Abstract
Tert-butylhydroquinone (tBHQ), the major metabolite of butylated hydroxyanisole, induces an antioxidant response through the redox-sensitive transcription factor, nuclear factor-E2-related factor-2 (Nrf2). However, the mechanism by which tBHQ induces Nrf2 activity is not entirely understood. Here, we show that tBHQ preferentially alters the redox status in the mitochondrial compartment in HeLa cells. HeLa cells treated with tBHQ showed a preferential oxidation of mitochondrial thioredoxin-2 (Trx2), while cellular glutathione and cytosolic thioredoxin-1 were not affected. Preferential mitochondrial oxidation by tBHQ was supported by detection of reactive oxygen species (ROS) specific to this compartment. To determine the role of Trx2 in regulating downstream effects of tBHQ, HeLa cells were transiently transfected with an empty, Trx2, or C93S (Cys93Ser) Trx2 dominant-negative mutant expression vector. Overexpression of Trx2 decreased basal mitochondrial ROS production, whereas expression of C93S Trx2 enhanced it. In addition, under untreated conditions, expression of C93S Trx2 led to an increase in the basal activities of Nrf2. With tBHQ treatments, Trx2 overexpression suppressed Nrf2 accumulation and activity, whereas expression of C93S Trx2 had no effect on the degree of inducibility or Nrf2 accumulation but did increase the overall activity of Nrf2. Quantitative polymerase chain reaction analysis of Nrf2-regulated gene expression corroborate Trx2 control of tBHQ-mediated Nrf2 activation. These data show a compartment-specific effect where tBHQ-induced Nrf2 signaling is mediated by Trx2 and suggest that antioxidant status in various compartments would provide different levels of control of redox signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cells have adapted responses to deal with their recurring interactions with their environment, including those that might induce oxidative stress, macromolecule damage, and cell death. While these responses are diverse, one response, the induction of phase II detoxification enzymes, relies heavily upon the redox-sensitive transcription factor, nuclear factor (erythroid-derived 2)-like 2 (Nrf2). Upon activation, Nrf2 has been shown to upregulate many genes involved in ROS and xenobiotic metabolism, including elements of the glutathione (GSH) and thioredoxin-1 (Trx1) systems (Solis et al. 2002; Nakaso et al. 2003; Petzer et al. 2003). Activation of Nrf2 has been shown to protect cells against carcinogens (Fahey et al. 2002) and xenobiotics (Kensler et al. 2007).
Nrf2 is a ubiquitously expressed transcription factor and is found in most tissues. Nrf2 is relatively inactive during unstressed conditions. Nrf2 is sequestered in the cytosol through interaction with an inhibitor protein, Kelch-like erythroid cell-derived protein with CNC homology (ECH)-associated protein1 (Keap1). While sequestered, Nrf2 is constitutively degraded through ubiquitination and subsequent proteosomal degradation. Keap1 contains many cysteine residues, of which approximately one third are cysteines with low predicted pK a (Copple et al. 2008), as they are flanked by at least one basic amino acid (Miseta and Csutora 2000). These cysteines act as redox sensors, which, upon changes to the redox environment and oxidative stress, are modified directly through either conjugation or oxidation to a sulfenic acid (−SOH). Modification causes a Keap1 conformational change resulting in the release of Nrf2, which translocates to the nucleus, binds to an antioxidant response element (ARE) sequence (5′-RTGACnnnGC-3′) (Rushmore et al. 1991), and causes an upregulation of phase II enzymes and antioxidant systems. There is some evidence that different activators of Nrf2 may act on different cysteine residues via the elicitation of an antioxidant response (Kobayashi et al. 2009). Having multiple mechanisms by which Keap1 can detect chemical insult provides a means by which Nrf2 can respond to many different types of stimuli.
Numerous inducers of Nrf2 have shown potential for therapeutic interventions to inhibit cancer and disease, including those that are natural products and synthetic derivatives. Tert-butylhydroquinone (tBHQ) is a metabolite of the chemical compound butylated hydroxyanisole and induces Nrf2 activation and conveys protection against hydrogen peroxide, 6-hydroxydopamine, the pesticidal deltamethrin, and other toxicants (Li et al. 2002a, 2007; Hara et al. 2003; Kraft et al. 2004). However, the mechanism by which tBHQ activates Nrf2 is not entirely understood. Reports differ on the probable mechanism of tBHQ-mediated Nrf2 activation, including those involving reactive oxygen species (ROS) (Pinkus et al. 1996; Itoh et al. 1999; Hara et al. 2003), where antioxidant supplementation was shown to inhibit tBHQ-induced gene induction in certain cell types but not in others. tBHQ-induced ROS production may be a consequence of redox cycling from a fully reduced form to an oxidized form as a semiquinone anion radical (Gharavi et al. 2007). A possible rationale for cell-specific differences may be due to variations in subcellular compartmentalized redox status. Previous work has shown that redox-sensitive transcription factor activity can be regulated by ROS production and redox status changes in specific subcellular compartments (Halvey et al. 2005, 2007; Hansen et al. 2006c, b, 2007).
