [34]. Tissue protein content was also determined according to the method described by [39] using bovine serum albumin as standard.
Histopathological Studies
Liver and kidney sections fixed in formalin solution were washed with distilled water and treated by a series of alcohol baths and embedded in paraffin. Sections of 4 to 6 μm thickness were made using microtome and stained with hematoxylin–eosin (HandE) and then observed with an Axiophot Zeiss Light Microscope at 40×, magnification (Zeiss, Germany).
Statistical Analysis
The results are expressed as mean ± standard error of the mean. The intergroup variation between various groups was measured by one-way analysis of variance (ANOVA) followed by Tukey's HSD Post-Hoc tests for multiple comparisons with statistical significance of p < 0.05. Data were analyzed using SPSS software, version 20.0. (Armonk, NY: IBM Corp).
Results
Fermentation Parameters
The results presented in Table 2 underscore a notable transformation in the samples obtained from the fermentation of sugarcane molasses and plant raw materials by the chosen consortium. This transformative process brought about a substantial reduction in pH values, accompanied by a subsequent elevation in titratable acidity. This shift can be attributed to the liberation of a range of organic acids, including lactic, acetic, formic, and malic acids, among others. These acids, generated during the fermentation, contribute to the observed changes in pH and acidity. Specifically, the pH levels of the samples underwent a significant drop from their initial values of 6.4 ± 0.2, 6.5 ± 0.2, and 5.8 ± 0.2 for NutrBio A, NutrBio B, and NutrBio C, respectively. This decrease culminated in pH values of 3.8 ± 0.2, 3.3 ± 0.3, and 4.2 ± 0.5 for the respective samples after fermentation. This shift in pH indicates the profound impact of the fermentation process on the chemical nature of the samples.
Furthermore, the concentration of sugars within these samples exhibited a discernible decline following the fermentation process. This reduction in sugar content led to the development of diverse compositions of hydrolyzed carbohydrates, which possess potential as a valuable fermentation medium.
The fermentation process orchestrated by the selected consortium brings about substantial modifications in pH, acidity, and sugar composition, ultimately fostering the development of a transformed medium rich in hydrolyzed carbohydrates.
Phenolics, Flavonoids, and Condensed Tannin Contents
The total Phenolics, flavonoids, and condensed tannins contents in the beverages are shown in Fig. 1. The FPBs exhibited a high content of polyphenolic compounds, with NutrBio B showing the highest levels of flavonoids and tannins. Moreover, the three FPBs exhibited high level of polyphenols: 183.5 ± 9.5 µg GAE/mL, 242.3 ± 12.4 µg GAE/mL and 217.6 ± 8.3 µg GAE/mL for NutrBio A, NutrBio B and NutrBio C, respectively. The results showed that NutrBio B has the highest level of flavonoids (106.4 ± 7.3 µg RE/mL), and tannins (94.2 ± 5.1 µg TA/mL).
Biochemical composition of three fermented beverages from aromatic and medicinal plants. * µg of gallic acid equivalent per ml of beverage; ** µg of rutin equivalent per ml of beverage; *** mg of catechin equivalent per ml of beverage. Results were expressed as the mean SD (n = 3). B; Significant difference at p < 0.05
It was observed that the FPBs are rich in phenolic compounds, especially NutrBio B, which could be an important starting point to explore their biological activities.
Volatile Compound Profiles
Thirty-one compounds were identified with remarkable differences between the three beverages (Table 3). Among the volatile compounds detected, some are typically produced by plants and other compounds are generated by the LAB metabolism. There were great variations in the composition of aroma components. Some compounds were detected only in NutrBio A (acetic acid ethyl ester, n-acetyl-propanamide, butanoic acid-3-methyl-propyl ester, and terpinen-4-ol). Some other compounds were detected only in NutrBio B (2-oxopentanedioic acid, 1,3-Dioxane,1-Butanol, 3-methyl-, acetate, Butanoic acid, Anethole, and propyl ester), and few others were detected only in NutrBio C (1,2-propanediol diformate, and 4-penten-2-ol). 2-amino-1,3-propanediol, n-propyl acetate, propanoic acid, propanoic acid propyl ester, lactic acid, benzaldehyde, eucalyptol, fenchone, 3-octadecyne, estragole, and benzenepropanoic acid 1-methylethyl ester were found in the three beverages with a significant difference between them (p < 0.05). The primary metabolic actions of the selected strains in food and beverage fermentation include their ability to predominantly ferment carbohydrates and, to a lesser degree, degrade proteins and fats in the raw materials. This could lead to the production of a broad range of metabolites, mainly organic acids (for example, lactic, acetic, formic, propionic), peptides, amino acids, along with many volatile and nonvolatile low-molecular-mass compounds, such as ketones and esters. These results suggest that the aromatic compounds generated by used mixed-culture (EM-1 AMA) could give positive aroma attributes to the flavor of the FPBs, depending on the substrate used.
