Abstract
Integrative processes for the production of bioenergy and biopolymers are gaining importance in recent years as alternatives to fossil fuels and synthetic plastics. In the present study, Bacillus thuringiensis strain EGU45 has been used to generate hydrogen (H2), polyhydroxybutyrate (PHB) and new co-polymers (NP). Under batch culture conditions with 250 ml synthetic media, B. thuringiensis EGU45 produced up to 0.58 mol H2/mol of glucose. Effluent from the H2 production stage was incubated under shaking conditions leading to the production of PHB up to 95 mg/l along with NP of levulinic acid up to 190 mg/l. A twofold to fourfold enhancement in PHB and up to 1.5 fold increase in NP yields was observed on synthetic medium (mixture of M-9+GM-2 medium in 1:1 ratio) containing at 1–2 % glucose concentration. The novelty of this work lies in developing modified physiological conditions, which induce bacterial culture to produce NP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Two major issues related to fossil fuels are their polluting nature and rapid depletion of their reservoirs [1]. The need is to look for non-polluting fuels and curtail the use of fossil fuels. The potential to circumvent these problems lies in exploiting bacterial metabolic activities especially those related to generation of bioenergy and biopolymers [2, 3]. Quite a few research efforts have gone into identifying bacteria with abilities to metabolize biowastes and generate hydrogen (H2) and polyhydroxyalkanoates (PHA) as end products [4]. Biological H2 production has been recognized as a clean fuel of the future and bioplastics will help to reduce the consumption of fossil fuel based plastics. Production of H2 and polyhydroxybutyrate (PHB—a class of PHA) in separate reactors and different sets of bacteria from pure substrates such as carbohydrates is limited by the high cost of feed which in turn hinders their production on a large scale for commercial use [5]. Another limiting factor associated with bacterial production of PHB is their brittle nature [2, 6]. Here, the prime need is to look for co-polymers which can impart greater strength to biopolymer, utilize a single bacterium and integrate these two processes to generate both the products. Although these concepts have been recognized quite early, however, very limited research has been carried out [5, 7–9]. Biological H2 and PHB production abilities of bacteria have been reported through in silico techniques and through wet experiments as well [4, 5, 10, 11].
Bacillus spp. have been reported to produce both H2 and PHB from glucose and biowastes [5, 12, 13]. A few Bacillus spp. have also been shown to produce co-polymers of PHA when co-fed with various substrates. In these works, co-polymer production was reported only from glucose supplemented with respective precursors [14, 15]. In the present work, we have been able to utilize a single bacterium to generate H2, PHB and new co-polymers (NP).
Materials and Methods
Organisms and Growth Conditions
Bacillus thuringiensis EGU45 (DQ508971) isolated previously in our laboratory was selected for producing H2 and PHB [5, 12]. Corresponding rrs (16S rRNA) gene sequence can be viewed at http://www.ncbi.nlm.nih.gov/ [4]. B. thuringiensis was grown in Himedia nutrient broth (13.0 g/l distilled water: composed of (g/l): Peptic digestion of animal tissue 5.0, NaCl 5.0, Beef extract 1.5 and Yeast extract 1.5) and incubated at 37 °C at 200 rpm for 16–20 h.
Hydrogen Production
For batch-culture H2 production, 250 ml of different concentrations (0.5, 1.0 and 2.0 %) of glucose in medium of composition (x) in g/l: (i) minimal medium (M-9)—Na2HPO4, 6.0 g; KH2PO4, 3.0 g; NaCl, 0.5 g; NH4Cl, 1.0 g; MgSO4, 1.0 mM and CaCl2, 0.1 mM), (ii) Growth medium (GM-2)—Yeast extract, 1.0; K2HPO4, 1.0 and MgSO4·7H2O, 0.5) and (iii) synthetic media (combinations M-9+GM-2) were added in 300 ml BOD bottles [5]. These were inoculated with B. thuringiensis at the rate of 10.0 μg cell protein/ml of glucose solution. The pH of medium was adjusted to 7.0. The bottles with provision for gas outlet and liquid sampling were made air tight with a glass stopper. All the bottles were then flushed with argon and incubated at 37 °C. Each day, the pH of the solution was checked by opening the bottle and readjusted to 7.0 with 2.0 N NaOH or 2.0 N HCl. After replacing the glass stopper, the bottles were reflushed with argon. The evolved gases were collected by water displacement method and residual glucose was measured by DNSA method. The process of gas collection and analysis was continued until H2 evolution ceased [5]. The results of this component of the Table 1 have been categorized as Experiment 1. The values presented here are based on three replications.
