Abstract
In order to survive living organisms have developed multiple mechanisms to deal with tough environmental conditions. Hormesis is defined as a process in which exposure to a low dose of a chemical agent or environmental factor that is damaging at higher doses induces an adaptive beneficial effect on the cell or organism. In this paper, we examine several ideas that might be taken into consideration before using hormesis as a therapeutic tool to improve health and life span, and hopefully will open the discussion for new and interesting debates regard hormesis. The first one is to understand that the same stressor or inductor can activate different pathways in a parallel or dual response, which might lead to diverse outcomes. Another idea is related to the mechanisms involved in activating Nrf2, which might be different and have diverse hormetic effects.
Last, we discuss mild oxidative stress in association to low-grade chronic inflammation as a stimulating avenue to be explored and the unexpected effects proposed by the obesity paradox theory. All the previous might help to clarify the reasons why centenarians are able to reach the extreme limits of human life span, which could probably be related to the way they deal with homeostasis maintenance, providing an opportunity for hormesis to intervene significantly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
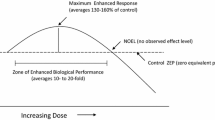
In order to survive, living organisms have developed multiple mechanisms to deal with tough environmental conditions. Many biological subdisciplines have identified and reported evolutionarily conserved processes in which a low dose of a stressful stimulus activates an adaptive response that increases the resistance of the cell or organism to a severe level of stress. This concept is not new, as Nietzsche said: “What doesn’t kill you makes you stronger”, however, due to a lack of frequent interaction among scientists from different areas, a broad range of terms that describe such “adaptive response” or “preconditioning” have emerged. Several years ago fifty recognized scientist from different fields, published a set of recommendations in order to unify the concepts and terminology for this response in cells and organisms after the disruption in their homeostasis and called it “hormesis” (Calabrese et al. 2010). Hormesis can be defined as: “a process in which exposure to a low dose of a chemical agent or environmental factor that is damaging at higher doses induces an adaptive beneficial effect on the cell or organism” (Calabrese and Baldwin 2003; Calabrese 2008; Calabrese et al. 2010; Mattson 2008; Rattan 2006; Hoffmann 2009)
There have been many hormetic agents identified so far (Calabrese and Baldwin 2003; Le Bourg and Rattan 2009), however here we will only focus on the hormetic effect induced by oxidant compounds and inflammation. We will discuss three hormetic scenarios that we have been studying in the past few years.
The first one is the classical oxidative conditioning hormesis (OCH) effect, where cells are subjected to an oxidant, such as hydrogen peroxide (H2O2), at a low dose to induce an hormetic response encompassing diverse antioxidant pathways that allow the cells to survive when they are re-exposed to the same oxidant. We will suggest the idea of “parallel response pathways” that are necessary in order to regulate simultaneously antioxidant and survival hormetic response against oxidative stress.
The second case is related to the nuclear factor erythroid-derived 2-like 2 (Nrf2) inductors. Nrf2 is an ubiquitous cytosolic transcription factor that is continuously degraded during cellular homeostasis; however, in response to modifications in cellular redox state, Nrf2 is released from its repressor Kelch-like ECH-associated protein 1(Keap-1), phosphorylated and translocated into the nucleus where it binds to the antioxidant response element (ARE) and induces antioxidant and phase II detoxifying enzymes expression (Itoh et al. 2004; Kobayashi and Yamamoto 2006; Kobayashi et al. 2006) and increases GSH content (Ishii and Mann 2014). Recently there has been an increasing interest in the use of molecules that can activate Nrf2 pathway. However, since most of them are oxidant molecules, their mechanism can be explained as an OCH: an oxidant low dose that induces an adaptive response, which protects the cells or organism against a higher oxidative insult. The mechanism involved in activating Nrf2 has mostly been related to modifications in redox state, however here we challenge that point of view in the light of the hormetic effects.
The third and last example that we will discuss is even more complicated because it involves the in vivo hormetic response to chronic inflammation and oxidative stress. This is discussed in relation to the “obesity paradox”. Obesity is a low-grade inflammation condition, which has also been related to low-grade oxidative stress. When it comes to animals or human context, there are many more variables to consider in order to understand the hormetic response, but some new results have shown an hormetic or at least and attenuation effect against metabolic and oxidative damaging outcomes during life span.
Parallel mechanisms during hormetic oxidative response
Traditionally hormetic response is induced by a homeostasis imbalance bring about by many different stressors such as chemicals, radiation, temperature changes and even exercise (Scannapieco et al. 2007; Choi et al. 2012; Bayod et al. 2012; Alarcón-Aguilar et al. 2014).
Hormetic stress response is characterized by activation of cellular defenses, in particular increasing antioxidant systems and proteins related to cellular survival. During OCH response changes in ROS/RNS levels and other metabolites, directly and indirectly, modify redox state (Ristow and Schmeisser 2011; Hoffmann et al. 2013; Speciale et al. 2011) activating the antioxidant response. Therefore, the mechanisms that have been described include different signaling pathways, among which are those where the transcription factors are sensitive to change due to redox state such as Nrf2, NFκB, AP-1, and HSF1. These transcription factors are known to simultaneously activate different cellular response to stress by triggering parallel response mechanisms, since most of them can regulate the expression of antioxidant enzymes and survival proteins (Mukherjee et al. 2013; Hsu et al. 2012; Jacobs and Marnett 2007; Zanotto-Filho et al. 2009).
In the last years many treatments to induce the hormetic response in order to counteract oxidative damage have been described (Hine and Mitchell 2012; Kubicova et al. 2013; Hashmi et al. 2014), however few of them have described in detail the molecular mechanisms during OCH.
Our group and others (Luna-López et al. 2010; Khoramian et al. 2011) have tried to explain this response more widely and not just since the perspective from the increment in the antioxidant response. Nevertheless while attempting to point out the complexity of potential hormetic mechanisms, we might fall into an oversimplification, as each of these responses are clearly multifaceted, and probably not the only two responses activated upon oxidative stress. Hence, just to increase the understanding about the parallel response that might be turning on during OCH and survival response, here we describe two examples:
-
a.
