Abstract
Polyhydroxyalkanoates (PHAs) are biodegradable polyesters accumulated in a wide variety of microorganisms as intracellular carbon and energy storage compounds. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) is one of the most valuable biopolymers because of its superior mechanical properties. Here, we developed a bioprocess utilizing recombinant Bacillus megaterium strain for PHBV over-production from glucose, without any precursor addition. PHA production was performed in a controlled bioreactor by batch and fed-batch modes using wild-type B. megaterium and rec-B. megaterium cells overexpressing the native phaC gene. The effect of oxygen transfer rate on biomass formation and PHA accumulation was also investigated, under different dissolved oxygen levels. Structural and thermal properties of PHA were characterized by GC–FID, 1H‐NMR, TGA and DSC analyses. Significantly, the copolymer produced from glucose as the carbon source in rec-B. megaterium was composed of 58 mol% of 3‐hydroxyvalerate monomers. After 66 h, rec-B. megaterium cells in fed-batch fermentation with a pre-determined growth rate µ0 = 0.1 h−1 produced the highest CDW (7.7 g L−1) and PHA concentration (6.1 g L−1). Moreover, an exponential glucose feeding profile resulted in 2.2-fold increase in PHA yield compared to batch cultivation. Overall, this study paves the way to an enhanced biopolymer production process in B. megaterium cells, where the highest product yield on cell was obtained as YP/X = 0.8 g g−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyhydroxyalkanoates (PHAs) have been considered as an eco-friendly alternative to petroleum-based plastics owing to their biorenewability, biocompatibility and biodegradability. PHAs are biodegradable polymers, accumulated in wide variety of microorganisms, including Gram-negative bacteria, Gram-positive bacteria, cyanobacteria and archaea as intracellular cytoplasmic inclusions for carbon and energy storage material, under environmental and nutritional stress conditions (limited nitrogen, phosphorus or oxygen) and excess carbon source [1, 2]. PHAs can be classified as short-chain length (SCL) (C3-C5), medium-chain length (MCL) (C6-C14) and SCL–MCL groups (C3 to C6–C14) depending on the monomer structure. Poly (3-hydroxybutyrate) (PHB) and poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) are the most well-known polyesters of the SCL-PHA family. PHB is a highly crystalline and brittle biopolymer with limited processability [3]. Hence, there have been attempts to improve the processability of PHB by incorporation of 3-hydroxyvalerate (3HV) through fermentation using expensive prescursors, such as sodium valerate [4, 5] or propionic acid [6, 7], unrelated carbon sources [8,9,10,11] or via limited number of attempts in metabolic engineering of Escherichia coli [12,13,14], Haloferax mediterranei [15], Rhodospirillum rubrum [16] or Ralstonia eutropha [17]. On the other hand, B. megaterium is a natural producer of P(3HB-co-3HV) from unrelated carbon sources [8, 10, 18,19,20] with a yield of up to 16.6 mol% of 3HV fraction, and this fraction goes up to 33 mol% for B. thuringiensis [21].
The incorporation of 3HV unit into the PHB crystal structure leads to dramatic changes in material properties of synthesized PHBV copolymers, such as low melting temperature, low crystallinity and toughness [22]. PHBV synthesis occurs via three-step enzymatic process catalyzed by β-ketothiolase (PhaA), β-ketoacyl-CoA reductase (PhaB) and PHA synthase (PhaC) sequentially [23]. PhaC enzyme is responsible for polymerization of PHA polyesters [24].
Currently, the major drawback for PHA production at large scale is the high production cost and low product yields compared with petroleum-based plastics [25]. Therefore, efforts have also been carried out to reduce the production cost using inexpensive and renewable carbon sources, the development of genetically-engineered better PHA-synthesizing strains and more efficient and sustainable bioprocess strategies for higher productivities [26,27,28,29].
There are various factors, such as microorganism selection, growth medium, nitrogen sources, carbon-to-nitrogen ratio, temperature, pH and dissolved oxygen demand, to obtain a higher polymer yield and volumetric productivity [25]. Various fed-batch fermentation strategies have been successfully used for PHA production by B. megaterium strains to achieve high cell densities and high PHA productivity [30, 31]. On the other hand, to maximize volumetric PHA productivity, oxygen transfer rate (OTR) is an important factor in fed-batch cultivation due to high oxygen demand [32]. Various studies have reported the increase in SCL-PHA synthesis in several microorganisms from sugars at low DO conditions [33, 34].
This study aimed to develop an engineered Bacillus megaterium NRRL B-14308 strain by overexpressing the native phaC gene for PHA production from glucose. Furthermore, the engineered B. megaterium strain was evaluated for PHBV production in laboratory-scale bioreactors by batch and fed-batch modes and compared with the wild-type B. megaterium NRRL B-14308 strain. Generally, PHBV copolymers were biologically produced by feeding its petroleum-based precursors like propionate or valeric acid, resulting in limited actual production due to their high cost and toxicity. This study is significant for production of PHBV by B. megaterium strain with the highest 3HV content from structurally unrelated carbon sources, such as glucose, without a 3HV precursor addition. The influence of oxygen transfer rate on the biomass production and PHA synthesis in laboratory-scale bioreactors under different DO concentrations was also investigated.
Materials and methods
Bacterial strains and plasmids
All bacterial strains and constructed plasmids used in this study are listed in Table 1. For construction of the recombinant plasmid of pC-HIS1623hp-phaC, the PHA synthase gene (phaC) (2590 bp) of B. megaterium NRRL B-14308 strain (GenBank Accession No. KGJ86215.1) was amplified from genomic DNA by colony PCR method, using the specific primers phaC_F (ATGACTACTAGTAAGGAGGTGAATATACAATGGCAATTCCTTACG-TGCAAG) and phaC_R (ATGATCGCATGCTTAGTGATGGTGATGGTGA-TGAGAACCGCCTTTAGAGCGTTTTTCTAGC) (underlined sequences indicate SpeI for phaC_F and SphI for phaC_R, respectively).