Here, we show that tBHQ induces mitochondrial oxidative stress en route to activation of Nrf2 in HeLa cells and that the mitochondria-specific antioxidant, thioredoxin-2 (Trx2), regulates tBHQ-induced stimulation of Nrf2 activity. These may provide a clearer paradigm to explain the mechanisms of tBHQ as an Nrf2 activator.
Methods
Cell culture
HeLa cells were purchased from American Type Culture Collection (Manassas, VA, USA) and were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics and maintained in humidified incubator at 5% CO2 and 20% O2.
Fluorescence detection of ROS
To determine ROS production as a consequence of tBHQ treatments, two different microplate fluorescent probes were used, dichlorofluorescein (DCF) for cellular hydrogen peroxide (Wang and Joseph 1999) and MitoSox Red for mitochondrial superoxide anion (Hansen et al. 2006c). For DCF assays, HeLa cells were grown until confluent and loaded with 100 µM DCF (Invitrogen) in loading medium (DMEM with 1% FBS) for 30 min. Cells were washed and then treated with tBHQ (0–15 μM in DMSO) for 1 h. DCF fluorescence was followed in a fluorometer (M2 microplate reader, Molecular Devices) at 488-nm excitation and 520-nm emission. Comparisons of tBHQ-treated cells were made with untreated cells.
For mitochondrial ROS production, HeLa cells were grown to confluence treated with tBHQ (0–15 μM) for 1 h, after which, they were loaded with 5 µM MitoSox Red in Hank's balanced salt solution for 10 min at 37°C. Cells were washed with warm buffer prior to measuring fluorescence in the microplate reader. MitoSox Red fluorescent intensity was determined at 510-nm excitation and 580-nm emission.
Glutathione redox measurements
GSH and GSSG were quantified by high-performance liquid chromatography with fluorescence detection, expressed as molar concentrations based on cell volume, which were used to calculate the steady-state redox potential values using the Nernst equation (Jones 2002).
Thioredoxin-1 redox measurements
Redox Western analysis of Trx-1 redox state was slightly modified from the original protocol as described previously (Watson et al. 2003; Hansen et al. 2004). Separation of Trx-1 oxidized and reduced forms is achieved via derivatization with iodoacetic acid (IAA) and separated based on differences in charge on a non-reducing non-denaturing gel. In brief, Trx-1 was carboxymethylated in guanidine-Tris solution (6 M guanidine-HCl, 50 mM Tris, pH 8.3, 3 mM EDTA, 0.5% (v/v) Triton X-100) containing 50 mM IAA and incubated for 30 min at 37°C. Excess IAA was removed by Sephadex chromatography (MicroSpin G-25 columns, Amersham Biosciences), after which, samples were diluted in 5× non-reducing sample buffer (0.1 M Tris–HCl, pH 6.8, 50% (v/v) glycerol, 0.05% (w/v) bromophenol blue) and separated on a native polyacrylamide gel (5% stacking gel, 15% resolving gel). Gels were electroblotted to nitrocellulose membrane and probed for Trx1 using anti-Trx1 primary antibody (American Diagnostica, Greenwich, CT, USA) and AlexaFluor 680 nm anti-goat IgG secondary antibody (Molecular Probes, Eugene, OR, USA). Membranes were scanned with an Odyssey infrared scanner (Li-Cor, Lincoln, NE, USA). Densitometric analysis was performed with the Odyssey scanning software. Redox potentials were determined using band intensities and the Nernst equation \( \left( {{E_{\rm{h}}} = {E_0} + 2.3 \times RT/nF \times \log } \right.\left( {\left[ {{\hbox{TRX}} - {\hbox{SS}}} \right]/\left[ {{\hbox{TRX}} - {\hbox{S}}{{\hbox{H}}_2}} \right];{E_0} = - 254\;{\hbox{mV}}\;{\hbox{at}}\;{\hbox{pH}}\;7.4} \right) \).