Alginate oligosaccharide.
Alginate oligosachharide (AOS) is a non-toxic, non-immunogenic and biodegradable polymer that has antioxidant and anti-inflammatory properties, and decreases advanced glycation end products (AGEs) formation. Strictly, AOS could not be considered an hormetic inductor since it is not a toxic chemical agent which at low doses might induce the hormetic response. However, the cellular responses it promotes might be helpful to understand the parallel mechanisms idea. Treating PC12 cell line with AOS protected it against apoptosis cell death induced with H2O2 by increasing antioxidant response. Nrf2 levels were augmented thus incrementing antioxidants enzymes as catalase (CAT) and superoxide dismutase (SOD), as well as GSH. Simultaneously, proteins related to cellular survival such as Bcl-2 and heat shock proteins HSP70 and HSP90 were also increased, and p53 phosphorylation, a mechanism related to apoptosis induction, was inhibited (Khoramian et al. 2011). These cellular protective mechanisms are similar to what is observed during OCH.
-
b.
Hydrogen peroxide
Hydrogen peroxide (H2O2) is highly utilized to generate oxidative damage. This molecule can induce different cellular responses depending on its concentration. Our group has established an oxidative conditioning hormesis (OCH) model (treating L929 cell line with H2O2 50 μM for 9 h), which has allowed us to establish different adaptive mechanisms in response to oxidative stress. OCH treatment simultaneously induces the activation of transcription factors that are sensible to redox state modifications like Nrf2 and NFκB. The first one is related to antioxidant response and phase II detoxification and the second one to increase proteins related to cellular survival, like Bcl-2, as a complementary part of cellular response to oxidative stress. Using the OCH model we also demonstrated the Bcl-2 participation is required to sustain Nrf2 activation during hormesis response linking this way both pathways. The previous suggest a parallel mechanism needed to activate a successful cellular response (Luna-López et al. 2010).
All the previous raise the question about the possible existence of some master regulators during cellular stress response, which might oppose the idea of different and independent response pathways. An interesting set of candidates for this role could be PKC isoforms. There are some partial evidences that point toward that approach. We have shown that during hormetic response PKC-α plays a preponderant role in NFκB activation to induce Bcl-2 expression as part of the survival response (Luna-López et al., 2013). It has been also reported (Niture et al. 2009) that PKC-δ is able to phosphorylate Nrf2 in Ser 40 once Cys 151 in Keap1 is oxidized, and therefore regulates this transcription factor as part of the antioxidant and survival responses. Still, if we consent with the idea of master regulators existence, then a new question may rise, is this master regulator also redox-sensitive?
Must PKC isoforms, but mainly PKC-α and PKC-δ, have a singular structural characteristic, which makes them susceptible to oxidative modifications (Jin et al. 2011; Lee et al. 2012; Gopalakrishna et al. 2013). PKC N-terminal presents regulatory domains that are bind to zinc and cysteine-rich motives, which are quickly oxidized by hydrogen peroxide. When these motives are oxidized, the auto-inhibitory function in the regulatory domain is compromised and consequently PKC activity is stimulated (Gopalakrishna and Jaken 2000; 2013; Mishra and Vinayak 2014).
Finally, it is also important to consider that most of the transcription factors that participate in this kind of processes (like Nrf2 and NFκB) are capable of regulating both responses simultaneously: antioxidant and survival (Niture and Jaiswal 2012; Luna López at al., 2013; Ravuri et al. 2013), suggesting that it might be fundamental to induce both of them concurrently in order to counteract changes in redox state. However, the dilemma if there are a few master regulators or there is a broad and independent spectrum of responses is still debatable.
Nrf2: multiple inductors and effects
Now it has become fashionable to induce Nrf2 pathway in order to activate phase II and antioxidant response. There are more than 4,000 papers in PubMed describing the beneficial effects of numerous recognized Nrf2 inductors such as curcumin (Grynkiewicz and Ślifirski 2012; Correa et al. 2013; García-Niño and Pedraza-Chaverrí 2014), tBHQ (Alarcón-Aguilar et al. 2014), sulforaphane (Santana-Martínez et al. 2014; Kleszczyński et al. 2013), ginseng (Park et al. 2010), resveratrol (Singh et al. 2014), etc. Moreover, every year more and more new Nrf2 inductors are discovered like luteolin (Xu et al. 2014), oxaliplatin (Wang et al. 2014b), aldosterone (Queisser, et al. 2014), etc. There are studies related to their beneficial and protective effects in all kind of models since renal failure (Trujillo et al. 2013), cardioprotection after reperfusion injury (Buelna-Chontal et al. 2014), atmospheric pollutant-induced toxicity (Rubio et al. 2010), impaired mitochondrial function (Pulliam et al. 2014) and even epilepsy therapies (Wang et al. 2014a, b).
The problem with all these studies is that because Nrf2 inductors are electrophiles and pro-oxidant molecules, their protective effects depend on a particular dose to activate Nrf2, without severely harming the cell or organism. Therefore to predict Nrf2 beneficial or harmful effects is a challenging task, since it involves other features besides determining the accurate dose, especially being that Nrf2 activators have different induction mechanisms. Moreover, beyond Nrf2 activation, it might also be important to take into consideration what else is going on during Nrf2 activation, and how it might affect cellular response.