Polyhydroxyalkanoate Production
The effluent from the H2 production stage was incubated under aerobic conditions for PHA production. PHA production was tested under three different set ups. In the first set up, the effluent from H2 production stage along with its bacterial biomass was utilized as such. For the other two set ups, effluent from H2 production stage was divided into two portions and each was centrifuged at 6,000 rpm at 4 °C for 20 min. For the second set up, bacterial biomass from one of the portions was mixed with supernatant from both the portions. For the third set up, the remaining bacterial biomass was mixed with fresh GM-2, supplemented with glucose (at a concentration similar to that in the initial H2 production stage). For batch culture PHA production, 200 ml of working volume was taken in 1.0 l conical flask. pH was set to 7.2 by 2.0 N NaOH or 2.0 N HCl and incubated at 37 °C in shaker at 200 rpm for 48 h. The results of this component of the Table 1 have been categorized as Experiments 2 and 3. The values presented here are based on three replications.
Gas Analysis
The gas composition was determined by gas chromatography (Nucon GC5765) using a thermal conductivity detector and argon as carrier gas at flow rate of 30 ml/min. Gas collection and analyses were done daily and H2 gas production was calculated from the head space measurement of gas composition and the total volume of biogas produced. Although no CH4 was expected to be evolved in the absence of any added methanogens at any stage, however, gas samples were also analyzed for its presence daily [5].
Protein Estimation
Actively growing cell cultures were centrifuged at 6,000 rpm at 4 °C for 20 min and protein content was estimated by Lowry’s method [16].
Polyhydroxyalkanoate Analysis
Samples (200 ml) were analyzed for cell dry weight (cdw) and PHA production. Aliquots (200 ml) were centrifuged at 6,000 rpm at 4 °C for 20 min. The pellet was washed with 10.0 ml saline solution (0.9 % NaCl) and re-centrifuged. The pellet was dried at 85 °C for 36 h and weighed to estimate cdw. About 40 mg of cdw was mixed with 2.0 ml chloroform, 2.0 ml propanol-hydrochloric acid solution [Propanol:hydrochloric acid (4:1) v/v] and 0.2 ml internal standard solution (40 g benzoic acid/l propanol) in a tightly sealed 25 ml test tube. The mixture was incubated in water bath at 100 °C for 2 h and then cooled to room temperature. The mixture was vortexed with 4.0 ml elix water. After the propanolysis of bacterial dry cell extract, the heavier phase containing chloroform solution with the esters of propanol and β-hydroxyl acids from PHA hydrolysis were analysed by gas chromatography (GC) using 10 % Reoplex 400 (length 30 m × OD 6.35 mm × ID 3 mm) with a mesh range of 80–100, dimethylpolysiloxane capillary column DB-1 (30 m × 0.25 mm × 0.25 μm) and flame ionization detector [5, 13]. The values for this new peak (NP) were calculated by taking 3-hydroxyvalerate peak in GC analysis as external standard. Thus, the values presented in table are only relative values and may not represent the exact amount.
GC–MS Analysis and Compound Identification
GC–MS analyses were carried out with Shimadzu 2010-MS Plus Gas-Chromatograph coupled with mass spectrophotometer (QP-2010) equipped with DB-5 (fused silica with 5 % phenylpolydimethylsiloxane) column (30 m × 0.32 mm × 0.25 μm). The carrier gas (Helium) inlet pressure was 84.8 kPa; the oven temperature was maintained at 80 °C for 5 min then programmed to 230 °C at 20 °C/min; flow controller: split, total flow 16.3 ml/min, column flow 1.21 ml/min, sampling time 0.5 min. The injected volume of each sample was 1.0 μl. Ionization energy for MS operation was 70 eV. The peaks were identified by comparing their fragmentation patterns with those of reference compounds in spectrum libraries Wiley08 and NIST08.