The amplified phaC fragment was digested with SpeI and SphI followed by purification, ligation and transformation of Escherichia coli DH5α by CaCl2 method, with 100 µg mL−1 ampicillin in selective solid medium. Single colonies were picked and the purified plasmids were confirmed via restriction digestion and DNA sequence analysis. Molecular biology protocols used in this study were as outlined by Sambrook and Russell [35]. B. megaterium protoplasts were transformed by a modified minimal media protoplast transformation protocol [36], and the selected colony was stored in glycerol stocks at − 86 °C.
Culture medium and growth conditions
The recombinant B. megaterium were grown in lysogeny broth (LB) medium containing 10 g L−1 peptone, 5 g L−1 yeast extract and 10 g L−1 NaCl and selective antibiotics to maintain the plasmid stability. The bacterial culture was inoculated using a 1:100 inoculum ratio into 20 mL LB medium in 250 mL Erlenmeyer flasks. The cells were centrifuged and re-suspended in 50 mL Minimal Medium (MM) [20] and the flasks were incubated at 37 °C and 200 rpm for 66 h. After fermentation, the cells were harvested by centrifugation at 10,000×g for 10 min.
Recombinant protein analysis
Whole-cell lysates were used for recombinant protein analysis, where total protein concentration was determined in duplicates using Bradford assay with bovine serum albumin (BSA, Sigma-Aldrich) as standard. The samples were subjected to SDS-PAGE (12%) and Western Blotting analysis. Proteins were visualized with Coomassie Blue (Bio-Rad) staining by GelDoc EZ imaging system (Bio-Rad). Then, proteins were transblotted onto PVDF membranes, blocked with blocking reagent (Bio-Rad) at room temperature (RT), and probed with anti-His (1:1000, Abcam) followed by anti-rabbit-HRP (1:2500, Promega) antibodies in 1% BSA-PBST. The immunoreactive bands were visualized using Opti-4CN colorimetric kit (Bio-Rad) according to manufacturer’s instructions.
Bioreactor operation in batch and fed-batch modes
All bioreactor experiments were carried out in a 500 mL fully controlled bioreactor system (My-Control miniBio, Applikon Biotechnology, Delft, Netherlands) with a working volume of 300 mL. The Minimal Medium (MM) was used as initial fermentation medium. A glucose solution of 100 g L−1 was fed separately for fed-batch cultivations. The 500 mL bioreactor was assembled with a pH sensor, optical DO sensor, L-type gas sparger and 2 Rushton impellers. The temperature and pH were maintained at 37 °C and 7.0 ± 0.1, respectively. The pH was controlled by 1 M HCl and 1 M NaOH solutions. The initial agitation rate was set at 300 rpm. Three different dissolved oxygen values (10%, 20% and 30% DO) were evaluated in this study and adjusted via agitation and/or aeration rates.
Fed-batch cultivations were performed using wild-type and recombinant Bacillus megaterium strains. The culture was initially carried out in a batch mode for 42 h and then fed-batch mode was initiated with exponential glucose feeding at a pre-determined specific growth rate (µ0) to achieve a higher PHA yields. During the exponential glucose feeding stage, feeding profile, F(t), was controlled based on the following equation [37]:
where F(t) (L h−1) is the total substrate feeding rate, µo (h−1) is the desired specific growth rate, V0 (L) is the initial volume, CX0 (g L−1) is the initial biomass concentration, CS0 (g L−1) is the feed substrate concentration and YX/S (g g−1) is the biomass yield on substrate. In the fed-batch phase, YX/S for the feeding profile equation was set as 0.36 g g−1, pre-determined from batch experiments. All runs were performed in duplicates.
Determination of oxygen transfer parameters
To determine the oxygen transfer parameters, such as volumetric mass transfer coefficient (kLa), biological enhancement factor (E), oxygen uptake rate (OUR), and oxygen transfer rate (OTR) for PHA production, the Dynamic method [38] was used.
Analytical procedures
Cell growth was analyzed by measuring the optical density at 600 nm (OD600) using a UV–visible spectrophotometer (Thermo Fisher Scientific Genesys 10S UV–VIS, USA). The cell dry weight was determined gravimetrically. The residual glucose concentration was determined spectrophotometrically using a modified microplate 3,5-dinitrosalicylic acid (DNS) assay [39]. PHA was measured spectrofluorometrically with fluorescence spectrophotometer (Varian Cary Eclipse, Agilent, Santa Clara, CA, USA) as given in our previous study [20].
PHA content and monomer determination
The PHA content quantification and monomer composition were determined by gas chromatography (GC) of methyl esters and verified by 1H-NMR. The lyophilized biomass was subjected to acidic methanolysis reaction [40]. The 3-hydroxyalkanoic acid methyl esters were analyzed by GC using an Agilent Technologies 6890 N chromatograph equipped with an HP-5 capillary column (25 m × 0.32 mm × 0.25 μm) and a flame ionization detector (FID). The temperatures of injection and detector were 300 °C. The oven temperature profile was: from 50 up to 100 °C at a rate of 5 °C min−1, then from 100 up to 300 °C at a rate of 20 °C min−1, and finally 300 °C for 5 min [41]. Helium was used as a carrier gas at a flow rate of 1 mL min−1. Methyl benzoate was used as an internal standard.
Polymer characterization
1H-NMR spectroscopy was used for determination of the chemical structure of PHA polymers. Briefly, about 20 mg of PHA sample was dissolved in deuterated chloroform and 1H-NMR spectra were recorded at 25 °C on Bruker (Billerica, MA, USA) 300 MHz spectrometer.