For example tert-butylhydroquinone (tBHQ) auto-oxidizes to tertbutylbenzoquinone (TBQ) entering a redox cycle reaction leading to the production of superoxide and hydrogen peroxide (Erlank et al. 2011), which modify redox state and activate Nrf2 (Taguchi et al. 2007; Imhoff and Hansen 2010) in the classical mechanism described for other Nrf2 traditional inductors such as curcumin and sulforaphane (Demirovic and Rattan 2011). Conversely, some authors have suggested that TBQ covalently binds to Keap1 through Cys23, Cys151, Cys226, and Cys368, activating Nrf2 directly through electrophilic Keap1 modification, rather than ROS formation (Abiko et al. 2011). Furthermore, there are additional Nrf2 activation mechanisms that can also occur through an autophagy impairment (Komatsu et al. 2010), for example as a result of rapamycin treatment, which inhibits mTOR activity leading to increased p62/SQSTM 1turnover, allowing Keap1 displacement from Nrf2 (Lerner et al. 2013).
Therefore, to determine the inductor hormetic dose to be used in animals and humans is a difficult matter, because even if the exact dose and mechanism were known, some other issues like individual genetic background and health status might modify the outcome. This would be an interesting thing to further review. In the case of Nrf2 inductors that modifying redox state, their protective-harmful effect might differ in function of age, health, nutritional state, habits, etc. Especially in the elderly, since old animals and cells are known to have decreased antioxidant systems and higher ROS production (Mendoza-Núñez et al. 2009; Calabrese et al. 2010; Vida et al. 2014) therefore, tBHQ optimal concentrations to induce Nrf2 might be different. The same applies for autophagy, which is known to be impaired with aging (Morimoto and Cuervo 2014)
Recent data from our lab (Alarcón-Aguilar et al. 2014) showed that when a tBHQ dose–response curve was performed in rat primary astrocytes the survival rate changed depending on the age of the donor animal. Astrocytes isolated from newborn animals (3–5 days), adult animals (9 month) and old animals (24 month) entirely survived to 10 and 25 mM tBHQ 24 h treatments, while approximately 50 % of the astrocytes derived from old and adult rats died after the 50-mM treatment, whereas at 100 mM more than 90 % of the cells from all age groups died. These results might not be suppressive in any way, since adults and old animals are more susceptible to oxidative stress, so tBHQ dose could have been very high for them. The interesting finding was observed when astrocytes were pre-treated with 25 mM tBHQ in order to activate Nrf2 survival pathway and generate an oxidative conditioning hormesis (OCH) response and obtain protection against a higher oxidative insult with the neurotoxic molecule MPP + (1-methyl-4-phenylpyridinium). Astrocytes derived from adult and old animals were able to activate Nrf2 (measured as Nrf2 translocation, Nrf2-ARE sequence binding and antioxidant enzymes increase) and showed 45 and 60 % protection against MPP + cell death respectively (p < 0.05) (quantified as cellular viability). Interestingly, astrocytes isolated from newborn rats pretreated with 25 mM tBHQ did not show a protective effect, and just when they were pretreated with a higher dose of 50 mM tBHQ, a 30 % protection was observed.
Against expected, these results showed that astrocytes from newborn animals required higher doses compared to the astrocytes from old animals in order to induce an hormetic protective response via Nrf2. This suggests that the beneficial-harmful effect cannot be predicted only based in high-low dose; however, in this case, age was a determinant matter.
Another important issue to discuss is Nrf2 beneficial-harmful effect during cancer development and expansion. On one hand, Nrf2 inductors have been used in clinical trials for cancer prevention and treatment; but, on the other hand, it has been reported that constitutive Nrf2 activation contributes to neoplasic cell survival and resistance to anticancer therapy (Namani et al. 2014). Even more, early studies from Yamamoto’s group showed that Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation (Wakabayashi et al. 2003).
Nrf2 cellular regulation is now known to be beyond Keap1 control, and the impact of nuclear receptors such as RARα, RXRα, PPARγ, ERα, ERRβ, and GR to functionally inhibit Nrf2 was recently reported (Namani et al. 2014).
Increased Nrf2 can help maintain an aggressive tumor phenotype by stimulating proliferation and offering protection from chemotherapy (Hayden et al. 2014; Mendelsohn and Larrick 2014). It has been reported recently that Nrf2 stimulates expression of transcription Kruppel-like factor 9 (Klf9). Klf9 suppress the expression of several important antioxidant enzymes such as thioredoxin reductase 2, resulting in further Klf9-dependent increases in ROS and subsequent cell death (Zucker et al. 2014).
Finally, the classical hormetic theory states that low-dose stimulation represents an adaptive biological response and high-dose stimulation results in disruption of homeostasis and deleterious and harmful effect (Satoh et al. 2013). As it has been also postulated that (Hoffmann 2009) the hormesis model runs counter to the assumption that effects at low doses can be predicted from those at high doses in a reasonably straightforward way. Nevertheless, when it comes to Nrf2 inductors and effects, the dose problem remains unsolved. Moreover, there are more features such as age (Alarcón el at. 2014), gender (Rohrer et al. 2014), and nutritional state that are just now starting to be pondered (Meakin et al. 2014), and should be seriously taken into consideration because they might modify the hormetic response.
The obesity paradox and the aging process
Classical hormesis states that low levels of environmental stress and toxins induce an hormetic response that has, in general, been conserved through natural selection and allows organisms to adapt or be protected against harder stress conditions.
However resistance to stress or hormetic preconditioning during pathological situations with chronic, low-grade inflammation and oxidative stress like obesity, have rarely studied, and it is not known if the organism is able to respond in a compensatory way that allows it to survive throughout aging despite its deterioration.
Even if the duration, severity and kind of stressor are supposed to determine if it has beneficial or harmful effects, there are still uncertainties to determine if a stressor will be hormetic or toxic. Wiese and coworkers (Wiese et al. 1995) utilized doses that caused toxicity instead of using doses in the hormetic zone, and observed adaptive responses that, in spite of the damage, were advantageous when a higher toxic challenge was used. Moreover, there are stress factors during normal metabolism and life that generate ROS/RNS that can lead to increased longevity (Epel and Lithgow 2014) or on the contrary, to bring about associated diseases (Shaw et al. 2014). Therefore it has been stated that the differences among individuals may account for those diverse responses; facing the same effector some individuals may develop pathologies and other generate adaptive responses (Mattson 2008).