Results and Discussion
In our recent attempts to produce H2 and PHB from glucose by Bacillus spp., we realized that M-9 medium was suitable for H2 production, where as GM-2 was fit for PHB production [5]. In order to improve the PHB yields, we attempted to mix M-9 and GM-2 in different combinations. Since GM-2 is more suitable for PHB production, we varied the concentration of GM-2 and kept M-9 concentration at fixed level (Table 1).
Hydrogen Production
H2 production was found to be influenced by glucose concentration and medium composition. With M-9 (1×) in the medium, H2 production varied from 50 to 150 ml at 0.25× GM-2, 30–195 ml at 0.5× GM-2, 35–245 ml at 1× GM-2 and 30–140 ml at 2× GM-2. It indicates that for high H2 production a combination of M-9 and GM-2 at 1× in 1:1 ratio is the best. Here, the second factor which influenced H2 yields was glucose concentration. H2 yields were comparable at 1.0–2.0 % glucose concentration, where it varied slightly between 0.42 and 0.43 mol/mol glucose. In almost all cases, H2 content of the evolved biogas was quite high in the range of 51–69 % and exceptionally it was low at 38.5 % in the case of 1× M-9 + 0.25× GM-2 at 2.0 % glucose concentration (Table 1).
Polyhydroxybutyrate Production
As the main objective of this work is to improve the PHB yields, we attempted to check the influence of nutrients and other intermediates which may be present in the effluent generated during H2 evolution.
With Same Bacterial Biomass
PHB production on subjecting effluent from H2 production stage to shaking conditions was observed to vary from 10 to 95 mg/l. Incidentally, the mixture of M-9 and GM-2 media resulted in the production of a NP which was otherwise not observable in all our previous works, where we have using either M-9 or GM-2 alone and not as a mixture [4, 5]. Here, three scenarios were evident: (i) PHB and no NP, (ii) no PHB and only NP, and (iii) both PHB and NP production. At the highest PHB production level of 95 mg/l, no NP was recorded. On the other hand, at the highest NP production level of 195 mg/l, no PHB was observable. At lower levels of PHB in the range of 10–15 mg/l, NP was also produced at 40–130 mg/l. These values were more than the PHB (80–85 mg/l) recorded previously with either M-9 or GM-2 alone [5].
With Half Bacterial Biomass
The production of PHB and NP was dramatically improved when the available nutrition level was higher as a result of reducing the bacterial biomass to half. In these cases PHB level was generally higher (up to 275 mg/l) than that observed in previous combinations where bacterial biomass was maintained as such (see previous paragraph). The production of NP was also quite high, up to 90 mg/l in most of the cases. Here the best combination was observed in M-9+GM-2 (1× + 1×) containing 1.0 % glucose with 275 mg/l of PHB and 80 mg/l of NP.
Half Bacterial Biomass with Fresh GM-2
Compared to the previous two combinations where effluent from H2 production stage was used, here we used only the bacterial biomass in combination with fresh GM-2. The purpose of replacing effluent from the previous stage with GM-2 was to increase the availability of nutrients and provide optimum C:N ratio, which is suitable for PHB production [13]. In these cases, there was a dramatic increase in production of PHB and NP. Here, PHB yields were found to achieve a level of 375 mg/l and NP up to 300 mg/l. It was quite interesting to observe that once more M-9+GM-2 at 1×:1× concentration proved to be the best. At 1.0 % glucose concentration, 185 + 300 mg/l of PHB + NP was observed and at 2 % glucose concentration 375 + 190 mg/l of PHB + NP was recorded (Table 1). An almost similar scenario was observed also in M-9 + GM-2 combination at 1× + 2× with 1.0 % glucose concentration, which yielded 325 + 100 mg/l of PHB + NP.
Identification of New Co-polymer
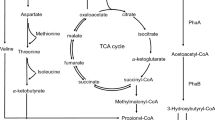
GC–MS analysis of this new monomer revealed that it is levulinic acid. Levulinic acid, also known as 4-oxopentanoic acid or 4-ketovaleric acid is a short chain fatty acid having a ketone carbonyl (CO) and an acidic carboxyl group (COOH) (Fig. 1).