In Vitro Antioxidant Activity
The antioxidant activity of natural products stems from their rich content of bioactive compounds, which neutralize harmful free radicals, contributing to overall health and disease prevention. The results presented in Table 4 showed that NutrBio B has the highest ability to quench DPPH and ABTS free radicals. Both in DPPH and ABTS assays, NutrBio B has the lowest IC50 values (13.4 ± 1.1 and 15.4 ± 1.5, respectively) which confirms that NutrBio B exhibited the highest scavenging activity compared to NutrBio A and NutrBio C. In addition, the FRPA was expressed as EC50 (µM Vit C/mL) of fermented beverages. The results showed that the three FPBs are able to reduce Fe+3/ferric cyanide complex to the ferrous form and exhibited different reducing activities (Table 4). However, NutrBio B has the lowest EC50 (8.3 ± 0.8 µg/mL) compared to NutrBio A and C, indicating its highest antioxidant activity and demonstrating the high correlation between total phenolics, total flavonoids, condensed tannin content, volatile compounds and the antioxidant activities.
Acute Toxicity
Using mice that weighed between 22 and 25 g, acute toxicity was examined after 72 h. Four groups of mice (each group comprised of six mice) were given oral administration of each of the three FPBs at dosages of 5, 10, 50, and 100 mg/kg of bw. The control group received physiological water (NaCl 0.9%). After administration of fermented beverages, the animals did not show any dangerous clinical symptoms of toxicity even with a dose as high as 100 mg/kg bw, since they keep their normal behavior. In addition, at this dose; no mortality was observed during the experiment. Accordingly, for the rest of the in vivo experiments, a dose of 5 mg/kg bw was chosen to investigate the antioxidant activity, hepatoprotective, and nephroprotective effects of all FPBs in experimental animals. Taking into account such findings together with the present results, it seems that the FPBs herein developed could be attributed a GRAS status since they don't show any toxicity effects. Nevertheless, deeper toxicological investigation should be considered to prove the safety of the products.
CCl4-Induced Hepato- and Nephro-Toxicity in Mice
Evaluation of Hepato- and Nephro-Protective Effects of the FPBs
The liver and kidney are the major organs of detoxification and metabolism of chemical xenobiotics which are toxic to human health. These processes of detoxification can be revealed by the alteration in liver biomarker enzymes including AST, and ALT. The results illustrated in Table 5 showed that the serum rates of AST, ALT, ALP, and T-BIL significantly decreased (p < 0.001) in the CCl4-treated groups administered with each of the three FPBs when compared to controls (receiving CCl4 and treated with saline) indicating a hepatocyte damage control. The treatment with FPBs restored significantly the serum parameter pathologic changes towards normal compared with CCl4 intoxicated group (p < 0.001). Functional beverages possess clear hepatoprotective effects, which could be attributed to the high content of bioactive compounds with high antioxidant activity. In addition, the results presented in Table 5 revealed that the CCl4 treatment induced a significant increase in plasma urea nitrogen level (14.5 ± 2 U/dL), and creatinine level (86.5 ± 5 U/dL) compared to control values (8.5 ± 1.3 U/dL, and 59.27 ± 0.66 U/ dL, for urea nitrogen, and creatinine, respectively), which confirms that the CCl4 induced nephrotoxic effects. However, pretreatment with each of the three FPBs restored the level of urea nitrogen and creatinine and thus renal functioning significantly (p < 0.01) compared to control group. The results showed that NutrBio B exhibited the highest protective effect compared to NutrBio A and NutrBio C. Similarly, glucuronic acid and malic acid are also by-products of the fermentation which helps in detoxifying the liver. Moreover, supporting the detoxification of the liver and kidney, the consumption of FPBs is also known to help excrete heavy xenobiotic substances and environmental pollutants from the human body through the kidneys. The results suggest that the FPBs exhibit high antioxidant activity and can enhance the nephron and hepato-functionality in mice treated with CCl4, which may strongly contribute to stabilize the serum parameters. Moreover, CCl4-induced hepatotoxicity and nephrotoxicity have been largely used in the experimental models for liver and kidney damage. The fermented beverage pretreatment significantly helped to drop activity in serum parameters such as AST, ALT, and ALP, and creatinine. This implies a potential hepatoprotective and nephroprotective effect of FPBs by a possible stabilization of the serum membrane mutually with a repair of hepatic tissue damage induced by CCl4. The FPBs were known to help in obtaining relief from gout, rheumatism, arthritis, and kidney stones, which are conditions associated with the accumulation of toxic substances in the body.
Effects of Fermented Beverages on Lipid Peroxidation, SOD, CAT, and GPx Activity
The cells are protected against the excess of Reactive Oxygen Species (ROS) by antioxidant enzymes (e.g., catalase, glutathione peroxidase (GPx), superoxide dismutase (SOD), and other antioxidants). Oxidative stress is one of the major consequences of the health problems caused by hepatotoxicity and nephrotoxicity. The results illustrated in Fig. 2, showed that treatment of mice with CCl4 induced significant increases of renal and hepatic lipid peroxidation levels as compared with the control group. However, FPBs treatment restored significantly the lipid peroxidation compared to control. Moreover, Fig. 2 clearly shows the effects of CCl4 on the antioxidant enzymes (SOD, CAT, and GPx) activities. Indeed, those activities were significantly decreased in CCl4 treated group compared to the control (not receiving CCl4). For the groups treated with FPBs, we observed an increase of SOD, CAT, and GPx activities compared to CCl4-treated animals. The data demonstrate the potential of the three FPBs to restore the activity of antioxidant enzymes. The administration of different FPBs reversed the damage caused by CCl4 to both liver and kidneys, thus confirming their antioxidant potential.