The thermal properties of PHA samples were analyzed by TGA (SII Exstar 6000 TG–DTA 6300, Perkin Elmer Inc, Waltham, MA, USA) and DSC (Diamond DSC, PerkinElmer Inc., Waltham, MA, USA). To determine the thermal degradation profile of the polymer, about 10 mg of PHA sample was heated from room temperature to 500 °C at a heating rate of 10 °C min−1 under nitrogen gas.
The melting temperatures (Tm) of the PHA polymers were determined by DSC analysis. Briefly, about 10 mg of PHA sample was encapsulated in an aluminum DSC pan and then heated from − 30 °C to 200 °C at a heating rate of 10 °C min−1 under nitrogen gas.
Statistical data analysis
Average and standard deviation values were calculated according to standard procedures and the results were analyzed by ANOVA test. Tukey’s test was used to compare mean values and to evaluate the significance of the differences between mean values to assess the PHA production. All statistical analyses were carried out using GraphPad Prism 8 software.
Results and discussion
The Gram-positive bacteria display the advantage of producing endotoxin-free PHAs, in contrast to Gram-negative organisms like E. coli. B. megaterium can grow on a wide variety of carbon sources and has no obvious alkaline proteases degrading recombinant gene products [42]. In the present study, the native PHA synthase genes of B. megaterium NRRL B-14308 were amplified by PCR and cloned into pC-HIS1623hp expression vector to construct the recombinant plasmid overexpressing its native PhaC enzyme, with the aim of enhancing PHA production. Successful construction of the recombinant plasmid pC-HIS1623hp-phaC was confirmed via DNA sequencing. The PhaC synthase was then overexpressed in the B. megaterium/pC-HIS1623hp-phaC system. SDS-PAGE analysis revealed the presence of the recombinant PhaC synthase gene with the expected molecular weight of 42 kDa (Fig. S1). The results were further confirmed by Western blot analysis using anti-His and anti-rabbit HRP antibodies and wild-type B. megaterium strain as the negative control.
The PHBV formation in the B. megaterium involves two parallel pathways, leading to C4 monomer (3-hydroxybutyrate) and C5 monomer (3-hydroxyvalerate) in the copolymer (Fig. 1). In the PHBV biosynthesis, first, two acetyl-CoA moieties or acetyl-CoA and propionyl-CoA are condensed to form thioester intermediates acetoacetyl-CoA and 3-ketovaleryl-CoA, respectively, by expressing phaA. Then, the thioester intermediates are reduced to two PHA monomers 3HB-CoA and 3HV-CoA by expressing phaB gene. Finally, by expressing phaC gene, the two PHA monomers are randomly polymerized to form PHBV copolymer [13, 43]. B. megaterium polyhydroxyalkanoate synthase gene complex is a heterodimer protein composed of PhaR and PhaC (catalytically active unit) subunits [44]. In this study, we overexpressed only the catalytically active unit, PhaC, where PhaR is not required for the expression of phaRBC operon. Similarly, there are several studies on overexpression of a single subunit from the heterodimeric forms [45,46,47]. Provided that the monomers are present, overexpressing this final enzyme in the pathway, PhaC, could enhance the PHA yields and change the copolymer composition. The former would especially hold true, if the polymerization step is the rate limiting step, and the latter if the PhaC enzyme has different affinities toward different monomers.
PHA production in batch mode
It has been clearly known that dissolved oxygen (DO) is one of the most important key parameters for cell growth and PHA accumulation. PHA synthesis can be triggered under oxygen-limited conditions by Bacillus megaterium strains [48]. The limitation of DO concentration results in the deviation of carbon flux from biomass production towards PHA accumulation [49].
B. megaterium NRRL B-14308 wild-type strain was cultivated in 500 mL scale, fully controlled bioreactor for 66 h using glucose as the carbon source to observe cell growth and PHA accumulation under different DO levels (10%, 20% and 30% DO) (Fig. S2) in batch cultivations. The fermentation kinetics and yields related to PHA production in the batch mode are shown in Table 2. The results showed that the highest PHA concentration and intracellular PHA content were reached at 20% DO set point compared to other DO levels. The 20% DO level showed higher cell dry weight than 10% DO level, whereas the lowest cell dry weight and PHA accumulation were obtained at 30% DO set point. On the other hand, the highest PHA yield, YP/S, was obtained for 20% DO set point. Based on these results, 20% DO was selected for further studies in bioreactor cultivations at batch and fed-batch modes with wild-type and rec-B. megaterium strains, to maximize the efficiency of the PHA accumulation.
The total cell dry weight (CDW), maximum PHA accumulation, PHA content, biomass yield (YX/S), product yields (YP/S and YP/X) and PHA volumetric productivity (qPHA) obtained from batch cultivations of wild-type B. megaterium and rec-B. megaterium cells are also summarized in Table 2. With a defined fermentation medium, cell dry weight reached 3.06 g L−1 with 2.43 g L−1 PHA accumulation for the wild-type B. megaterium strain. However, the highest CDW (3.52 g L−1) and maximum PHA accumulation (2.76 g L−1) were achieved when fermentation was carried out using rec-B. megaterium cells after 66 h of cultivation. The highest final PHA content and the highest PHA volumetric productivity were also attained using rec-B. megaterium cells as 78 wt% and 0.15 g L−1 h−1, respectively. Consequently, there is 1.2-fold increase in the PHA final concentration for rec-B. megaterium cells compared to wild-type B. megaterium cells in the batch mode.
PHA accumulation reached a steady state after approximately 66 h of batch cultivation, even when glucose was not consumed totally by the cells, confirming that glucose was not the limiting nutrient in the minimal medium (Fig. 2). PHA synthesis continued in the stationary phase (t = 42–66 h), confirming that B. megaterium accumulated PHAs by adopting growth-associated and non-growth-associated mechanisms [50, 51].