GC–MS analysis of polyhydroxyalkanoate produced by Bacillus thuringiensis: i Chromatographs of standard and sample showing esters of (A) 3-Hydroxybutyric acid; (B) 3-Hydroxyvaleric acid; (C) Benzoic acid (Internal standard) and (D) 4-Oxo-pentanoic acid; and ii Mass spectra of peaks with major fragmentation patterns and corresponding structures (A–D)
In conclusion, it was observed that although M-9+GM-2 (1× + 0.5×) medium combination was better for H2 production but M-9+GM-2 (1× + 1×) medium combinations were working best for overall integrated approach for both H2 and PHA production. 1.0 % glucose concentration was found optimum for both H2 and PHA production and use of fresh medium with corresponding glucose promoted new co-polymer to a significant extent. Production of PHA co-polymer has been reported from various Bacillus strains, however, this usually require supplementation of precursor substrates [14, 15]. This is the first study of PHA co-polymer production with a new composition that too without supplementation of precursor sources.
Strategies to improve waste treatment processes aim at generating multiple products, each with its own commercial value. Initially, methane generation alone was found to be the only aspect of waste/wastewater treatment through anaerobic digestion. However, it was soon realized that to make the whole process economical, we need to derive other products as well such as H2 and PHB [1, 5]. Efforts to produce H2, PHB and methane in different stages from the same feed have been found to be feasible [13, 17–19]. Now the issue is to improve the quality of PHB, which is otherwise very brittle in nature. In order to circumvent this limitation of PHB, various studies have revealed that co-polymers are better materials in terms of their crystallinity, molding and ductility [2, 6, 20]. Previously, a few photosynthetic organisms: Rhodopseudomonas palustris, Rhodospirillum rubrum and Rhodobacter sphaeroides were the only organisms known to produce both the bioproducts [1, 4, 5]. Bacillus spp. have been shown to be unique in their abilities as they can produce both H2 and PHB from a wide range of substrates such as glucose and biowastes [4, 5, 12, 13, 17, 21]. A few of these Bacillus spp. have potential to produce co-polymers of PHA. In most of the studies, the feed material needed a supplementation with precursor to produce a co-polymer along with hydroxybutyrate (3HB) e.g. valerate (3HV), 3-hydroxyhexanoate (3HHx), 6-hydroxyhexanoate (6HHx) or caprolactone were added to produce P(3HB-co-3HV), P(3HB-co-3HHx), P(3HB-co-6HHx) or P(3HB-co-6HHx-co-3HHx), respectively [14]. In the present work, we have been able to utilize a single bacterium to generate H2, PHB and NP.
Although, we have demonstrated H2 and PHB production as one of the value addition processes to the overall degradation process, however, the emphasis of the present study has been on NP production. It may be remarked here that in all our previous studies, we did not observe co-polymer production from glucose as the starting material. Here, we have been primarily targeting to enhance the yield of PHB [12, 13]. In our previous works, we had observed that on M-9 medium, H2 yield was consistent at different concentration (0.5–2.0 %) of glucose but PHB yield was low. On the other hand, on GM-2 medium, H2 yield was poor at higher concentration (2.0 %) of glucose but PHB yield was high [5]. Since we intended to produce H2 followed by PHB, we combined the two media i.e., M-9 and GM-2. However, we could observe that keeping concentration of M-9 constant and varying GM-2 resulted in almost similar H2 yields at each of the glucose concentrations tested in the present study e.g. at 0.5 % glucose it was in the range of 0.22 to 0.36 mol/mol glucose; at 1.0 % glucose it varied from 0.43 to 0.58 mol/mol glucose and at 2.0 % glucose it was between 0.26 and 0.42 mol/mol glucose. Among these 1.0 % glucose proved to be the best (Table 1, Experiment 1). The effluent from the H2 production stage resulted in poor PHB yields. At this stage, we varied two factors: (i) reduced the bacterial biomass to half such that the available nutrient level goes up and (ii) reduced the bacterial biomass but completely replaced the effluent from H2 stage with fresh GM-2 which thus further increased the availability of nutrients and also provided a C:N ratio which is suitable for PHB production [4, 12]. As a consequence of reducing the bacterial biomass to half, we did not observe any significant increase in PHB in comparison to the previous situation, where the bacterial biomass present in effluent of H2 production stage was exposed to PHB production conditions. On the other hand, fresh GM-2 proved to be significantly better in terms of enhanced PHB and NP yields.