Levels of lipid peroxidation (expressed as TBARS, nmol/mg of protein) and antioxidants enzymes (SOD, CAT, GPx) activity (U/mg protein) in liver and kidney (lower) in control and experimental treated mice. Control: (salin); Tox: (salin and CCl4) received normal salin (10 ml/kgbw) each Meanwhile; A (NutriBio A, and CCl4); B (NutriBio B and CCl4); and C (NutriBio C and CCl4), groups were administered with 5 mg/kg bw of NurtiBio A, NutriBio B and NutriBio C, respectively. Results were expressed as the mean SD (n = 6). B; Significant difference at p < 0.001, A; Significant difference at p < 0.001, compared to CCl4 treated group
Previous studies have also been carried out to find the protective effects of FPBs against CCl4-induced hepatotoxicity, and the observed effects were attributed to the antioxidant capacity of the beverages, along with their ability to enhance hepatic and renal functionality.
Histopathological Studies
Previous results showed that FPBs present a potent protective effect against nephrotoxicity and hepatotoxicity induced by CCl4. In order to validate these effects, comprehensive histopathological evaluations were conducted on kidney and liver tissues. The photomicrographs presented in Fig. 3 represent the liver and kidney sections of different groups. It clearly appears that the liver section of the control group showed a normal histological structure. However, the CCl4-treated group showed significant morphological changes. These alterations were distinguished by membrane cell degradation, focal necrosis, and major vascular congestion that may be attributed to the toxic effects of CCl4 by the generation of ROS, and accordingly causing damage to different membrane constituents of the hepatocytes. The liver sections of the groups pretreated with FPBs had an architecture nearly comparable to the control group without any signs of vascular or membrane cell changes (Fig. 3A–C).
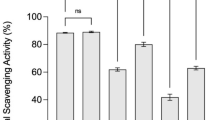
Photomicrographs of renal tissue and hepatic tissue in control and experimental treated mice. kidney and liver sections stained with Masson’s trichrome and Sirius red stain (G × 200) (A) and quantified by Image J (B). Control: (salin); Tox: (salin and CCl4) received normal salin (10 ml/kgbw) each Meanwhile; A: (NutriBio A, and CCl4); B: (NutriBio B and CCl4); and C: (NutriBio C and CCl4), groups were administered with 5 mg/kg bw of NurtiBio A, NutriBio B and NutriBio C, respectively
In addition, histopathological studies of the glomeruli and tubules in the kidney sections of the control (Fig. 3 control) and FPBs groups (Fig. 3A–C) did not show any abnormalities, which confirmed the results obtained for the serum parameters of nephron functioning, MDA levels, and the antioxidant enzymes SOD, CAT and GPx activities. However, CCl4-treated mice showed visible pathological changes, including glomerular atrophy, degenerated tubular structure, leukocyte infiltration, and numerous tubular casts (Fig. 2. Tox). These renal lesions were protected by treatment with fermented beverages (Fig. 2A–C).
Discussion
The fermentation process induced notable changes in the pH values and acidity of the samples. The decrease in pH and increase in titratable acidity were attributed to the generation of various organic acids during fermentation, including lactic, acetic, formic, and malic acids [40]. The results presented in Table 2, showed that the pH levels dropped significantly, reflecting the profound impact of fermentation on the chemical nature of the beverages. Additionally, the concentration of sugars in the samples decreased, leading to the development of a medium rich in hydrolyzed carbohydrates. This transformation suggests that the fermentation process enhances the potential of the beverages as a valuable fermentation medium [40].
Polyphenols constitute one of the most abundant groups of substances in plants, including a wide variety of bioactive molecules that contain at least one aromatic ring with one or more hydroxyl groups in addition to other substituents exhibiting large spectra of biological activities. During fermentation, many changes of composition occur, leading to a modified ratio of nutrients and antinutrients and therefore the properties of the product, such as bioactivity and digestibility are modified [4]. Lactic fermentation of plants has been shown to increase the concentration of several phenolic compounds [41]. It was shown that the three fermented beverages exhibited high content of polyphenols around 242.3 ± 12.4 µg GAE/mL. Also, the NutrBio B possess the high content of polyphenol, flavonoids and tannins to compare with the other fermented beverages. The FPBs exhibited a high content of polyphenolic compounds, with NutrBio B showing the highest levels of flavonoids and tannins. These compounds have potential biological activities and might contribute to the health benefits of the beverages. NutrBio B, in particular, stood out as having a rich composition of phenolic compounds, suggesting its potential as a source of bioactive compounds for exploring various health-related applications.