Glucose consumption (Cs, dashed line), cell (OD600, gray line) and PHA (CPHA, solid line) accumulations during batch production by a B. megaterium NRRL B-14308 wild-type strain; b recombinant B. megaterium strain. Data expressed are the mean values and all experiments were performed in duplicate (n = 2, maximum SD = ± 21.2%, minimum ± 1.9%)
Fed-batch cultivations for PHA production
Fed-batch cultivations have been extensively employed to reach high cell density cultures and improve polymer productivity by monitoring DO, pH and carbon levels as feed-back control parameters [52]. In this study, exponential glucose feeding profiles at several pre-determined specific growth rates were implemented to achieve higher PHA production in fed-batch fermentation.
The cultivation was initiated with a glucose concentration of 10 g L−1, and the reactor was operated in a batch mode for 42 h, followed by a fed-batch phase (42–66 h) (Fig. 3). Ammonium sulfate (1 g L−1) as the nitrogen source was only supplemented at the beginning of the cultivation, to promote cell growth.
The fed-batch phase was initiated after the batch phase (at t = 42 h) by automatically feeding the fermentation medium with glucose solution under exponential feeding profiles, controlled 20% DO and pH 7.0 for wild-type and rec-B. megaterium cells.
The three different exponential glucose feeding profiles were based on the pre-determined specific growth rates: µ0 = 0.05 h−1, µ0 = 0.075 h−1, µ0 = 0.1 h−1. Therefore, a series of batch cultivations in the bioreactor were performed to determine the initial biomass yield on substrate (YX/S), as YX/S = 0.36 g biomass g−1 glucose. This value was applied in fed-batch experiments to evaluate the different exponential glucose feeding strategies and was consistent with previous studies [53, 54] for PHA production.
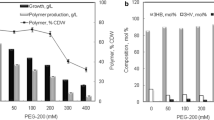
Comparing the fermentation profiles, the trends of cell growth (Fig. 3) and PHA concentration (Fig. 4) were similar in the batch phase, whereas the accumulation curves diverged for the fed-batch phase, i.e. after 42 h, because of the increasing PHA accumulation and a reduced biomass formation as a consequence of the exponential glucose feeding and nitrogen limitation. During the fed-batch stage, the PHA concentration increased exponentially to 6.15 g L−1 as the highest level, by the end of the fermentation (Fig. 4). The high rate of PHA accumulation observed in rec-B. megaterium strain at the higher pre-determined specific growth rate (µ0 = 0.1 h−1) towards the end of the process was potentially due to the high exponential glucose overfeeding and nitrogen depletion in the growth medium.
The kinetic and stoichiometric parameters for the fed-batch cultivation of wild-type and rec-B. megaterium cells at different exponential glucose feeding rates are demonstrated in Fig. 5. The exponential fed-batch strategy resulted in a 2.2-fold increase in PHA production compared to batch cultivations. The highest final cell concentration (7.68 g L−1), PHA content of the cells (80%), volumetric PHA productivity (0.54 g L−1 h−1), as well as cell and product yields on substrate (0.74 g g−1 and 0.62 g g−1, respectively) were obtained with rec-B. megaterium cells at the higher pre-determined specific growth rate (µ0 = 0.1 h−1), confirming the positive effect of genetic modification for PHA biosynthesis. The results also showed that rec-B. megaterium NRRL B-14308 strain could accumulate higher amount of PHA content compared to other Bacillus strains (Table 3). Moreover, if a high cell density fed-batch strategy is applied, the PHA yields could be even further improved. Nevertheless, a relatively high YP/X value, 0.8 g g−1, was obtained via strain and bioprocess improvements.
The kinetic and stoichiometric parameters for the fed-batch cultivation of wild-type and rec-B. megaterium cells at different exponential glucose feeding rates. Asterisks indicate the significance levels of two-way ANOVA test comparisons with Tukey test, *p < 0.1, **p < 0.01, ***p < 0.001, ****p < 0.0001
Oxygen transfer parameters of fed-batch fermentations
During PHA production by wild-type and rec-B. megaterium cells in fed-batch cultivation using different glucose feeding rates, the dynamic method was applied to determine the oxygen transfer parameters, such as volumetric mass transfer coefficient (kLa), enhancement factor (E), oxygen uptake rate (OUR) and oxygen transfer rate (OTR). The oxygen transfer parameters were determined for all fed-batch fermentations and are summarized in Table 4.
The kLa values first increased and then decreased with the cultivation time. The kLa values are considerably affected by a lot of factors, including geometrical characteristics of bioreactors, viscosity and surface tension of the broth, temperature, aeration rate, agitation speed, foam formation, and microorganisms’ morphology [55]. The bioreactor operational parameters, such as temperature, agitation speed, aeration rate, were kept constant throughout the bioprocesses and an antifoam agent was not used. Therefore, this decrease can be related to the viscosity of the fermentation broth. The viscosity of fermentation broth increased as the B. megaterium cells were in exponential growth phase and during PHA accumulation. The increase in broth viscosity reduced oxygen transfer to the cells, resulting in a resistance zone for mass transfer [37, 56].
The oxygen uptake rate (OUR) is one of the most fundamental parameters in fermentation processes and depends on metabolic activity of cells. OUR increases in the exponential growth phase due to the high substrate consumption at the start of the cultivation process. The OUR was at its maximum for 18 h of the fermentation processes because of the high-specific oxygen demand for culture viability. After exponential growth phase, OUR decreased because of decreasing metabolic activity of cells [57]. The oxygen transfer rate (OTR) in fermentation processes has been related to volumetric mass transfer coefficient, kLa and oxygen consumption by the microorganism. The OTR increased because of high oxygen demand for 18 h. The OTR is a critical factor for PHA biosynthesis, because OTR is equal to OUR under oxygen limitation. Thus, variation in cellular respiration could increase the reducing power (NADPH/NADP+), which is an important cofactor involved in PHA biosynthesis and regulation [5].