Although production of a wide range of co-polymers of PHA by Bacillus has been reported from pure sugars and biowaste materials [14, 15, 22, 23]. The detection of levulinic acid without any supplement in the feed (glucose) is a new observation. The levulinic acid component in the PHA co-polymer produced by B. thuringiensis strain EGU45 is likely to impart strength to the PHA [2]. The novelty of this work is that this co-polymer was not generated if we did not introduce the innovative steps of modifying the physiological conditions, which arise due to (i) exposure of the bacterial culture to mixture of two synthetic media (M-9+GM-2) and (ii) subjecting the cultures to H2 production conditions, such that it allowed Bacillus to express its co-polymer producing ability under defined physiological conditions. This innovative strategy has not been reported previously. In addition to the NP observed in combinations of GM-2 and M-9 medium, another feature which demands attention is enhancement in bacterial growth. Here we observed DCM to be as high as 3,000 mg/l in comparison to 1,050–1,190 mg/l on either GM-2 or M-9 [5]. It showed a further 1.6-fold enhancement (4,800 mg/l) when fresh GM-2 medium was used (Table 1, Experiment 3). It thus provides an opportunity to further enhance the PHB and NP yields.
PHB is brittle in nature and has low extension to break as compared to co-polymers, which have lower melting point, less crystalline, more ductile and easier to mold. The interest in application of PHAs in medicine, pharmacology, agricultural, food and cosmetic industries has been primarily due to their biodegradability and biocompatibility. Among the various applications, the most attractive is their use in tissue engineering and drug delivery systems. In agriculture, their application as carriers for slow and controlled release of insecticides and pesticides has large commercial potential. In food industries it has been used as flavor delivering agent and dairy cream substitutes. In case of cosmetic industries it has been used as manufacturing of disposable articles such as shampoo bottles and cosmetic materials [2, 20]. Bacillus has some unique features, which make it a strong candidate for use in this integrated approach, such as survival under both aerobic and anaerobic conditions [24]. Their ability to undergo sporulation, antibiotics production and quorum quenching enzymes, allow them to compete well their competitors [25, 26]. We can take advantage of these properties of Bacillus spp. especially while treating unsterilized biowastes, which invariably are accompanied by a large number of bacteria of diverse origins.
References
Kalia VC, Purohit HJ (2008) Microbial diversity and genomics in aid of bioenergy. J Ind Microbiol Biotechnol 35:403–419
Singh M, Patel SKS, Kalia VC (2009) Bacillus subtilis as potential producer for polyhydroxyalkanoates. Microb Cell Factories 8:38
Hallenbeck PC, Ghosh D, Skonieczny MT, Yargeau V (2009) Microbiological and engineering aspects of biohydrogen production. Indian J Microbiol 49:48–59
Porwal S, Kumar T, Lal S, Rani A, Kumar S, Cheema S, Purohit HJ, Sharma R, Patel SKS, Kalia VC (2008) Hydrogen and polyhydroxybutyrate producing abilities of microbes from diverse habitats by dark fermentative process. Bioresour Technol 99:5444–5451
Patel SKS, Singh M, Kalia VC (2011) Hydrogen and polyhydroxybutyrate producing abilities of Bacillus spp. from glucose in two stage system. Indian J Microbiol 51:418–423
Reddy CSK, Ghai R, Rashmi, Kalia VC (2003) Polyhydroxyalkanoates: an overview. Bioresour Technol 87:137–146
Ntaikou I, Kourmentza C, Koutrouli EC, Stamatelatou K, Zampraka A, Kornaros M, Lyberatos G (2009) Exploitation of olive oil mill wastewater for combined hydrogen and biopolymer production. Bioresour Technol 100:3724–3730