For example, there are aminobutyric acid, polyphenols, and flavonoids in fermented pepper leaves beverage prepared by [42]. Other investigations tried to use combined techniques to enhance the polyphenols contents in FPBs and FPEs. Furthermore, other compounds may be found in FPE including caffeic acid, in fermented dandelion beverage [43]. In addition, Among the volatile compounds, alcohols are regarded as the predominant compounds in the aromatic profile of FPBs and are a common terminal end product in the degradation of sugar and catabolism of amino acids. Volatile ester compounds are generated by the esterification of free acids with alcohol [44]. The mixed-culture fermentation resulted in the production of a range of metabolites, including organic acids, peptides, amino acids, ketones, and esters, which could contribute positively to the sensory attributes of the beverages. The study evaluated the antioxidant activity of the FPBs using various assays. NutrBio B exhibited the highest ability to quench DPPH and ABTS free radicals, indicating strong antioxidant potential. The Ferric Reducing Power Assay (FRPA) results also demonstrated the antioxidant capacity of the fermented beverages. The high antioxidant activity was correlated with the content of phenolic compounds, flavonoids, and condensed tannins, as well as the presence of volatile compounds. These findings suggest that the FPBs could be valuable sources of antioxidants, contributing to overall health and disease prevention.
Nowadays, it’s well known that the nutritional value of phenolic compounds is strongly related to their antioxidant activity or their ability to counteract oxidative stress, which is expressed by excessive production of ROS. Metabolization and depolymerization of phenolic compounds correlated with an increase in antioxidant activity that has been observed during lactic acid fermentation of plants [33]. These correlations have been described by many authors [45, 46].
Acute toxicity testing revealed that the FPBs did not induce toxic effects in mice even at relatively high doses. This suggests a potential GRAS status for the beverages. Further toxicological investigations are recommended for comprehensive safety assessment. The xenobiotic such as the CCl4 present a high dangers effect on human health, it’s well known that it can induce the hepatotoxicity and nephrotoxicity [47,48,49]. These processes of detoxification revealed by the alteration in liver biomarker enzymes including AST, and ALT [34]. Similar to glucuronic acid, malic acid is also a byproduct of the fermentation which helps in detoxifying the liver [50], Moreover supporting the detoxification of the liver and kidney, the consumption of fermented plants beverages also known to help excrete heavy xenobiotic substances and environmental pollutants from the human body through the kidneys [51]. Several authors have successfully determined that CCl4 may induce hepatic cell destruction and cause an increase in serum enzyme levels [52]. The results showed that the NutrBio B exhibited the highest protective effect compared to NutrBio A and NutrBio C. CCL4-induced kidney damage is characterized by increase in serum parameter (creatinine), due to tubular necrosis which can induce a renal failure [53]. The results suggested that the fermented beverages possessing high antioxidant activities can enhance the nephron and hepato protective effect of mice treated with CCl4, which may stabilize the serum parameter [54]. Moreover, CCl4, induced hepatotoxicity, and nephrotoxicity have been largely used by experimental model for liver and kidney damage [55]. The active metabolites of CCl4 can induce hepatic cell destruction, which in turn enhance serum enzymes, such as AST and ALT [56]. In this study the fermented beverages pretreatment significantly reduced the increase of activities in serum parameter such as AST, ALT, and ALP, and creatinine due to CCl4 administration. This implies a potential hepatoprotective and nephroprotective effect of fermented beverages by a possible stabilization of serum membrane mutually with a repair of hepatic tissue damaged induced by CCl4, as has been suggested by other authors [57]. Fermented beverages consumption has been demonstrated to inhibit the activity of CCl4 and prevent liver injury in rats [53]. In vivo studies have suggested that Kombucha tea is capable of preventing paracetamol induced hepatotoxicity [54]. The fermented plants beverages were known to help in obtaining relief from gout, rheumatism, arthritis, and kidney stones which are conditions associated with the accumulation of toxic substances in the body [58].
The cells are protected against the excess of ROS by antioxidant enzymes (e.g., catalase, glutathione peroxidase (GPx), superoxide dismutase (SOD), and other antioxidants). However, when ROS overwhelms antioxidant capacity, the cell functions are affected by this imbalance [49]. The free radicals and ROS have the ability to start multiple chain reactions which will eventually lead to cell damage or the death of the affected cell [55]. During the fermentation process, many compounds with radical scavenging properties are released from the plant’s raw material [59]. Polyphenols and flavonoids are the main group of compounds which are found in plants belonging to flavanol group [59]. Polyphenols are considered as having high levels of broad antioxidant capacity since they have the ability to scavenge free radicals and ROS [60]. Therefore, during the fermentation process, the total phenolic content increases [9, 60], the production of compounds possessing radical scavenging properties depends on the metabolites produced [61]. This suggests that the fermented functional beverages presented nephroprotective and hepatoprotective properties due to their antioxidant capacity, which is in accord with previous results [62].