Besides, kLa, OTR and OUR values in rec-B. megaterium cells were generally higher than wild-type cells. This can be explained by rec-B. megaterium cells having a higher metabolic activity, especially in biopolymer synthesis. Moreover, with the increasing glucose feeding rates, higher oxygen transfer parameters were attained, due to the increased oxygen demand of the cells, as expected.
Polymer characterization
1H-NMR spectroscopy was performed to identify the chemical structure of the produced PHA polymers, as shown in Fig. 6. The peak at 0.90 ppm corresponds the protons of the terminal methyl group of 3HV monomer unit. The peaks at 1.25 ppm, 1.55 ppm, 2.58 ppm and 5.27 ppm correspond to the methyl group of 3HB unit, internal –CH2 group of 3HV unit, the –CH2 groups and the –CH groups of 3HB and 3HV monomer units, respectively [58]. The molar fraction of 3HV monomer unit was determined from the relative intensities of methyl groups of 3HV (0.90 ppm) and 3HB (1.25 ppm) monomer units in the 1H-NMR spectra according to the following equation [53]:
The 1H-NMR spectrum confirms that the chemical structure of the biopolymers produced in this study corresponds to PHBV copolymer. According to the above equation, integrating the area under the peaks at 0.90 ppm and 1.25 ppm, the PHBV copolymer was found to be composed of 58 mol% of 3HV monomer units. The content of 3HV monomer units in PHBV copolymer is important for industrial applications because P(3HB-co-3HV) copolymers containing more than 20 mol% 3HV monomers exhibit superior material properties, such as impact strength and polymer flexibility, suitable for manufacturing of films and fibers [59, 60]. This study is significant for production of PHBV by B. megaterium strain with the highest 3HV content from unrelated carbon sources, without a precursor addition (Table 3).
Although there is no clear evidence from metabolic pathway analysis on the increase of 3HV content in recombinant B. megaterium cells only overexpressing phaC gene, it could be explained by the increment in microbial growth, which may digest organic acids and release odd-fatty acid compounds as metabolites, precursors for 3HV production. On the other hand, the observed increase in the 3HV fraction could also be due to the higher PHA synthase activity from the additional copy of phaC. In recombinant strains, the higher PHA synthase activity would promote the polymerization of 3HV-CoA in the medium. Briefly, the higher PhaC activity in recombinant B. megaterium cells pulls more C5 intermediates into P(3HB-co-3HV) synthesis prior to conversion into shorter C4 intermediates, resulting in a higher 3HV fraction than wild-type strain [61, 62]. Moreover, a further study on enzyme kcat values for each enzyme in the PHA synthesis pathway, especially for PhaA, PhaB and PhaC, the phenomenon could be better explained, and accordingly plan a better strategy for metabolic engineering. This could also be performed via an initial metabolic flux analysis.
To determine the PHA content and monomer composition within bacterial cells, GC analysis was performed after methanolysis. GC analysis also confirmed the produced polymer to be PHBV copolymer, as two different peaks were observed at retention times of ~ 0.9 min and ~ 1.7 min, corresponding to 3HB and 3HV methyl esters in the PHA sample, respectively (Fig. S3). From the GC peak areas, the PHA produced from recombinant B. megaterium cells on fed-batch fermentation mode was found to contain 54 mol% HV and 46 mol% HB, where the monomer ratios were consistent with the results obtained from 1H-NMR analysis.
The thermophysical properties of the produced PHA polymers were analyzed by TGA and DSC (Table 5). PHA polymers showed two main degradation temperature ranges in agreement with previous studies [20, 63, 64]. The TGA curves for produced PHA showed a gradual weight loss with the increasing temperature, which started at around 260 °C and entirely degrading at around 455 °C. Two-step degradation temperatures are likely due to the incorporation of different monomers, such as 3HV, in the PHA samples. The first-step degradation temperature can be ascribed to decomposition of the crosslinked polymer chain. The second-step degradation is due to the decomposition of the main block of PHBV polymer chain [65].
The melting temperatures of PHBV polymers were examined using DSC analysis. The DSC thermogram showed two melting points for the produced PHBV polymers (Table 5). It can be seen that the two melting temperatures were both lower than the melting temperatures of PHB homopolymer, ca. 170 °C [66]. As the proportion of 3HV monomer units in the PHBV polymer chain increases, the melting temperature of the polymer decreases, resulting in an improvement in impact strength and polymer flexibility and broader applications [67]. The occurrence of two melting temperatures could be observed due to melting–re-cyrstallization–re-melting process of PHBV polymers [68].
Conclusion
PHAs are currently being produced at about 270,000 tons year−1 with an increasing demand [29]. The present study aimed to produce PHA biopolymer from Bacillus megaterium NRRL B-14308 strain with a higher production efficiency, by investigating the bioprocess design parameters, including dissolved oxygen level and fermentation mode. Also, aiming to increase the production of PHA synthase enzyme (PhaC), recombinant strains were constructed via phaC overexpression in B. megaterium.
The batch experiments showed that the highest PHA concentration and intracellular PHA content were reached at 20% DO set point. Furthermore, in the batch fermentation mode, there was 1.2-fold increase in the PHA final concentration for rec-B. megaterium cells against wild-type B. megaterium cells. To maximize PHA productivity, fed-batch fermentations with different exponential glucose feeding rates based on desired specific growth rate were performed. The results demonstrated that fed-batch cultivation increased the volumetric PHA productivity 2.2 times compared to batch cultivation. By controlling the DO level, the highest cell biomass (7.7 g L−1), final PHA concentration (6.1 g L−1), volumetric PHA productivity (0.54 g L−1 h−1) as well as cell and product yields on substrate (0.74 g g−1 and 0.62 g g−1, respectively) were achieved at the end of the fed-batch cultivation by rec-B. megaterium strain at µ0 = 0.1 h−1. Also, the experimental data indicated that well-tuned DO level and availability of excess carbon source and nitrogen limitation enhance the synthesis of PHAs in B. megaterium. The characterization of synthesized PHAs was performed by 1H-NMR, GC-FID, TGA and DSC analyses. The results revealed that the synthesized PHAs composed of 42 mol% of 3HB and 58 mol% of 3HV monomers. Thus, this study is significant for the production of PHBV copolymer with a high 3HV content by B. megaterium from an unrelated, simple carbon source, glucose, with no need of precursor addition.