This is also true for the same organism over time; it has been suggested that adaptation capacity declines during aging and the vigor loss to deal with stress accelerates the aging process exposing the organism to degenerative diseases. However, not all the tissues and organs might deteriorate at the same rate, and those stressors, allowing the organism to survive, might strengthen some of them.
Inflammation is a protective response against noxious stimuli that mostly involves a loss of function in the place where it is produced. This equilibrium between costs and benefits during the inflammatory response has been evolutionary optimized in the face of the different environmental conditions. Chronic inflammation is classically described as a response against infection or injury; nevertheless it has been recently related to ailments like obesity (Hotamisligil and Erbay 2008). When the diseases are caused by a wrong regulated inflammatory response, such as inflammatory tissue damage or sepsis, inflammation becomes prejudicial and even more damaging when this response exacerbates in magnitude and duration. Hence it is important to study and understand the different and particular processes that occur in the individuals that contribute to the development of inflammatory pathologies (Okin and Medzhitov 2012).
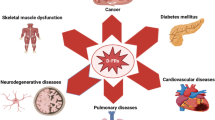
During obesity the excessive fat accumulation induces a low-grade chronic inflammation, which has been associated to the induction of several diseases such as diabetes, dyslipidemias, atherosclerosis, cancer, etc. Obese organisms show high systemic oxidative stress (Matsuda and Shimomura 2013) and altered cellular functions for example reticulum endoplasmic stress, mitochondrial dysfunction, insulin resistance and excessive storage of ectopic fat. Chronic inflammation during obesity also correlates with C-reactive protein, IL-6, IL-8 and TNF-α increase in serum (Shoelson et al. 2006; Kahn et al. 2006). But despite the changes described, it has been found that not all obese individuals exhibit increased risk of significant inflammation or metabolic disorders and on the contrary, there is a protective effect given by obesity against chronic diseases. Thirty percent of obese patients are metabolically healthy, with equal insulin sensibility to slim individuals, low liver fat and no carotid artery thickness. Recent studies have even suggested that there is a protection against steatosis development, visceral adipose tissue inflammation and less mortality in obese patients. Altogether these findings are proposed to contribute to “healthy obesity” in what has been called the “obesity paradox” (Bluher 2012) (Fig. 1).
Hormetic mechanism during the “obesity paradox”. All the organisms are exposed to a great collection of stressors during their lifespan, which might promote oxidative stress, cellular damage and inflammation. However, young organisms use inflammation as a beneficial signal to deal against different kind of stressors and stimulate tissue repair (turquoise). During the adulthood of an obese individual (green), inflammation signals produced by enlarged adipocytes might not only impair normal tissue repair, but might broaden oxidative stresses and cellular injury, leading to metabolic disorders and disease. The obesity paradox theory proposes that life time exposure to low-grade inflammation produced by adipocytes might induce an hormetic mechanism which could be able to have a better glucose metabolism, damage responses etc., which would protect the organism against chronic diseases during old age (lilac)
Albeit some physiological and endocrine mechanisms in adipose tissue have been proposed to be defensive, like leptin and adiponectin as cardio-protectors (Flegal and Kalantar-Zadeh 2013), the actual causes for this paradox are still unknown.
It is possible that the stress resistance to the obesity metabolic effects might be mediated by the cellular effective adaptation to stress, which counteracts the mechanisms that lead to insulin signaling alteration and β cells dysfunction. One important feature to mention is that most of the studies supporting the obesity paradox were performed in elderly patients cohorts (Beddhu 2004; Lainscak et al. 2012; Landbo et al. 1999; Uretsky et al. 2007). It seems the obesity may play a more important role in the elevated mortality risk in young people than in the elderly (Lee et al. 2010).
Aging is still one of the uncomprehended biological phenomena mainly because its inherent and complex multifunctional etiology and by the difficulty to dissociate normal aging effects from the aging that manifests as a consequence of age-associated disease (Kregel and Zhang 2007). Nevertheless, many authors have defined aging as a progressive decline in tissue homeostasis maintenance associated to a propensity to degenerative disease and death (Hayflick 1998). Numerous studies have shown that oxidative stress and mitochondrial dysfunction are two important elements that contribute to the aging process (Barbieri et al. 2009; Cui et al. 2011).
Mild oxidative stress has been proposed as a positive hormetic event; minor protein and lipid oxidative damage may have prophylactic effects over certain diseases associated to aging, that otherwise could cause cellular death and tissue damage (Yan 2014). Aging has also been associated with a pro-inflammatory phenotype in mammals due to the incremented expression of genes related to immune and inflammatory response like NFκB, as well as high-serum cytokine levels such IL-6 and TNF-α. This low-grade pro-inflammatory state during aging has been named “Inflammaging” and it has been proposed that macrophages participation, cellular stress and genetic factors of such condition contribute to predispose the organism to develop age-related diseases (de Magalhaes et al. 2009).
Appling the hormesis theory to the aging research, Rattan and coworkers in various sets of experiments performed within human fibroblasts in vitro, showed that repetitive exposure to mild heat as stressor has hormetic effects against several biochemical and aging parameters such as lower protein oxidation increased proteasome activity and antioxidant enzymes, resistance against ethanol, H2O2 and UV-B radiation, etc. (Rattan 2006).
Little has been studied about the obesity-aging process and many studies are missing in order to determine if oxidative or hormetic stress, or mild inflammation can influence the organism to have a better adaptation during lifespan and survive.