However, CCl4-treated mice showed visible pathological changes, including glomerular atrophy, degenerated tubular structure, leukocyte infiltration and numerous tubular casts Fig. 3 Studies have also been carried out to find protective effects of fermented plants beverages against CCL4-induced hepatotoxicity and the results have shown that the antioxidant activity of polyphenol substances of fermented beverages is responsible for this function [33]. These studies have further explained that a functional beverage prevents the apoptotic cell death of the hepatocytes which is triggered from the exposure of the liver to the environmental toxins [33]. These renal lesions were protected by treatment with fermented beverages. The kidney and liver lesions confirm their Hepatoprotective and Nephroprotective effect.
The limitation of this study lies in the influence of various factors on the fermentation process, including plant materials, microbial diversity, and fermentation duration. These factors might lead to variations in the composition and bioactivity of FPBs, which could affect their consistency and reproducibility. Notably, the results showed that the three beverages exhibited high content of bioactive metabolites and high antioxidant and biological activity. In fact, these works need to be done in the future by multiple analyses in biochemistry, microbiology, animal model, and even clinical studies for determination other benefits effect on wellbeing of FPBs.
Conclusion
In this work, it was demonstrated for the first time, that the antioxidant, hepatoprotective, nephroprotective properties and the bioactive composition of the three fermented plants beverages. The physiochemical parameter showed that the pH, reducing sugar, and total sugar decreased after fermentation and creased of titratable acidity in three fermented beverages. On the other hand, the three fermented beverages revealed an enhanced level of polyphenols, flavonoids and tannins, especially, NutrBio B. In addition, the GC–MS analyses of the three fermented plants beverages showed thirty-one interesting volatile compounds, which could give pleasant-positive aroma attributes to the flavor of the beverages making fermented aromatic and medicinal plants more acceptable to consumers. The highest antioxidant activity was observed in the NutrBio B. The three fermented plants beverages have the potential to become a functional food with both strong antioxidant activity and good flavor.
In addition, the three fermented plant beverages (FPBs) possess high hepatoprotective, and nephroprotective, effects against CCl4. They can restore the rates of biochemical indicators, antioxidant, lipid peroxidation, and enzyme activities to almost their normal values and prevent kidney and liver lesions.
Accordingly, these results indicate that this innovative approach, based on fermentation process of aromatic and medicinal plants is an innovative and interesting process which can be used to enhance and ameliorate secondary metabolites production and extraction. Indeed, fermentation appears to be able to increase the contents of bioactive compounds in those beverages, thus improving their biological activities which exhibited an excellent protective effect and may be considered as a useful source of cellular defense agent in liver and kidney tissues against CCL4.
Data availability
All data generated or analyzed during this study are included in this published article and all related data and information are available from the corresponding author on reasonable request.
References
Petrova P, Petrov K (2020) Lactic acid fermentation of cereals and pseudocereals: ancient nutritional biotechnologies with modern applications. Nutrients 12:1118. https://doi.org/10.3390/nu12041118
Wang Q, Fu H, Zhang G et al (2023) Efficient chain elongation synthesis of n-caproate from shunting fermentation of food waste. Bioresour Technol 370:128569. https://doi.org/10.1016/j.biortech.2022.128569
Tamang JP, Cotter PD, Endo A et al (2020) Fermented foods in a global age: east meets West. Compr Rev Food Sci Food Saf 19:184–217. https://doi.org/10.1111/1541-4337.12520
Barba FJ, Brianceau S, Turk M et al (2015) Effect of alternative physical treatments (ultrasounds, pulsed electric fields, and high-voltage electrical discharges) on selective recovery of bio-compounds from fermented grape pomace. Food Bioprocess Technol 8:1139–1148. https://doi.org/10.1007/s11947-015-1482-3
Szopa A, Klimek-Szczykutowicz M, Kokotkiewicz A et al (2019) Phenolic acid and flavonoid production in agar, agitated and bioreactor-grown microshoot cultures of schisandra chinensis cv. Sadova No. 1—a valuable medicinal plant. J Biotechnol 305:61–70. https://doi.org/10.1016/j.jbiotec.2019.08.021