References
Krishnan S, Chinnadurai GS, Perumal P (2017) Polyhydroxybutyrate by Streptomyces sp.: production and characterization. Int J Biol Macromol 104:1165–1171
Kumar M, Singhal A, Verma PK, Thakur IS (2017) Production and characterization of polyhydroxyalkanoate from lignin derivatives by Pandoraea sp. ISTKB ACS Omega 2:9156–9163
Ferre-Guell A, Winterburn J (2018) Biosynthesis and characterization of polyhydroxyalkanoates with controlled composition and microstructure. Biomacromol 19:996–1005
Koller M, Hesse P, Bona R, Kutschera C, Atlic A, Braunegg G (2007) Potential of various archae- and eubacterial strains as industrial polyhydroxyalkanoate producers from whey. Macromol Biosci 7:218–226
Urtuvia V, Maturana N, Peña C, Díaz-Barrera A (2020) Accumulation of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Azotobactervinelandii with different 3HV fraction in shake flasks and bioreactor. Bioprocess Biosyst Eng 43:1469
Marangoni C, Furigo A, de Aragão GMF (2002) Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Ralstoniaeutropha in whey and inverted sugar with propionic acid feeding. Process Biochem 38:137–141
Suhazsini P, Keshav R, Narayanan S, Chaudhuri A, Radha P (2020) A study on the synthesis of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) by Bacillusmegaterium utilizing cheese whey permeate. J Polym Environ 28:1390–1405
Güngörmedi G, Demirbilek M, Mutlu MB, Denkbaş EB, Çabuk A (2014) Polyhydroxybutyrate and hydroxyvalerate production by Bacillusmegaterium strain A1 isolated from hydrocarbon-contaminated soil. J Appl Polym Sci 131:40530
Haywood GW, Anderson AJ, Roger Williams D, Dawes EA, Ewing DF (1991) Accumulation of a poly(hydroxyalkanoate) copolymer containing primarily 3-hydroxyvalerate from simple carbohydrate substrates by Rhodococcus sp. NCIMB 40126. Int J Biol Macromol 13:83–88
Porras MA, Ramos FD, Diaz MS, Cubitto MA, Villar MA (2019) Modeling the bioconversion of starch to P(HB-co-HV) optimized by experimental design using Bacillusmegaterium BBST4 strain. Environ Technol 40:1185–1202
Valappil SP, Rai R, Bucke C, Roy I (2008) Polyhydroxyalkanoate biosynthesis in Bacillus cereus SPV under varied limiting conditions and an insight into the biosynthetic genes involved. J Appl Microbiol 104:1624–1635
Aldor IS, Kim S-W, Prather KLJ, Keasling JD (2002) Metabolic engineering of a novel propionate-independent pathway for the production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in recombinant Salmonellaenterica serovar typhimurium. Appl Environ Microbiol 68:3848–3854
Chen Q, Wang Q, Wei G, Liang Q, Qi Q (2011) Production in Escherichiacoli of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with differing monomer compositions from unrelated carbon sources. Appl Environ Microbiol 77:4886–4893
Yang JE, Choi YJ, Lee SJ, Kang K-H, Lee H, Oh YH, Lee SH, Park SJ, Lee SY (2014) Metabolic engineering of Escherichiacoli for biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from glucose. Appl Microbiol Biotechnol 98:95–104
Han J, Hou J, Zhang F, Ai GM, Li M, Cai SF, Liu HL, Wang L, Wang ZJ, Zhang SL, Cai L, Zhao DH, Zhou J, Xiang H (2013) Multiple propionyl coenzyme a-supplying pathways for production of the bioplastic poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in Haloferaxmediterranei. Appl Environ Microbiol 79:2922–2931
Heinrich D, Raberg M, Steinbuchel A (2015) Synthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from unrelated carbon sources in engineered Rhodospirillumrubrum. FEMS Microbiol Lett 362:fnv038
Zhang YZ, Liu GM, Weng WQ, Ding JY, Liu SJ (2015) Engineering of Ralstoniaeutropha for the production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from glucose. J Biotechnol 195:82–88
Reddy SV, Thirumala M, Mahmood S (2009) Production of PHB and P (3HB-co-3HV) biopolymers by Bacillusmegaterium strain OU303A isolated from municipal sewage sludge. World J Microbiol Biotechnol 25:391–397
Porras MA, Vitale C, Villar MA, Cubitto MA (2017) Bioconversion of glycerol to poly(HB-co-HV) copolymer in an inexpensive medium by a Bacillusmegaterium strain isolated from marine sediments. J Environ Chem Eng 5:1–9
Akdoğan M, Çelik E (2018) Purification and characterization of polyhydroxyalkanoate (PHA) from a Bacillusmegaterium strain using various dehydration techniques. J Chem Technol Biotechnol 93:2292–2298
Ray S, Kalia VC (2017) Co-metabolism of substrates by Bacillusthuringiensis regulates polyhydroxyalkanoate co-polymer composition. Bioresour Technol 224:743–747
Luzier WD (1992) Materials derived from biomass/biodegradable materials. Proc Natl Acad Sci USA 89:839–842
Sim SJ, Snell KD, Hogan SA, Stubbe J, Rha C, Sinskey AJ (1997) PHA synthase activity controls the molecular weight and polydispersity of polyhydroxybutyrate in vivo. Nat Biotechnol 15:63–67
Chek MF, Hiroe A, Hakoshima T, Sudesh K, Taguchi S (2019) PHA synthase (PhaC): interpreting the functions of bioplastic-producing enzyme from a structural perspective. Appl Microbiol Biotechnol 103:1131–1141