Thioredoxin-2 redox measurements
Trx2 redox methods used were as previously described (Halvey et al. 2005). Resolution of oxidized and reduced Trx2 is achieved with derivatization with 4-acetoamido-4′-maleimidylstilbene-2,2′-disulphonic acid (AMS) and separated based on protein size on a non-reducing sodium dodecyl sulfate (SDS) gel. Following tBHQ treatment, HeLa cells were precipitated with ice-cold trichloroacetic acid (10%) for 30 min at 4°C then centrifuged, resuspended in 100% acetone, and incubated at 4°C for 30 min. Following centrifugation, acetone was removed, and protein pellets were dissolved in lysis/derivatization buffer [20 mM Tris/HCl, pH 8, 15 mM AMS (Invitrogen) with 1% SDS] and incubated at room temperature for 3 h. Oxidized and reduced Trx2 were separated on an SDS/15% polyacrylamide gel in the presence of non-reducing loading buffer. Immunoblotting was performed as described above but utilized rabbit anti-Trx2 as the primary antibody. AlexaFluor 680-conjugated anti-rabbit antibody (Invitrogen) was used as the secondary antibody. Integrated densitometries of oxidized and reduced Trx2 bands were used with the Nernst equation to calculate E h values where E 0 = −330 mV at pH 7.6 (mitochondrial pH).
Vector transfections and ARE-driven luciferase activity measurements
HeLa cells were transfected with combinations of either an empty, human Trx2, or mutant C93S (Cys → Ser) Trx2 expression vector (Chen et al. 2002), a human NQO1-ARE (NADPH quinone:oxidoreductase 1-ARE, 5′-TCACAGTGACTCAGCAAA-3′) luciferase reporter (vector construction described elsewhere (Erickson et al. 2002)), and a pLacZ vector for at least 24 h using Fugene6 transfection reagent (Roche) per the manufacturer's instructions. The C93S Trx2 mutant act as a dominant-negative by binding with Trx2-related substrates, but due to the lack of the other vicinal thiol found in functional Trxs, the C93S Trx2 is unable to completely reduce the substrate and remains bound through the formed disulfide. Thus, the C93S Trx2 mutant may effectively inhibit Trx2 substrate function, inhibit the function of endogenous Trx2, and promote oxidative stress. In the case of peroxiredoxins, the overall peroxide elimination power in the mitochondria could be decreased. Increased Trx2 (wild-type and C93S) expression of approximately 35% was verified via immunoblot analysis following 24 h of transfection, normalized to β-actin expression. After transfection, cells were treated with tBHQ (0–15 μM) for 8 h, washed with phosphate buffer solution, and then collected in reporter lysis buffer (Promega). Luciferase assay reagent (Promega) was added to lysates, and luminescence was measured on a luminometer (Lumicount). The NQO1-ARE luciferase reporter activity was normalized to β-galactosidase activity using a kit for the colorimetric measurement of 2-nitrophenyl-β-d-galactopyranoside (ONPG) hydrolysis product (Sigma-Aldrich).
Assessment of Nrf2 protein levels was performed via SDS-polyacrylamide gel electrophoresis and immunoblotting techniques. Cells were transfected with empty, Trx2, or C93S Trx2 mutant expression vectors for at least 24 h, after which, they were treated with 15 μM tBHQ for 2 h. Cells were collected in RIPA buffer (Sigma) with protease inhibitors. Nrf2 protein levels were detected with a rabbit Nrf2 primary antibody (Santa Cruz Biotechnology) and a goat anti-rabbit AlexaFluor 680 secondary antibody (Invitrogen). Blots were visualized via the Odyssey Scanning system (Li-Cor) via methods provided by the manufacturer.
SiRNA knockdown of Trx2
siRNA and related transfection reagents were purchased from Santa Cruz Biotechnologies and used per the manufacturer's instructions. After transfection, quantification of Trx2 was determined via immunoblotting techniques to verify a decrease in Trx2 protein levels and standardized to β-actin expression. Immunoblot visualization of Trx2 was performed as previously outlined above.
Quantitative real-time fluorescence PCR of Nrf2-regulated genes
RNA from transfected (control, Trx2 or C93S vectors, or siRNA) cells with and without tBHQ (15 µM) treatments was collected with the RNAEasy Kit (Qiagen). cDNA was synthesized from collected RNA with the Quantitect DNA synthesis kit (Qiagen) and prepared for real-time fluorescence polymerase chain reaction (PCR) analysis of the expression of glutamylcysteine ligase (Gclc), NADPH quinone:oxidoreductase 1 (NQO1), and heme oxygenase-1 (HO1), which are genes known to be regulated by Nrf2 (Petzer et al. 2003). Primers and SYBR green real-time PCR kit were purchased from SABiosciences and were used per the manufacturer's instructions.
Statistical analysis
Each measurement is the result of at least three independently performed experiments. The one-way analysis of variance was employed to determine whether the means of different groups were significantly different. The Tukey's post hoc test was used to determine the significance for all pairwise comparisons of interest.