In our lab we have used a model where obesity is generated by neonatal neurointoxication with monosodium glutamate (MSG) to study if chronic low-grade inflammation generated by obesity might induce an hormetic effect on life and health span, in mice from 4 to 20 months. Our results showed that during the first 16 months, MSG-mice presented metabolic alterations like higher weight gain, higher Lee index, glucose intolerance, insulin resistance and other biochemical parameters like higher cholesterol and triglycerides levels and IL-6, TNF- α, but lower adiponectin (Hernández-Bautista et al. 2014). At tissue level, heart, lung, liver and kidney showed progressive lipoperoxidation and protein carbonylation (manuscript in preparation), however all metabolic and oxidative damages did not show significant differences when compared with control mice after 16 months, and MSG-mice even showed higher adiponectin levels with age. Recently, Song and coworkers reported that adiponectin plasma levels negatively correlated with fat percentage in male patients over 65 years old, but not in female elderly patients (Song et al. 2014); thus, they suggested the existence of certain gender-regulated mechanisms that might be affecting the relationship among changes in adiponectin levels, age and fat body composition. These observations agree with our results, where a relation between weight loss and increased adiponectin was observed from 16-months on, in both, control and MSG-obese female mice, but not in males (Hernández-Bautista et al. 2014). These results show that low adiponectin levels might be associated with chronic inflammation and insulin resistance.
On the other hand, some research has been done studying human elderly populations with paradoxical results. For example, it has been shown that centenaries have high adiponectin plasma levels which has been associated with longevity; however in other studies, the high adiponectin levels is a marker in patients with cardiovascular disease and is correlated with increased mortality. One explanation that has been suggested to explain these discrepancies is the possible deregulation of adiponectin due to inflammation, but the real explanation for these inconsistencies is still unknown (Yadav et al. 2013).
All the previous based the obesity-paradox proposing that suggesting that obesity effects might be attenuated during aging, pointing toward a possible hormetic mechanism mediated by mild inflammation, which might allow the organism to adapt to an adverse and deteriorated state.
Conclusions and perspectives
From our point of view the hormesis theory has to overcome several conceptual and practical challenges in order to be applied as a therapeutic tool to improve both, health and life span. It might be important to understand the different pathways that can be activated by the same stressor or inductor, and the diverse outcomes that this could lead. Mild oxidative stress in association to low-grade chronic inflammation is a stimulating avenue to be explored and surprising results might be achieved in tissues and organisms.
The reasons why centenarians and super-centenarians are able to reach the extreme limits of human life span are still unknown, but probably are related to the why they deal with homeostasis maintenance and here is where hormesis might have a significant interference.
Abbreviations
- OCH :
-
Oxidative conditioning hormesis
- H 2 O 2 :
-
Hydrogen peroxide
- Nrf2 :
-
Nuclear factor erythroid-derived 2-like 2
- Keap1 :
-
Kelch-like ECH-associated protein 1
- ROS/RNS :
-
Reactive oxygen species/Reactive nitrogen species
- CAT :
-
Catalase
- SOD :
-
superoxide dismutase
- GSH :
-
Reduced glutathione
- PKC :
-
Protein kinase C
- AOS :
-
Alginate oligosachharide
- AGEs :
-
advanced glycation end products
- tBHQ :
-
tert-butylhydroquinone
- TBQ :
-
tertbutylbenzoquinone
- MPP+ :
-
1-methyl-4-phenylpyridinium
References
Abiko Y, Miura T, Phuc BH, Shinkai Y, Kumagai Y (2011) Participation of covalent modification of Keap1 in the activation of Nrf2 by tert-butylbenzoquinone, an electrophilic metabolite of butylated hydroxyanisole. Toxicol Appl Pharmacol 255:32–39
Alarcón-Aguilar A, Luna-López A, Ventura-Gallegos JL, Lazzarini R, Galván-Arzate S, González-Puertos VY, Morán J, Santamaría A, Königsberg M (2014) Primary cultured astrocytes from old rats are capable to activate the Nrf2 response against MPP + toxicity after tBHQ pretreatment. Neurobiol Aging 35:1901–1912
Barbieri M, Boccardi V, Papa M, Paolisso G (2009) Metabolic journey to healthy longevity. Horm Res 71:24–27
Bayod S, Del Valle J, Lalanza JF, Sanchez-Roige S, de Luxán-Delgado B, Coto-Montes A, Canudas AM, Camins A, Escorihuela RM, Pallàs M (2012) Long-term physical exercise induces changes in sirtuin 1 pathway and oxidative parameters in adult rat tissues. Exp Gerontol 47:925–935
Beddhu S (2004) The body mass index paradox and an obesity, inflammation, and atherosclerosis syndrome in chronic kidney disease. Semin Dial 17:229–232
Bluher M (2012) Are there still healthy obese patients? Curr Opin Endocrinol Diabetes Obes 19:341–346
Buelna-Chontal M, Guevara-Chávez JG, Silva-Palacios A, Medina-Campos ON, Pedraza-Chaverri J, Zazueta C (2014) Nrf2-regulated antioxidant response is activated by protein kinase C in postconditioned rat hearts. Free Radic Biol Med [Epub ahead of print]