Raza ZA, Tariq MR, Majeed MI, Banat IM (2019) Recent developments in bioreactor scale production of bacterial polyhydroxyalkanoates. Bioprocess Biosyst Eng 42:901–919
Huschner F, Grousseau E, Brigham CJ, Plassmeier J, Popovic M, Rha C, Sinskey AJ (2015) Development of a feeding strategy for high cell and PHA density fed-batch fermentation of Ralstonia eutropha H16 from organic acids and their salts. Process Biochem 50:165–172
Suwannasing W, Imai T, Kaewkannetra P (2015) Cost-effective defined medium for the production of polyhydroxyalkanoates using agricultural raw materials. Bioresour Technol 194:67–74
Choi SY, Rhie MN, Kim HT, Joo JC, Cho IJ, Son J, Jo SY, Sohn YJ, Baritugo K-A, Pyo J, Lee Y, Lee SY, Park SJ (2020) Metabolic engineering for the synthesis of polyesters: a 100-year journey from polyhydroxyalkanoates to non-natural microbial polyesters. Metab Eng 58:47–81
Choi SY, Cho IJ, Lee Y, Kim Y-J, Kim K-J, Lee SY (2020) Microbial polyhydroxyalkanoates and nonnatural polyesters. Adv Mater 32:1907138
Kanjanachumpol P, Kulpreecha S, Tolieng V, Thongchul N (2013) Enhancing polyhydroxybutyrate production from high cell density fed-batch fermentation of Bacillusmegaterium BA-019. Bioprocess Biosyst Eng 36:1463–1474
RamKumar Pandian S, Deepak V, Kalishwaralal K, Rameshkumar N, Jeyaraj M, Gurunathan S (2010) Optimization and fed-batch production of PHB utilizing dairy waste and sea water as nutrient sources by Bacillusmegaterium SRKP-3. Bioresour Technol 101:705–711
Blunt W, Sparling R, Gapes DJ, Levin DB, Cicek N (2018) The role of dissolved oxygen content as a modulator of microbial polyhydroxyalkanoate synthesis. World J Microbiol Biotechnol 34:106
de Almeida A, Giordano AM, Nikel PI, Pettinari MJ (2010) Effects of aeration on the synthesis of poly(3-hydroxybutyrate) from glycerol and glucose in recombinant Escherichiacoli. Appl Environ Microbiol 76:2036
Senior PJ, Beech GA, Ritchie GA, Dawes EA (1972) The role of oxygen limitation in the formation of poly-β-hydroxybutyrate during batch and continuous culture of Azotobacterbeijerinckii. Biochem J 128:1193–1201
Sambrook JF, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Woodbury
Moore SJ, Lawrence AD, Biedendieck R, Deery E, Frank S, Howard MJ, Rigby SEJ, Warren MJ (2013) Elucidation of the anaerobic pathway for the corrin component of cobalamin (vitamin B12). Proc Natl Acad Sci 110:14906
Çelik E, Çalık P, Oliver SG (2009) Fed-batch methanol feeding strategy for recombinant protein production by Pichiapastoris in the presence of co-substrate sorbitol. Yeast 26:473–484
Taguchi H (1966) Dynamic measurement of the volumetric oxygen transfer coefficient in a fermentation system. J Ferment Technol 44:881–889
Yıldırım Z, Çelik E (2017) Periplasmic and extracellular production of cellulase from recombinant Escherichiacoli cells. J Chem Technol Biotechnol 92:319–324
Braunegg G, Sonnleitner B, Lafferty RM (1978) A rapid gas chromatographic method for the determination of poly-β-hydroxybutyric acid in microbial biomass. Eur J Appl Microbiol Biotechnol 6:29–37
Fei T, Cazeneuve S, Wen Z, Wu L, Wang T (2016) Effective recovery of poly-β-hydroxybutyrate (PHB) biopolymer from Cupriavidusnecator using a novel and environmentally friendly solvent system. Biotechnol Progress 32:678–685
Bunk B, Schulz A, Stammen S, Münch R, Warren MJ, Rohde M, Jahn D, Biedendieck R (2010) A short story about a big magic bug. Bioeng Bugs 1:85–91
Srirangan K, Liu X, Tran TT, Charles TC, Moo-Young M, Chou CP (2016) Engineering of Escherichiacoli for direct and modulated biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer using unrelated carbon sources. Sci Rep 6:36470
McCool GJ, Cannon MC (2001) PhaC and PhaR are required for polyhydroxyalkanoic acid synthase activity in Bacillusmegaterium. J Bacteriol 183:4235–4243
Chabes A, Domkin V, Larsson G, Liu A, Gräslund A, Wijmenga S, Thelander L (2000) Yeast ribonucleotide reductase has a heterodimeric iron-radical-containing subunit. Proc Natl Acad Sci 97:2474
Laszlo V, Hoda MA, Garay T, Pirker C, Ghanim B, Klikovits T, Dong YW, Rozsas A, Kenessey I, Szirtes I, Grusch M, Jakopovic M, Samarzija M, Brcic L, Kern I, Rozman A, Popper H, Zöchbauer-Müller S, Heller G, Altenberger C, Ziegler B, Klepetko W, Berger W, Dome B, Hegedus B (2015) Epigenetic down-regulation of integrin α7 increases migratory potential and confers poor prognosis in malignant pleural mesothelioma. J Pathol 237:203–214
Liberal V, Martinsson-Ahlzén H-S, Liberal J, Spruck CH, Widschwendter M, McGowan CH, Reed SI (2012) Cyclin-dependent kinase subunit (Cks) 1 or Cks2 overexpression overrides the DNA damage response barrier triggered by activated oncoproteins. Proc Natl Acad Sci 109:2754
Faccin DJL, Rech R, Secchi AR, Cardozo NSM, Ayub MAZ (2013) Influence of oxygen transfer rate on the accumulation of poly(3-hydroxybutyrate) by Bacillus megaterium. Process Biochem 48:420–425
Koller M, Braunegg G (2015) Potential and prospects of continuous polyhydroxyalkanoate (PHA) production. Bioengineering 2:94–121