Results
tBHQ induces mitochondrial reactive oxygen species production
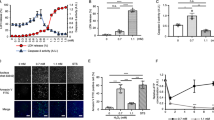
DCF assays have been used in multiple studies to illustrate the increase in production of ROS, primarily hydrogen peroxide, on a cellular level. In our studies, DCF fluorescence did not change with increasing concentrations of tBHQ (Fig. 1). However, DCF fluorescence does not quantify changes in ROS production in subcellular compartments, such as the mitochondria; but rather, it usually is typical of whole cell ROS production. Thus, we used MitoSox Red as a mitochondria-specific superoxide anion indicator and treated cells with tBHQ, demonstrating a dose-dependent increase in fluorescence (Fig. 1). These results are indicative of an increase in mitochondrial ROS production.
Compartmentalized tert-butylhydroquinone (tBHQ)-induced reactive oxygen species (ROS) generation in HeLa cells. Cells were treated with varying concentrations of tBHQ (0–15 μM) for 1 h. ROS was detected by dichlorofluorescein (whole cells) or MitoSox (mitochondrial specific) fluorescent dyes. tBHQ caused a dose-dependent increase in ROS preferential to the mitochondria, where little change was detected on the whole cell level. Asterisks denote a statistically significant difference (p < 0.05). Results are from three independently run experiments
Trx2, but not Trx1 or GSH, redox potential becomes oxidized with tBHQ treatment
Previous work has shown that redox couples are not in equilibrium but rather are independently regulated (Hansen et al. 2006a; Jones 2006). Thus, we evaluated the redox potentials (E h) of GSH, Trx1, and Trx2. GSH E h was unchanged after 1 h of tBHQ treatments (Fig. 2a) and remained at approximately −230 mV. Similarly, Trx1 E h did not change as well (Fig. 2b) and remained at approximately −288 mV. However, Trx2 E h became oxidized with tBHQ treatment (Fig. 2b). Untreated cells had a Trx2 E h of −357 mV but significantly increased to approximately −330 mV with tBHQ treatments. Oxidation of Trx2 relative to GSH and Trx1 suggests that tBHQ preferentially causes oxidative stress in the mitochondrial compartment.
a Redox status of Trx2, Trx1, and glutathione (GSH) with tert-butylhydroquinone (tBHQ) treatment. Cells were treated with tBHQ (0–15 μM) for 1 h and then assayed for each individual redox couple (see “Methods”). tBHQ treatment did not affect the redox status of either Trx1 or GSH, but did cause a significant oxidation of Trx2. b Redox western blots of Trx1 and Trx2 in HeLa cells treated with tBHQ for 1 h. Trx1 bands did not shift as a consequence of tBHQ treatments, which is indicative of oxidation, but rather remained unaffected. Trx2 bands did shift, indicating oxidation of Trx2 with tBHQ exposures. Asterisks denote a statistically significant difference (p < 0.05). Results are from three independently run experiments
Trx2 modulates mitochondrial ROS production
Transfection resulted in an increase (by approximately 50% based on densitometry) in total Trx2 protein as compared to basal levels of expression (Fig. 3a). Overexpression of Trx2 causes a decrease in ROS as detected by MitoSox Red compared to empty vector controls, where Trx2 overexpressing cultures showed a 50% decrease in ROS production (Fig. 3b). Conversely, overexpression of C93S Trx2 caused a significant increase in mitochondrial ROS production, measured as a 50% increase from empty vector controls. These results were confirmed via fluorescence microscopy (Fig. 3c). These results show that Trx2 is an important regulator of basal mitochondrial ROS production.
a Immunoblot analysis of cultures transfected with either the human Trx2 or C93S Trx2 overexpression vectors. Transient transfection demonstrates an increase of approximately 35% from empty vector (pcDNA 3.1) transfected controls. b Overexpression of Trx2 decreases mitochondrial reactive oxygen species (ROS) production. HeLa cells were transiently transfected with a Trx2 overexpression vector and were then stained with MitoSox Red, an indicator of mitochondrial ROS production. Cells overexpressing Trx2 had less mitochondrial ROS than empty vector controls. Conversely, overexpression of the C93S Trx2 mutant exacerbated mitochondrial ROS production as compared to the control. Asterisks denote a statistically significant difference (p < 0.05) from controls. Results are from three independently run experiments. c MitoSox Red staining of HeLa cells transfected with empty, Trx2 overexpressing, or C93S Trx2 mutant vectors. The intensity of MitoSox Red fluorescence is indicative of ROS production
Trx2 modulates tBHQ-mediated Nrf2 activity
An accepted mechanism by which Nrf2 activity is increased is through the stabilization of Nrf2 (Hayes and McMahon 2009). When Nrf2 is bound to Keap1, it is continuously ubiquitinated and degraded (McMahon et al. 2003). Following activation, Nrf2 is no longer degraded but rather accumulates within the cell. Thus, Nrf2 activation is often measured through an increase in protein levels. HeLa cells were transfected with either empty, Trx2, or C93S Trx2 mutant expression vectors. Treatment with tBHQ (15 μM) showed an increase in Nrf2 protein after 2 h in the empty vector controls (Fig. 4). However, in Trx2 transfected cultures, there was not an increase in Nrf2 protein levels, suggesting that Nrf2 transactivation did not occur. Expression of the C93S Trx2 mutant increased Nrf2 levels at baseline compared to untreated empty vector controls. tBHQ treatments in the C93S Trx2 transfected cultures showed an increase in Nrf2 concentrations that were similar to the level of induction in tBHQ-treated controls.