Das D, Sarkar S, Borsingh Wann S et al (2022) Current perspectives on the anti-inflammatory potential of fermented soy foods. Food Res Int 152:110922. https://doi.org/10.1016/j.foodres.2021.110922
Fentie EG, Jeong M, Emire SA et al (2022) Physicochemical properties, antioxidant activities and microbial communities of Ethiopian honey wine. Tej Food Res Int 152:110765. https://doi.org/10.1016/j.foodres.2021.110765
Rupprecht L, Rojas EM, Glass S, Garbe LA (2017) Fructans juice as fermentable raw material to improve a beverage with prebiotic and probiotic properties. J Biotechnol 256:S28. https://doi.org/10.1016/j.jbiotec.2017.06.646
Gadhoumi H, Gullo M, De Vero L et al (2021) Design of a new fermented beverage from medicinal plants and organic sugarcane molasses via lactic fermentation. Appl Sci 11:6089. https://doi.org/10.3390/app11136089
Fessard A, Kapoor A, Patche J et al (2017) Lactic fermentation as an efficient tool to enhance the antioxidant activity of tropical fruit juices and teas. Microorganisms 5:23. https://doi.org/10.3390/microorganisms5020023
Cuadrado C, Hajos G, Burbano C et al (2002) Effect of natural fermentation on the lectin of lentils measured by immunological methods. Food Agric Immunol 14:41–49. https://doi.org/10.1080/09540100220137655
Nkhata SG, Ayua E, Kamau EH, Shingiro J-B (2018) Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci Nutr 6:2446–2458. https://doi.org/10.1002/fsn3.846
Jang CH, Oh J, Lim JS et al (2021) Fermented soy products: beneficial potential in neurodegenerative diseases. Foods 10:636. https://doi.org/10.3390/foods10030636
Zhu Y-Y, Thakur K, Feng J-Y et al (2020) Riboflavin-overproducing lactobacilli for the enrichment of fermented soymilk: insights into improved nutritional and functional attributes. Appl Microbiol Biotechnol 104:5759–5772. https://doi.org/10.1007/s00253-020-10649-1
An X, Wang Z, Li J et al (2022) Analysis of flavor-related compounds in fermented persimmon beverages stored at different temperatures. LWT 163:113524. https://doi.org/10.1016/j.lwt.2022.113524
Zheng X, Wu F, Hong Y et al (2018) Developments in taste-masking techniques for traditional chinese medicines. Pharmaceutics 10:157. https://doi.org/10.3390/pharmaceutics10030157
Zhu W, Jiang B, Zhong F et al (2021) Effect of microbial fermentation on the fishy-odor compounds in kelp (laminaria japonica). Foods 10:2532. https://doi.org/10.3390/foods10112532
Yan X-T, Zhang Z, Wang Y et al (2023) Antioxidant capacity, flavor and physicochemical properties of FH06 functional beverage fermented by lactic acid bacteria: a promising method to improve antioxidant activity and flavor of plant functional beverage. Appl Biol Chem 66:7. https://doi.org/10.1186/s13765-022-00762-2
Gulcin İ (2020) Antioxidants and antioxidant methods: an updated overview. Arch Toxicol 94:651–715. https://doi.org/10.1007/s00204-020-02689-3
Daud S, Abid O-R, Sardar A et al (2022) Design, synthesis, in vitro evaluation, and docking studies on ibuprofen derived 1,3,4-oxadiazole derivatives as dual α-glucosidase and urease inhibitors. Med Chem Res. https://doi.org/10.1007/s00044-021-02814-6
Tonolo F, Moretto L, Folda A et al (2019) Antioxidant properties of fermented soy during shelf life. Plant Foods Hum Nutr 74:287–292. https://doi.org/10.1007/s11130-019-00738-6
AL Zahrani AJ, Shori AB (2023) Viability of probiotics and antioxidant activity of soy and almond milk fermented with selected strains of probiotic Lactobacillus spp. LWT 176:114531. https://doi.org/10.1016/j.lwt.2023.114531
Voss GB, Monteiro MJP, Jauregi P et al (2021) Functional characterisation and sensory evaluation of a novel synbiotic okara beverage. Food Chem 340:127793. https://doi.org/10.1016/j.foodchem.2020.127793
Bonciani T, De Vero L, Giannuzzi E et al (2018) Qualitative and quantitative screening of the β -glucosidase activity in Saccharomyces cerevisiae and Saccharomyces uvarum strains isolated from refrigerated must. Lett Appl Microbiol 67:72–78. https://doi.org/10.1111/lam.12891
Rul F, Béra-Maillet C, Champomier-Vergès MC et al (2022) Underlying evidence for the health benefits of fermented foods in humans. Food Funct 13:4804–4824. https://doi.org/10.1039/D1FO03989J
Horlacher N, Oey I, Agyei D (2023) Learning from tradition: health-promoting potential of traditional lactic acid fermentation to drive innovation in fermented plant-based dairy alternatives. Fermentation 9:452. https://doi.org/10.3390/fermentation9050452
Koriem KMM, Arbid MS, Asaad GF (2013) Chelidonium majus leaves methanol extract and its chelidonine alkaloid ingredient reduce cadmium-induced nephrotoxicity in rats. J Nat Med 67:159–167. https://doi.org/10.1007/s11418-012-0667-6
Yao H-T, Luo M-N, Li C-C (2015) Chitosan oligosaccharides reduce acetaminophen-induced hepatotoxicity by suppressing CYP-mediated bioactivation. J Funct Foods 12:262–270. https://doi.org/10.1016/j.jff.2014.11.014