Mohapatra S, Maity S, Dash HR, Das S, Pattnaik S, Rath CC, Samantaray D (2017) Bacillus and biopolymer: prospects and challenges. Biochem Biophys Rep 12:206–213
Lee SY (1996) Plastic bacteria? Progress and prospects for polyhydroxyalkanoate production in bacteria. Trends Biotechnol 14:431–438
Ryu HW, Hahn SK, Chang YK, Chang HN (1997) Production of poly(3-hydroxybutyrate) by high cell density fed-batch culture of Alcaligeneseutrophus with phosphate limitation. Biotechnol Bioeng 55:28–32
García C, Alcaraz W, Acosta-Cárdenas A, Ochoa S (2019) Application of process system engineering tools to the fed-batch production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from a vinasses–molasses mixture. Bioprocess Biosyst Eng 42:1023–1037
Sun ZY, Ramsay JA, Guay M, Ramsay BA (2006) Automated feeding strategies for high-cell-density fed-batch cultivation of Pseudomonasputida KT2440. Appl Microbiol Biotechnol 71:423–431
Zhou Y, Han L-R, He H-W, Sang B, Yu D-L, Feng J, Zhang X (2018) Effects of agitation, aeration and temperature on production of a novel glycoprotein GP-1 by Streptomyceskanasenisi ZX01 and scale-up based on volumetric oxygen transfer coefficient. Molecules 23:125
Sinha J, Tae Bae J, Pil Park J, Hyun Song C, Won Yun J (2001) Effect of substrate concentration on broth rheology and fungal morphology during exo-biopolymer production by Paecilomyces japonica in a batch bioreactor. Enzyme Microb Technol 29:392–399
Garcia-Ochoa F, Gomez E, Santos VE, Merchuk JC (2010) Oxygen uptake rate in microbial processes: an overview. Biochem Eng J 49:289–307
Wang C, Zheng Y, Sun Y, Fan J, Qin Q, Zhao Z (2016) A novel biodegradable polyurethane based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(ethylene glycol) as promising biomaterials with the improvement of mechanical properties and hemocompatibility. Polym Chem 7:6120–6132
Alsafadi D, Al-Mashaqbeh O (2017) A one-stage cultivation process for the production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) from olive mill wastewater by Haloferax mediterranei. N Biotechnol 34:47–53
de Paula FC, de Paula CBC, Gomez JGC, Steinbüchel A, Contiero J (2017) Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production from biodiesel by-product and propionic acid by mutant strains of Pandoraea sp. Biotechnol Progress 33:1077–1084
Han J, Qiu Y-Z, Liu D-C, Chen G-Q (2004) Engineered Aeromonas hydrophila for enhanced production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) with alterable monomers composition. FEMS Microbiol Lett 239:195–201
Fukui T, Kichise T, Iwata T, Doi Y (2001) Characterization of 13 kDa granule-associated protein in Aeromonascaviae and biosynthesis of polyhydroxyalkanoates with altered molar composition by recombinant bacteria. Biomacromol 2:148–153
Kuciel S, Mazur K, Jakubowska P (2019) Novel biorenewable composites based on poly (3-hydroxybutyrate-co-3-hydroxyvalerate) with natural fillers. J Polym Environ 27:803–815
Singh S, Mohanty AK, Sugie T, Takai Y, Hamada H (2008) Renewable resource based biocomposites from natural fiber and polyhydroxybutyrate-co-valerate (PHBV) bioplastic. Compos A 39:875–886
Hasan SK, Zainuddin S, Tanthongsack J, Hosur M, Allen L (2018) A study of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) biofilms’ thermal and biodegradable properties reinforced with halloysite nanotubes. J Compos Mater 52:3199–3207
Salgaonkar BB, Bragança JM (2015) Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Halogeometricumborinquense strain E3. Int J Biol Macromol 78:339–346
Luangthongkam P, Laycock B, Evans P, Lant P, Pratt S (2019) Thermophilic production of poly(3-hydroxybutyrate-co-3-hydrovalerate) by a mixed methane-utilizing culture. N Biotechnol 53:49–56
Don T-M, Chen CW, Chan T-H (2006) Preparation and characterization of poly(hydroxyalkanoate) from the fermentation of Haloferaxmediterranei. J Biomater Sci Polym Ed 17:1425–1438
Alkotaini B, Sathiyamoorthi E, Kim BS (2015) Potential of Bacillusmegaterium for production of polyhydroxyalkanoates using the red algae Gelidiumamansii. Biotechnol Bioprocess Eng 20:856–860
Alkotaini B, Koo H, Kim BS (2016) Production of polyhydroxyalkanoates by batch and fed-batch cultivations of Bacillusmegaterium from acid-treated red algae. Korean J Chem Eng 33:1669–1673
Rodriguez-Contreras A, Koller M, Dias MMD, Calafell-Monfort M, Braunegg G, Marques-Calvo MS (2013) High production of poly(3-hydroxybutyrate) from a wild Bacillusmegaterium Bolivian strain. J Appl Microbiol 114:1378–1387
Acknowledgements
This work was supported by Hacettepe University Scientific Research Projects Coordination Unit (Grant number FBB-2016-11851). We would like to thank Belgin Aslan for her help in TG and DSC analyses and Beray Temelli for her help in 1H-NMR analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Akdoğan, M., Çelik, E. Enhanced production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) biopolymer by recombinant Bacillus megaterium in fed-batch bioreactors. Bioprocess Biosyst Eng 44, 403–416 (2021). https://doi.org/10.1007/s00449-020-02452-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02452-z