Nrf2 protein levels in transfected cells treated with tert-butylhydroquinone (tBHQ). Cells transfected with an empty vector showed an increase in Nrf2 protein levels with tBHQ (15 μM) after 2 h. However, in cells transfected with Trx2, no increase in Nrf2 levels was observed with tBHQ treatment. Overexpression of C93S Trx2 showed an increase in Nrf2 activation with tBHQ treatment. β-actin was used as a loading control. The blot shows typical results from three independently perform experiments
To verify ARE involvement, we utilized ARE luciferase reporter constructs. Transient co-transfections of the human NQO1-ARE luciferase reporter, β-gal reporter, and either empty, Trx2, or C93S Trx2 expression vectors were achieved in HeLa cells. Luciferase activity showed that basal ARE activity was increased as a consequence of C93S Trx2 overexpression as compared to empty vector controls (Fig. 5a). Overexpression of Trx2 did not affect basal ARE activity, which was comparable to the activity in the empty vector controls. Increased ARE activity due to C93S Trx2 overexpression may be related to an overall increase in ROS as a mechanism by which the ARE is activated, whereas under unstimulated basal conditions, overexpression of Trx2 does not affect ARE responses. These results suggest that Trx2-mediated ROS activation of the ARE may be more important during periods of mitochondrial stress than regulation of basal Nrf2 activities.
Tert-butylhydroquinone (tBHQ)-stimulated antioxidant response element (ARE) activity in HeLa cells transfected with Trx2 or C93S Trx2 expression vectors. a In empty vector controls, tBHQ activated human NQO1-ARE luciferase reporting in a dose-dependent manner. Similarly, in cells expressing C93S Trx2, human NQO1-ARE luciferase reporting increased in a dose-dependent manner but when compared to empty vector controls, absolute levels were significantly higher. In cells transfected with the Trx2 expression vector, tBHQ was unable to stimulate the ARE. Asterisks denote statistically significant changes (p < 0.05) in Nrf2 activity in the transfected cultures as compared to the empty vector control under basal conditions only. b Induction of the ARE in cells overexpressing Trx2 or C93S Trx2. Normalizing changes to percent change from untreated tBHQ had a very similar effect in both the empty vector control and the C93S Trx2 transfected cells, where a dose-dependent increase in the induction of the NQO1-ARE was noted, that were virtually identical. In cells transfected with Trx2 overexpressing vectors, tBHQ-initiated induction was blocked, showing no significant changes from untreated cultures. Asterisks denote a statistically significant difference (p < 0.05) from empty vector controls treated at the same concentration of tBHQ
With tBHQ treatments, absolute activities of ARE, based on luciferase activity/β-gal activity, were very different (Fig. 5a). While empty vector controls and Trx2 transfected cultures showed similar activity in untreated cultures, tBHQ treatments caused a significant increase in ARE activity, starting at the lowest concentration of 2.5 μM tBHQ in empty vector controls. Trx2 transfected cultures did not respond to tBHQ, even at the highest concentration of 15 μM. In C93S Trx2 transfected cells, basal activities were already elevated compared to empty vector control cultures, but responded in a dose-dependent manner to tBHQ treatments.
While these data show changes in ARE activity, it is also important to quantitate ARE induction. To measure ARE induction, data were normalized to their respective untreated measurements. Dosing empty vector control cultures with increasing concentrations of tBHQ showed a dose-dependent increase in ARE activity via increased luciferase reporting (Fig. 5b). A statistically significant induction was achieved at 10 μM tBHQ. In C93S Trx2 transfected cultures, tBHQ showed a very similar dose-dependent ARE induction pattern, being nearly identical to the empty vector controls. Interestingly, in Trx2 transfected cultures, ARE induction was inhibited regardless of the tBHQ treatments, demonstrating the regulatory nature of Trx2 in tBHQ-induced ARE activation.