Calabrese EJ (2008) Converging concepts: adaptive response, preconditioning, and the Yerkes–Dodson law are manifestations of hormesis. J Ageing Res Rev 7:8–20
Calabrese EJ, Baldwin LA (2003) Hormesis at the National Toxicology Program (NTP): evidence of hormetic dose responses in NTP dose-range studies. Nonlinear Biol Toxicol Med 1:455–467
Calabrese V, Cornelius C, Mancuso C, Lentile R, Stella AM, Butterfield DA (2010) Redox homeostasis and cellular stress response in aging and neurodegeneration. Methods Mol Biol 610:285–308
Choi VW, Cheung AL, Cheng SH, Yu KN (2012) Hormetic effect induced by alpha-particle-induced stress communicated in vivo between zebrafish embryos. Environ Sci Technol 46:11678–11683
Correa F, Buelna-Chontal M, Hernández-Reséndiz S, García-Niño WJ, Roldán F, Soto V, Silva-Palacios A, Amador A, Pedraza-Chaverrí J, Tapia E, Zazueta C (2013) Curcumin maintains cardiac and mitochondrial function in chronic kidney disease. Free Radic Biol Med 61C:119–129
Cui H, Kong Y, Zhang H (2011) Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct 2012:646354
de Magalhaes JP, Curado J, Church GM (2009) Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics 25:875–881
Demirovic D, Rattan SI (2011) Curcumin induces stress response and hermetically modulates wound healing ability of human skin fibroblasts undergoing ageing in vitro. Biogerontology 12:437–444
Epel ES, Lithgow GJ (2014) Stress biology and aging mechanisms: toward understanding the deep connection between adaptation to stress and longevity. J Gerontol A Biol Sci Med Sci 69:S10–S16
Erlank H, Elmann A, Kohen R, Kanner J (2011) Polyphenols activate Nrf2 in astrocytes via H2O2, semiquinones, and quinones. Free Radic Biol Med 51:2319–2327
Flegal KM, Kalantar-Zadeh K (2013) Overweight, mortality and survival. Obesity 21:1744–1745
Hayflick L (1998) How and why we age. Exp Gerontol 33:639–653
García-Niño WR, Pedraza-Chaverrí J (2014) Protective effect of curcumin against heavy metals-induced liver damage. Food Chem Toxicol 69C:182–201
Gopalakrishna R, Jaken S (2000) Protein kinase C signaling and oxidative stress. Free Radic Biol Med 28:1349–1361
Gopalakrishna R, McNeill TH, Elhiani AA, Gundimeda U (2013) Methods for studying oxidative regulation of protein kinase C. Meth Enzymol 528:79–98
Grynkiewicz G, Ślifirski P (2012) Curcumin and curcuminoids in quest for medicinal status. Acta Biochem Pol 59:201–212
Hashmi MZ, Khan KY, Hu J, Naveedullah, Su X, Abbas G, Yu C, Shen C (2014) Hormetic effects of noncoplanar PCB exposed to human lung fibroblast cells (HELF) and possible role of oxidative stress. Environ Toxicol [Epub ahead of print]
Hayden A, Douglas J, Sommerlad M, Andrews L, Gould K, Hussain S, Thomas GJ, Packham G, Crabb SJ (2014) The Nrf2 transcription factor contributes to resistance to cisplatin in bladder cancer. Urol Oncol [Epub ahead of print]
Hernández-Bautista RJ, Alarcón-Aguilar FJ, Del C, Escobar-Villanueva M, Almanza-Pérez JC, Merino-Aguilar H, Fainstein MK, López-Diazguerrero NE (2014) Biochemical alterations during the obese-aging process in female and Male Monosodium Glutamate (MSG)-treated mice. Int J Mol Sci 15:11473–11494
Hine CM, Mitchell JR (2012) NRF2 and the phase II response in acute stress resistance induced by dietary restriction. J Clin Exp Pathol S4:7329
Hoffmann GR (2009) A perspective on the scientific, philosophical, and policy dimensions of hormesis. Dose Response 7:1–51
Hoffmann GR, Moczula AV, Laterza AM, Macneil LK, Tartaglione JP (2013) Adaptive response to hydrogen peroxide in yeast: induction, time course, and relationship to dose–response models. Environ Mol Mutagen 54:384–396
Hotamisligil GS, Erbay E (2008) Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol 8:923–934
Hsu YY, Chen CS, Wu SN, Jong YJ, Lo YC (2012) Berberine activates Nrf2 nuclear translocation and protects against oxidative damage via a phosphatidylinositol 3-kinase/Akt-dependent mechanism in NSC34 motor neuron-like cells. Eur J Pharm Sci 46:415–425
Imhoff BR, Hansen JM (2010) Tert-butylhydroquinone induces mitochondrial oxidative stress causing Nrf2 activation. Cell Biol Toxicol 26:541–5
Ishii T, Mann GE (2014) Redox status in mammalian cells and stem cells during culture in vitro: critical roles of Nrf2 and cystine transporter activity in the maintenance of redox balance. Redox Biol 2:786–794. doi:10.1016/j.redox.2014.04.008
Itoh K, Tong KI, Yamamoto M (2004) Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med 36:1208–1213
Jacobs AT, Marnett LJ (2007) Heat shock factor 1 attenuates 4-Hydroxynonenal-mediated apoptosis: critical role for heat shock protein 70 induction and stabilization of Bcl-XL. J Biol Chem 282:33412–33421
Jin H, Kanthasamy A, Anantharam V, Rana A, Kanthasamy AG (2011) Transcriptional regulation of pro-apoptotic protein kinase C delta: implications for oxidative stress-induced neuronal cell death. J Biol Chem 286:19840–19859
Kahn SE, Zinman B, Haffner SM, O’Neill MC, Kahn SE1, Zinman B, Haffner SM, O’Neill MC, Kravitz BG, Yu D, Freed MI, Herman WH, Holman RR, Jones NP, Lachin JM, Viberti GC. Obesity is a major determinant of the association of C-reactive protein levels and the metabolic syndrome in type 2 diabetes. Diabetes 55:2357–2364
Khoramian TS, Khalaj L, Ashabi G, Kiaei M, Khodagholi F (2011) Alginate oligosaccharide protects against endoplasmic reticulum- and mitochondrial-mediated apoptotic cell death and oxidative stress. Biomaterials 32:5438–545