Wannes WA, Tounsi MS (2022) Tunisian nephroprotective plants: a review. J Explor Res Pharmacol. https://doi.org/10.14218/JERP.2022.00031
Aruoma OI, Deiana M, Rosa A et al (2002) Assessment of the ability of the antioxidant cocktail-derived from fermentation of plants with effective microorganisms (EM-X) to modulate oxidative damage in the kidney and liver of rats in vivo: studies upon the profile of poly- and mono-unsaturated fatty acids. Toxicol Lett 135:209–217. https://doi.org/10.1016/S0378-4274(02)00261-8
Mercado-Pacheco J, Julio-Altamiranda Y, Sánchez-Tuirán E et al (2020) Variables affecting delignification of corn wastes using urea for total reducing sugars production. ACS Omega 5:12196–12201. https://doi.org/10.1021/acsomega.0c00645
Hayouni E, Abedrabba M, Bouix M, Hamdi M (2007) The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian quercus coccifera l. and juniperus phoenicea l. fruit extracts. Food Chem 105:1126–1134. https://doi.org/10.1016/j.foodchem.2007.02.010
Dewanto V, Wu X, Adom KK, Liu RH (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50:3010–3014. https://doi.org/10.1021/jf0115589
Tlili N, Feriani A, Saadoui E et al (2017) Capparis spinosa leaves extract: Source of bioantioxidants with nephroprotective and hepatoprotective effects. Biomed Pharmacother 87:171–179. https://doi.org/10.1016/j.biopha.2016.12.052
Tian H, Shen Y, Yu H et al (2017) Effects of 4 probiotic strains in coculture with traditional starters on the flavor profile of yogurt: role of probiotics on yogurt flavor…. J Food Sci 82:1693–1701. https://doi.org/10.1111/1750-3841.13779
Shi X, Chen F, Xu Y et al (2015) Aromatic components produced by non- Saccharomyces Cerevisiae derived from natural fermentation of grape. Nat Prod Res 29:1870–1873. https://doi.org/10.1080/14786419.2015.1008475
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin e. Anal Biochem 269:337–341. https://doi.org/10.1006/abio.1999.4019
Tipoe GL, Leung TM, Liong EC et al (2010) Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology 273:45–52. https://doi.org/10.1016/j.tox.2010.04.014
OliverH L, NiraJ R, Farr AL, RoseJ R (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275. https://doi.org/10.1016/S0021-9258(19)52451-6
Sõukand R, Pieroni A, Biró M et al (2015) An ethnobotanical perspective on traditional fermented plant foods and beverages in Eastern Europe. J Ethnopharmacol 170:284–296. https://doi.org/10.1016/j.jep.2015.05.018
Mantzourani I, Terpou A, Bekatorou A et al (2020) Functional pomegranate beverage production by fermentation with a novel synbiotic L. paracasei biocatalyst. Food Chem 308:125658. https://doi.org/10.1016/j.foodchem.2019.125658
Song Y-R, Shin N-S, Baik S-H (2014) Physicochemical and functional characteristics of a novel fermented pepper (Capsiccum annuum L.) leaves-based beverage using lactic acid bacteria. Food Sci Biotechnol 23:187–194. https://doi.org/10.1007/s10068-014-0025-4
Brianceau S, Turk M, Vitrac X, Vorobiev E (2015) Combined densification and pulsed electric field treatment for selective polyphenols recovery from fermented grape pomace. Innov Food Sci Emerg Technol 29:2–8. https://doi.org/10.1016/j.ifset.2014.07.010
Urbonaviciene D, Viskelis P, Bartkiene E et al (2015) The use of lactic acid bacteria in the fermentation of fruits and vegetables—technological and functional properties. In: Ekinci D (ed) Biotechnology. InTech, Rijeka
Mezzetti F, Fay JC, Giudici P, De Vero L (2017) Genetic variation and expression changes associated with molybdate resistance from a glutathione producing wine strain of Saccharomyces cerevisiae. PLoS ONE 12:e0180814. https://doi.org/10.1371/journal.pone.0180814
Uchida M, Kurushima H, Ishihara K et al (2017) Characterization of fermented seaweed sauce prepared from nori (Pyropia yezoensis). J Biosci Bioeng 123:327–332. https://doi.org/10.1016/j.jbiosc.2016.10.003
Rita R-D, Zanda K, Daina K, Dalija S (2011) Composition of aroma compounds in fermented apple juice: effect of apple variety, fermentation temperature and inoculated yeast concentration. Procedia Food Sci 1:1709–1716. https://doi.org/10.1016/j.profoo.2011.09.252
Yoshioka H, Tanaka M, Fujii H, Nonogaki T (2016) Sasa veitchii extract suppresses carbon tetrachloride-induced hepato- and nephrotoxicity in mice. Environ Health Prev Med 21:554–562. https://doi.org/10.1007/s12199-016-0581-8
Mbarki S, Alimi H, Bouzenna H et al (2017) Phytochemical study and protective effect of Trigonella foenum graecum (Fenugreek seeds) against carbon tetrachloride-induced toxicity in liver and kidney of male rat. Biomed Pharmacother 88:19–26. https://doi.org/10.1016/j.biopha.2016.12.078