Nrf2-regulated gene expression is modulated by Trx2 expression
While overexpression of Trx2 appears to alter Nrf2 as indicated through luciferase reporting construct, quantitative fluorescence real-time PCR was also performed to confirm changes in Nrf2-related genes. Results show that all three genes evaluated, Gclc, NQO1, and HO-1, were significantly upregulated by C93S expression, even without tBHQ stimulation (Fig. 6a). The addition of tBHQ showed an increase in control cultures where the expression of these same genes was significantly increased (Fig. 6b). Interestingly, fold changes from tBHQ-treated cultures were altered by overexpression of Trx2 or C93S Trx2. Overexpression of Trx2 resulted in a significant decrease of Gclc and NQO1 expression, but HO-1 was unaffected (Fig. 6c). Conversely, overexpression of the C93S Trx2 mutant resulted in the enhanced expression compared to the tBHQ-treated empty vector control cultures, demonstrating the capacity of Trx2 to regulate tBHQ-mediated gene expression.
Quantitative analysis of Nrf2-regulated gene expression. Gclc, NQO1, and HO-1 gene expression was measured post-transfection with either empty vector controls, Trx2, or C93S Trx2 expression vectors. a Prior to tert-butylhydroquinone (tBHQ) treatment, basal levels of Gclc, NQO1, and HO-1 were determined. Overexpression of Trx2 did not significantly affect the overall expression of any of the genes measured. Overexpression of the C93S Trx2 mutant showed a substantial increase in all genes measured. Asterisks denote a statistically significant difference (p < 0.05) from basal levels of gene expression in the untreated, empty vector control cultures. b tBHQ induction of Gclc, NQO1, and HO-1 expression in empty vector control cells. As expected, tBHQ caused a substantial increase in all three genes measured compared to non-tBHQ-treated cultures. Asterisks denote a statistically significant difference (p < 0.05) from untreated and tBHQ-treated (15 µM) empty vector control cultures. c Changes in tBHQ-induced levels of Gclc, NQO1, and HO-1 gene expression in cells overexpressing either Trx2 or mutant C93S Trx2. Compared to control cultures treated with tBHQ, Trx2 overexpression significantly decreased tBHQ-mediated induction of both Gclc and NQO1 but not HO-1. Conversely, overexpression of C93S Trx2 potentiated tBHQ-mediated induction of the expression of all three genes. Asterisks denote a statistically significant difference (p < 0.05) from tBHQ-treated empty vector control cultures and the Trx2 or C93S Trx2 transfected cultures
siRNA knockdown experiments were performed, and Trx2 expression was decreased by approximately 70% (Fig. 7a). Loss of Trx2 expression resulted in an increase expression of Gclc, NQO1, and HO-1 even without tBHQ stimulation (Fig. 7b). NQO1 expression was significantly increased in Trx2-deficient cultures treated with tBHQ as compared to those not receiving tBHQ. Interestingly, in these same cultures, tBHQ treatment did not significantly increase Gclc or HO-1 expression significantly, suggesting that in cultures lacking adequate Trx2 function and at 15 µM, tBHQ cannot potentiate the expression of these genes.
siRNA knockdown of Trx2 and expression of tert-butylhydroquinone (tBHQ)-stimulated and untreated cultures. a Verification of Trx2 knockdown by siRNA methodologies. Protein levels of Trx2 are significantly decreased with siRNA transfection, measured as approximately 30% of original basal levels (based on densitometry). b Changes in Nrf2-regulated gene expression in Trx2-deficient cultures. tBHQ induces a significant increase in the expression of Gclc, NQO1, and HO-1 in non-transfected cultures. Untreated siRNA transfected cultures demonstrated a significant increase in all three genes measured. tBHQ treatment in siRNA transfected cultures showed as significant increase in NQO1 expression but not in Gclc or HO-1 expression. Asterisks denote a statistically significant difference (p < 0.05) from untreated, control transfected cultures. Crosses denote a statistically significant difference (p < 0.05) between untreated and tBHQ-treated siRNA transfected cultures
Discussion
While there are a multitude of compounds capable of Nrf2 activation, tBHQ at low doses conveys similar levels of protection through the antioxidant response (Li et al. 2002a). Studies where tBHQ was added to the diet showed that tBHQ supplementation decreased colon adenocarcinomas, hepatocellular carcinomas, and liver preneoplastic foci in animal models (Fukushima et al. 1991; Hasegawa et al. 1992; Hirose et al. 1993), but tBHQ is also associated with esophageal hyperplasia, forestomach papillomas, and hyperplasia of mucosa (Altmann et al. 1985; Hirose et al. 1993). While much of tBHQ protection, and possibly its carcinogenic effect, is believed to be the consequence of ROS generation via quinone/semiquinone redox cycling, our results showed very little cellular ROS production, but rather very discrete ROS production within the mitochondria.