Venkata Mohan S, Reddy MV, Subhash GV, Sarma PN (2010) Fermentative effluents from hydrogen producing bioreactor as substrate for poly(β-OH) butyrate production with simultaneous treatment: an integrated approach. Bioresour Technol 101:9382–9386
Yan Q, Zhao M, Miao H, Ruan W, Song R (2010) Coupling of the hydrogen and polyhydroxyalkanoates (PHA) production through anaerobic digestion from Taihu blue algae. Bioresour Technol 101:4508–4512
Kalia VC, Lal S, Ghai R, Mandal M, Chauhan A (2003) Mining genomic databases to identify novel hydrogen producers. Trends Biotechnol 21:152–156
Kalia VC, Chauhan A, Bhattacharyya G, Rashmi (2003) Genomic databases yield novel bioplastic producers. Nat Biotechnol 21:845–846
Kumar T, Singh M, Purohit HJ, Kalia VC (2009) Potential of Bacillus sp. to produce polyhydroxybutyrate from biowaste. J Appl Microbiol 106:2017–2023
Patel SKS, Singh M, Kumar P, Purohit HJ, Kalia VC (2012) Exploitation of defined bacterial cultures for production of hydrogen and polyhydroxybutyrate from pea-shells. Biomass Bioenergy 36:218–225
Tajima K, Igari T, Nishimura D, Nakamura M, Satoh Y, Munekata M (2003) Isolation and characterization of Bacillus sp. INT005 accumulating polyhydroxyalkanoate (PHA) from gas field soil. J Biosci Bioeng 95:77–81
Valappil SP, Rai R, Bucka C, Roy I (2008) Polyhydroxyalkanoate biosynthesis in Bacillus cereus SPV under varied limiting conditions and an insight into the biosynthetic genes involved. J Appl Microbiol 104:1624–1635
Patel SKS, Purohit HJ, Kalia VC (2010) Dark fermentative hydrogen production by defined mixed microbial cultures immobilized on ligno-cellulosic waste materials. Int J Hydrogen Energy 35:10674–10681
Kalia VC, Joshi AP (1995) Conversion of waste biomass (pea-shell) into hydrogen and methane through anaerobic digestion. Bioresour Technol 53:165–168
Patel SKS, Kumar P, Kalia VC (2012) Enhancing biological hydrogen production through complementary microbial metabolisms. Int J Hydrogen Energy 37:10590–10603
Patel SKS, Kalia VC (2012) Integrative biological hydrogen production: an overview. Indian J Microbiol. doi:10.1007/s12088-012-0287-6
Kalia VC, Raizada N, Sonakya V (2000) Bioplastics. J Sci Ind Res 59:433–445
Kalia VC, Jain SR, Kumar A, Joshi AP (1994) Fermentation of biowaste to hydrogen by Bacillus licheniformis. World J Microbiol Biotechnol 10:224–227
Labuzek S, Radecka I (2001) Biosynthesis of copolymers of PHB tercopolymer by Bacillus cereus UW85 strain. J Appl Microbiol 90:353–357
Mizuno K, Ohta A, Hyakutake M, Ichinomiya Y, Tsuge T (2010) Isolation of polyhydroxyalkanoate-producing bacteria from a polluted soil and characterization of the isolated strain Bacillus cereus YB-4. Polym Degrad Stab 95:1335–1339
Van der Voort M, Abee T (2009) Transcriptional regulation of metabolic pathways, alternative respiration and enterotoxin genes in anaerobic growth of Bacillus cereus ATCC 14579. J Appl Microbiol 107:795–804
Kalia VC, Purohit HJ (2011) Quenching the quorum sensing system: potential antibacterial drug targets. Crit Rev Microbiol 37:121–140
Kalia VC, Raju SC, Purohit HJ (2011) Genomic analysis reveals versatile organisms for quorum quenching enzymes: acyl-homoserine lactone-acylase and -lactonase. Open Microbiol J 5:1–13
Acknowledgments
The authors wish to thank Director of CSIR-Institute of Genomics and Integrative Biology and Department of Biotechnology, Government of India for providing the necessary funds, facilities and moral support. M. Singh and P. Kumar are thankful to UGC and CSIR for Senior Research Fellowships and S.K.S. Patel to CSIR for Research Associate Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, M., Kumar, P., Patel, S.K.S. et al. Production of Polyhydroxyalkanoate Co-polymer by Bacillus thuringiensis . Indian J Microbiol 53, 77–83 (2013). https://doi.org/10.1007/s12088-012-0294-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-012-0294-7