Li S, Khafipour E, Krause DO et al (2012) Effects of subacute ruminal acidosis challenges on fermentation and endotoxins in the rumen and hindgut of dairy cows. J Dairy Sci 95:294–303. https://doi.org/10.3168/jds.2011-4447
Mathew S, Abraham TE (2006) In vitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food Chem Toxicol 44:198–206. https://doi.org/10.1016/j.fct.2005.06.013
Naczk M, Shahidi F (2006) Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J Pharm Biomed Anal 41:1523–1542. https://doi.org/10.1016/j.jpba.2006.04.002
Miller RP, Tadagavadi RK, Ramesh G, Reeves WB (2010) Mechanisms of cisplatin nephrotoxicity. Toxins 2:2490–2518. https://doi.org/10.3390/toxins2112490
Katanić J, Mihailović V, Matić S et al (2015) The ameliorating effect of Filipendula hexapetala extracts on hepatorenal toxicity of cisplatin. J Funct Foods 18:198–212. https://doi.org/10.1016/j.jff.2015.07.004
Kuriakose GC, Kurup MG (2011) Antioxidant and antihepatotoxic effect of spirulina laxissima against carbon tetrachloride induced hepatotoxicity in rats. Food Funct 2:190. https://doi.org/10.1039/c0fo00163e
Jayakumar T, Ramesh E, Geraldine P (2006) Antioxidant activity of the oyster mushroom, pleurotus ostreatus, on CCl4-induced liver injury in rats. Food Chem Toxicol 44:1989–1996. https://doi.org/10.1016/j.fct.2006.06.025
Ravikumar S, Gnanadesigan M (2011) Hepatoprotective and antioxidant activity of a mangrove plant Lumnitzera racemosa. Asian Pac J Trop Biomed 1:348–352. https://doi.org/10.1016/S2221-1691(11)60078-6
Rice-Evans C, Miller N, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2:152–159. https://doi.org/10.1016/S1360-1385(97)01018-2
Gadhoumi H, Hayouni ELA, Martinez-Rojas E et al (2022) Biochemical composition, antimicrobial and antifungal activities assessment of the fermented medicinal plants extract using lactic acid bacteria. Arch Microbiol 204:374. https://doi.org/10.1007/s00203-022-02985-9
Luengo E, Álvarez I, Raso J (2013) Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov Food Sci Emerg Technol 17:79–84. https://doi.org/10.1016/j.ifset.2012.10.005
Prakash Maran J, Sivakumar V, Thirugnanasambandham K, Sridhar R (2013) Optimization of microwave assisted extraction of pectin from orange peel. Carbohydr Polym 97:703–709. https://doi.org/10.1016/j.carbpol.2013.05.052
Rjeibi I, Feriani A, Ben Saad A et al (2017) Phytochemical characterization and bioactivity of Lycium europaeum: a focus on antioxidant, antinociceptive, hepatoprotective and nephroprotective effects. Biomed Pharmacother 95:1441–1450. https://doi.org/10.1016/j.biopha.2017.09.035
Acknowledgements
We are grateful to the Honorable Vice Institute of Pasteur, Tunis, Tunisia. This study was supported by a grant from the Ministry of Tunisia, Laboratory of Aromatic and Medicinal Plants, Center of Biotechnology (LR15CBBC06) at the Ecopark of Borj-cédria. BP-901, 2050 Hammam-Lif. Tunisia.Authors are very grateful to Dr. Saito Ari Directo: Sanko Sangyo Co., Okinawa, Japan for kindly providing saion-EM inocula.
Funding
This study was supported by University of Tunis EL Manar, and Laboratory of Aromatic and Medicinal Plants, Center of Biotechnology (LR15CBBC06) of Borj-cédria. BP-901, 2050 Hammam-Lif. Tunisia and Experimental Commodities and Animal Care Service: Institute of Pasteur, Tunis, Tunisia.
Author information
Authors and Affiliations
Contributions
H. G conceptualization methodology, performed the majority of the experiments and revised the original manuscript. Z.D. assisted with data analysis and write the original manuscript, W.Y. validation, data curation. R. S. designed the study and performed the experiments. K. M. performed the animal experiment, M. T. performed the statistical data analyses, A. C. contributed to the design and analysed the manuscript, M. S. T. contributed to drafting the manuscript, validation, data curation, visualization, E.A.H contributed to drafting the manuscript. All authors have read and approved the final version of the submitted manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript certify that they have NO affiliation with or involvement in any organization or entity with any financial interest. I certify that I am submitting the manuscript on behalf of all the authors.
Ethical approval
This study obtained the ethical approval from the Bio-Medical Ethics Committee of the Pasteur Institute of Tunis, Tunisia 2019/2/I/ LR16IPT09/V2.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gadhoumi, H., Dhouafli, Z., Yeddes, W. et al. Biochemical Composition, Antioxidant Capacity and Protective Effects of Three Fermented Plants Beverages on Hepatotoxicity and Nephrotoxicity Induced by Carbon Tetrachloride in Mice. Indian J Microbiol 64, 229–243 (2024). https://doi.org/10.1007/s12088-023-01172-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-023-01172-8