There are many examples of the independent effects of various redox couples and their compartmentation on redox signaling. For example, GSH is a capable inhibitor of NF-κB activation by various inducers of oxidative stress. However, the window of activation can be closed under severe oxidative stress, where cysteine residues in the DNA binding domain of NF-κB can be oxidized and gene transactivation is inhibited. Nuclear Trx1 has been shown to increase the effectiveness of NF-κB signaling by reducing oxidized cysteines and thereby enhancing NF-κB signals (Hirota et al. 1999). Thus, GSH and Trx1 have very distinct roles in the regulation of NF-κB in two very different compartments.
In the present study, tBHQ induces the oxidation of mitochondrial Trx2 redox status, while cellular GSH and extramitochondrial thioredoxin, Trx1, redox states are unchanged. tBHQ-induced ROS production is believed to be, in part, due to copper (Cu)-, prostaglandin H synthase-, or lipoxygenase-mediated redox cycling from the quinone to the semiquinone and is hypothesized to play a role in tBHQ carcinogenicity (Schilderman et al. 1993; Li et al. 2002b). There are few studies where the effect of tBHQ on the GSH redox status has been fully characterized. Studies are contradictory, where tBHQ induces a decrease in GSH in certain cell types but causes an increase in others (Liu et al. 1994; Nakagawa et al. 1994; Nakagawa 1996; Hara et al. 2003; Okubo et al. 2003). While our studies did not show a very profound effect on the GSH system, there have been reports of tBHQ-related GSH conjugates in animal models, demonstrating tBHQ's interaction with GSH (Peters et al. 1996). However, because there is little effect on GSH redox status, it is unlikely that tBHQ is activating downstream signaling pathways through a GSH redox-mediated mechanism. Other studies support this hypothesis, where ROS may not be the signaling mechanism but rather signaling may be initiated by tBHQ oxidation products directly (Nakamura et al. 2003).
However, these data show that there is a preferential tBHQ-induced oxidation of the mitochondria, as demonstrated by Trx2 oxidation. Trx2 is a mitochondrial specific protein that can actively participate in ROS removal in conjunction with mitochondria-specific peroxiredoxins (Prxs) and thioredoxin reductase-2 (TR2) (Rhee et al. 2001, 2005). While it is not clear how the interaction between tBHQ and Trx2 occurs, oxidation of Trx2 does coincide with changes in mitochondrial ROS production, namely, superoxide anion (O ·−2 ). Furthermore, the origins of O ·−2 are unclear as it could be the result of possible redox cycling within the mitochondria. Potentially, tBHQ causes changes in mitochondrial metabolism or electron transport chain function, yielding O ·−2 in the process. Clearly, further work is required to fully understand the mechanism of tBHQ-induced oxidation in the mitochondria.
In regards to cell signaling, this study shows that Trx2 is a critical factor in mediating the mechanism by which tBHQ induces Nrf2 activity. Overexpression of Trx2 reduces tBHQ-induced Nrf2 activation, while overexpression of the dominant-negative mutant, C93S Trx2, enhances activation. Overexpression of Trx2 in other signaling paradigms has been shown to alter cell signaling. In HeLa cells, Trx2 overexpression decreased basal NF-κB activities and inhibited TNF-α-induced activation (Hansen et al. 2006c). Here, it appears that activation of Nrf2 is either directly or indirectly mediated by factors originating from the mitochondria that may be the result of tBHQ-induced oxidation, which is regulated by Trx2 and possibly other mitochondrial antioxidants.
However, another important function of Trx2 is the regulation of the mitochondrial permeability transition (MPT). The activation of the MPT results in the initiation of apoptotic processes. In U937 monocytes, tBHQ altered mitochondrial membrane potential and resulted in the release of cytochrome c (Okubo et al. 2003). This is another process where tBHQ-mediated injury and Trx2 function intersects and may relate to factors involved in Nrf2 activation and signaling.
In summary, tBHQ appears to generate mitochondrial ROS that plays a key role in the regulation of the Nrf2 signaling pathway. Moreover, mitochondrial antioxidants, such as Trx2, contribute to tBHQ induction of Nrf2. Understanding how mitochondrial antioxidants function in regulating these processes would provide a foundation to making compounds like tBHQ more useful in practical applications, identifying possible target tissues and decreasing its toxicity and carcinogenic potential.
References
Altmann HJ, Grunow W, Wester PW, Mohr U. Induction of forestomach lesions by butylhydroxyanisole and structurally related substances. Arch Toxicol Suppl. 1985;8:114–6.
Chen Y, Cai J, Murphy TJ, Jones DP. Overexpressed human mitochondrial thioredoxin confers resistance to oxidant-induced apoptosis in human osteosarcoma cells. J Biol Chem. 2002;277:33242–8.
Copple IM, Goldring CE, Kitteringham NR, Park BK. The Nrf2-Keap1 defence pathway: role in protection against drug-induced toxicity. Toxicology. 2008;246:24–33.