Kleszczyński K, Ernst IM, Wagner AE, Kruse N, Zillikens D, Rimbach G, Fischer TW (2013) Sulforaphane and phenylethyl isothiocyanate protect human skin against UVR-induced oxidative stress and apoptosis: role of Nrf2-dependent gene expression and antioxidant enzymes. Pharmacol Res 78:28–40
Kobayashi A, Yamamoto M (2006) Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzym Regul 46:113–140
Kobayashi A, Kang M, Watai Y, Tong KI, Shibata T, Uchida K, Yamamoto M (2006) Oxidative and electrophilicstresses activate Nrf2 through inhibition of ubiquitination activity of Keap. Mol Cel Biol 26:221–229
Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M (2010) The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol 12:213–223
Kregel KC, Zhang HJ (2007) An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol 292:18–36
Kubicova L1, Hadacek F, Chobot V (2013) Quinolinic acid: neurotoxin or oxidative stress modulator? Int J Mol Sci 14(11):21328–38
Lainscak M, von Haehling S, Doehner W, Anker SD (2012) The obesity paradox in chronic diseases:facts and numbers. J Cachex Sarcopenia Muscle 3:1–4
Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP (1999) Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 160:1856–1861
Le Bourg E, Rattan SI (2009) Is hormesis applicable as a pro-healthy aging intervention in mammals and human beings, and how? Introduction to a special issue of Dose-Response. Dose Response 8:1–3. doi:10.2203/dose-response.09-052
Lee MJ, Wu Y, Fried SK (2010) Adipose tissue remodeling in pathophysiology of obesity. Curr Opin Clin Nutr Metab Care 13:371–376
Lee SK, Shehzad A, Jung JC, Sonn JK, Lee JT, Park JW, Lee YS (2012) Protein kinase Cα protects against multidrug resistance in human colon cancer cells. Mol Cells 34:61–69
Lerner C, Bitto A, Pulliam D, Nacarelli T, Konigsberg M, Van Remmen H, Torres C, Sell C (2013) Reduced mammalian target of rapamycin activity facilitates mitochondrial retrograde signaling and increases life span in normal human fibroblasts. Aging Cell 12:966–977
Luna-López A, Triana-Martínez F, López-Diazguerrero NE, Ventura-Gallegos JL, Gutiérrez-Ruiz MC, Damián-Matsumura P, Zentella A, Gómez-Quiroz LE, Königsberg M (2010) Bcl-2 sustains hormetic response by inducing Nrf-2 nuclear translocation in L929 mouse fibroblasts. Free Radic Biol Med 49:1192–1204. doi:10.1016/j.freeradbiomed.2010.07.004
Matsuda M, Shimomura I (2013) Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract 7:330–341
Mattson MP (2008) Hormesis and disease resistance: activation of cellular stress response pathways. Hum Exp Toxicol 27:155–162
Meakin PJ, Chowdhry S, Sharma RS, Ashford FB, Walsh SV, McCrimmon RJ, Dinkova-Kostova AT, Dillon JF, Hayes JD, Ashford ML (2014) Susceptibility of Nrf2-null mice to steatohepatitis and cirrhosis upon consumption of a high-fat diet is associated with oxidative stress, perturbation of the unfolded protein response, and disturbance in the expression of metabolic enzymes but not with insulin resistance. Mol Cell Biol 34:3305–3320
Mendelsohn AR, Larrick JW (2014) Paradoxical effects of antioxidants on cancer. Rejuvenation Res 17:306–311
Mendoza-Núñez VM, Sánchez-Rodríguez MA, Correa-Muñoz E (2009) Undernutrition and oxidative stress as risk factors for high blood pressure in older Mexican adults. Ann Nutr Metab 54:119–123
Mishra S, Vinayak M (2014) Ellagic acid inhibits PKC signaling by improving antioxidant defense system in murine T cell lymphoma. Mol Biol Rep 4:4187–4197
Morimoto RI, Cuervo AM (2014) Proteostasis and the aging proteome in health and disease. J Gerontol A Biol Sci Med Sci 69(Suppl 1):S33–8
Mukherjee S, Ghosh S, Choudhury S, Adhikary A, Manna K, Dey S, Sa G, Das T, Chattopadhyay S (2013) Pomegranate reverses methotrexate-induced oxidative stress and apoptosis in hepatocytes by modulating Nrf2-NF-κB pathways. J Nutr Biochem 24:2040–2050
Namani A, Li Y, Wang XJ, Tang X (2014) Modulation of NRF2 signaling pathway by nuclear receptors: implications for cancer. Biochim Biophys Acta 1843:1875–1885
Niture SK, Jaiswal AK (2012) Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J Biol Chem 287:9873–9886
Niture SK, Jain AK, Jaiswal AK (2009) Antioxidant-induced modification of INrf2 cysteine 151 and PKC-delta-mediated phosphorylation of Nrf2 serine 40 are both required for stabilization and nuclear translocation of Nrf2 and increased drug resistance. J Cell Sci 122:4452–4464
Nunn AVW, Bell JD, Guy GW (2009) Lifestyle-induced metabolic inflexibility and accelerated ageing syndrome: insulin resistance, friend or foe? kNutr Metab 6:16
Okin D, Medzhitov R (2012) Evolution of inflammatory diseases. Curr Biol 22:733–740
Park SH, Jang JH, Chen CY, Na HK, Surh YJ (2010) A formulated red ginseng extract rescues PC12 cells from PCB-induced oxidative cell death through Nrf2-mediated upregulation of heme oxygenase-1 and glutamate cysteine ligase. Toxicology 278:131–139
Pulliam DA, Deepa SS, Liu Y, Hill S, Lin AL, Bhattacharya A, Shi Y, Sloane L, Viscomi C, Zeviani M, Van Remmen H (2014) Complex IV deficient surf1−/− mice initiate mitochondrial stress responses. Biochem J. [Epub ahead of print]
Queisser N, Oteiza PI, Link S, Hey V, Stopper H, Schupp N (2014) Aldosterone activates transcription factor Nrf2 in kidney cells both in vitro and in vivo. Antioxid Redox Signal. [Epub ahead of print]
Rattan SI (2006) Hormetic modulation of aging and longevity by mild heat stress. Dose Response 3:533–546
Ravuri C1, Svineng G, Huseby NE (2013) Differential regulation of γ-glutamyltransferase and glutamate cysteine ligase expression after mitochondrial uncoupling: γ-glutamyltransferase is regulated in an Nrf2- and NFκB-independent manner. Free Radic Res 47:394–403
Ristow M, Schmeisser S (2011) Extending life span by increasing oxidative stress. Free Radic Biol Med 51:327–336
Rohrer PR, Rudraiah S, Goedken MJ, Manautou JE (2014) Is nuclear factor erythroid 2-related factor 2 responsible for sex differences in susceptibility to acetaminophen-induced hepatotoxicity in mice? Drug Metab Dispos 42:1663–1674
Rubio V, Valverde M, Rojas E (2010) Effects of atmospheric pollutants on the Nrf2 survival pathway. Environ Sci Pollut Res Int 17:369–382
Santana-Martínez RA, Galván-Arzáte S, Hernández-Pando R, Chánez-Cárdenas ME, Avila-Chávez E, López-Acosta G, Pedraza-Chaverrí J, Santamaría A, Maldonado PD (2014) Sulforaphane reduces the alterations induced by quinolinic acid: modulation of glutathione levels. Neuroscience 272:188–198
Satoh T, McKercher SR, Lipton SA (2013) Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic Biol Med 65:645–57
Scannapieco AC, Sørensen JG, Loeschcke V, Norry FM (2007) Heat-induced hormesis in longevity of two sibling drosophila species. Biogerontology 8:315–325
Shaw PX, Werstuck G, Chen Y (2014) Oxidative medicine and cellular longevity. Oxi Med Cel Longev 569146
Shoelson SE, Lee J, Goldfine AB (2006) Inflammation and insulin resistance. J Clin Investig 7:1793–1801
Singh B, Shoulson R, Chatterjee A, Ronghe A, Bhat NK, Dim DC, Bhat HK (2014) Resveratrol inhibits estrogen-induced breast carcinogenesis through induction of NRF2-mediated protective pathways. Carcinog. [Epub ahead of print]
Song HJ, Oh S, Quan S, Ryu OH, Jeong JY, Hong KS, Kim DH (2014) Gender differences in adiponectin levels and body composition in older adults: hallym aging study. BMC Geriatr 14:8
Speciale A, Chirafisi J, Saija A, Cimino F (2011) Nutritional antioxidants and adaptive cell responses: an update. Curr Mol Med 11:770–789
Taguchi K, Fujii S, Yamano S, Cho AK, Kamisuki S, Nakai Y, Sugawara F, Froines JR, Verhagen H, Furnee C, Schutte B, Hermans R, Kumagai Y (2007) An approach to evaluate two-electron reduction of 9,10-phenanthraquinone and redox activity of the hydroquinone associatedwith oxidativestress. Free Radic Biol Med 43:789–799
Trujillo J1, Chirino YI, Molina-Jijón E, Andérica-Romero AC, Tapia E, Pedraza-Chaverrí J (2013) Renoprotective effect of the antioxidant curcumin: recent findings. Redox Biol 1:448–456
Uretsky S, Messerli FH, Bangalore S, Champion A, Cooper-Dehoff RM, Zhou Q, Pepine CJ (2007) Obesity paradox in patients with hypertension and coronary artery disease. Am J Med 120:863–870
Vida C, Gonzalez EM, Fuente MD (2014) Increase of oxidation and inflammation in nervous and immune systems withaging and anxiety. Curr Pharm Des [Epub ahead of print]
Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, Harada T, Engel JD, Yamamoto M (2003) Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet 35:238–245
Wang W, Wu Y, Zhang G, Fang H, Wang H, Zang H, Xie T, Wang W (2014a) Activation of Nrf2-ARE signal pathway protects the brain from damage induced by epileptic seizure. Brain Res 1544:54–61
Wang XJ, Li Y, Luo L, Wang H, Chi Z, Xin A, Li X, Wu J, Tang X (2014b) Oxaliplatin activates the Keap1/Nrf2 antioxidant system conferring protection against the cytotoxicity of anticancer drugs. Free Radic Biol Med 70:68–77. doi:10.1016/j.freeradbiomed.2014.02.010
Wiese AG, Pacifici RE, Davies KJA (1995) Transcient adaptation to oxidative stress in mammalian cells. Arch Biochem Biophys 318:231–240
Xu J, Wang H, Ding K, Zhang L, Wang C, Li T, Wei W, Lu X (2014) Luteolin provides neuroprotection in models of traumatic brain injury via the Nrf2–ARE pathway. Free Radic Biol Med 71:186–195
Yadav A, Kataria MA, Saini V, Yadav A (2013) Role of leptin and adiponectin in insulin resistance. Clin Chim Acta 417:80–84
Yan L (2014) Positive oxidative stress in aging and aging-related disease tolerance. Redox Biol 2:165–169
Zanotto-Filho A, Gelain DP, Schröder R, Souza LF, Pasquali MA, Klamt F, Moreira JC (2009) The NF kappa B-mediated control of RS and JNK signaling in vitamin A-treated cells: duration of JNK-AP-1 pathway activation may determine cell death or proliferation. Biochem Pharmacol 77:1291–1301
Zucker SN, Fink EE1, Bagati A, Mannava S, Bianchi-Smiraglia A, Bogner PN, Wawrzyniak JA, Foley C, Leonova KI, Grimm MJ, Moparthy K, Ionov Y, Wang J, Liu S, Sexton S, Kandel ES, Bakin AV, Zhang Y, Kaminski N, Segal BH, Nikiforov MA (2014) Nrf2 amplifies oxidative stress via induction of Klf9. Mol Cell 53:916–928
Acknowledgments
The authors want to acknowledge M. in BE Luis A. Maciel for his comments to this paper. This work was supported by CONACyT’s grant CB-2012-1-178349. As well as the “Red Temática de Investigación en Salud y Desarrollo Social” from CONACYT and INGER DI-PI004/2012.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luna–López, A., González-Puertos, V.Y., López-Diazguerrero, N.E. et al. New considerations on hormetic response against oxidative stress. J. Cell Commun. Signal. 8, 323–331 (2014). https://doi.org/10.1007/s12079-014-0248-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-014-0248-4