Erickson AM, Nevarea Z, Gipp JJ, Mulcahy RT. Identification of a variant antioxidant response element in the promoter of the human glutamate-cysteine ligase modifier subunit gene. Revision of the ARE consensus sequence. J Biol Chem. 2002;277:30730–7.
Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, et al. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci U S A. 2002;99:7610–5.
Fukushima S, Shibata MA, Hirose M, Kato T, Tatematsu M, Ito N. Organ-specific modification of tumor development by low-dose combinations of agents in a rat wide-spectrum carcinogenesis model. Jpn J Cancer Res. 1991;82:784–92.
Gharavi N, Haggarty S, El-Kadi AO. Chemoprotective and carcinogenic effects of tert-butylhydroquinone and its metabolites. Curr Drug Metab. 2007;8:1–7.
Halvey PJ, Watson WH, Hansen JM, Go YM, Samali A, Jones DP. Compartmental oxidation of thiol-disulphide redox couples during epidermal growth factor signalling. Biochem J. 2005;386:215–9.
Halvey PJ, Hansen JM, Johnson JM, Go YM, Samali A, Jones DP. Selective oxidative stress in cell nuclei by nuclear-targeted D-amino acid oxidase. Antioxid Redox Signal. 2007;9:807–16.
Hansen JM, Watson WH, Jones DP. Compartmentation of Nrf-2 redox control: regulation of cytoplasmic activation by glutathione and DNA binding by thioredoxin-1. Toxicol Sci. 2004;82:308–17.
Hansen JM, Go YM, Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol Toxicol. 2006a;46:215–34.
Hansen JM, Zhang H, Jones DP. Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions. Free Radic Biol Med. 2006b;40:138–45.
Hansen JM, Zhang H, Jones DP. Mitochondrial thioredoxin-2 has a key role in determining tumor necrosis factor-alpha-induced reactive oxygen species generation, NF-kappaB activation, and apoptosis. Toxicol Sci. 2006c;91:643–50.
Hansen JM, Moriarty-Craige S, Jones DP. Nuclear and cytoplasmic peroxiredoxin-1 differentially regulate NF-kappaB activities. Free Radic Biol Med. 2007;43:282–8.
Hara H, Ohta M, Ohta K, Kuno S, Adachi T. Increase of antioxidative potential by tert-butylhydroquinone protects against cell death associated with 6-hydroxydopamine-induced oxidative stress in neuroblastoma SH-SY5Y cells. Brain Res Mol Brain Res. 2003;119:125–31.
Hasegawa R, Tiwawech D, Hirose M, Takaba K, Hoshiya T, Shirai T, et al. Suppression of diethylnitrosamine-initiated preneoplastic foci development in the rat liver by combined administration of four antioxidants at low doses. Jpn J Cancer Res. 1992;83:431–7.
Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34:176–88.
Hirose M, Yada H, Hakoi K, Takahashi S, Ito N. Modification of carcinogenesis by alpha-tocopherol, t-butylhydroquinone, propyl gallate and butylated hydroxytoluene in a rat multi-organ carcinogenesis model. Carcinogenesis. 1993;14:2359–64.
Hirota K, Murata M, Sachi Y, Nakamura H, Takeuchi J, Mori K, et al. Distinct roles of thioredoxin in the cytoplasm and in the nucleus. A two-step mechanism of redox regulation of transcription factor NF-kappaB. J Biol Chem. 1999;274:27891–7.
Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86.
Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112.
Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–79.
Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116.
Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, et al. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol. 2009;29:493–502.
Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci. 2004;24:1101–12.
Li J, Lee JM, Johnson JA. Microarray analysis reveals an antioxidant responsive element-driven gene set involved in conferring protection from an oxidative stress-induced apoptosis in IMR-32 cells. J Biol Chem. 2002a;277:388–94.
Li Y, Seacat A, Kuppusamy P, Zweier JL, Yager JD, Trush MA. Copper redox-dependent activation of 2-tert-butyl(1, 4)hydroquinone: formation of reactive oxygen species and induction of oxidative DNA damage in isolated DNA and cultured rat hepatocytes. Mutat Res. 2002b;518:123–33.
Li HY, Zhong YF, Wu SY, Shi N. NF-E2 related factor 2 activation and heme oxygenase-1 induction by tert-butylhydroquinone protect against deltamethrin-mediated oxidative stress in PC12 cells. Chem Res Toxicol. 2007;20:1242–51.
Liu RM, Vasiliou V, Zhu H, Duh JL, Tabor MW, Puga A, et al. Regulation of [Ah] gene battery enzymes and glutathione levels by 5, 10-dihydroindeno[1, 2-b]indole in mouse hepatoma cell lines. Carcinogenesis. 1994;15:2347–52.
McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem. 2003;278:21592–600.
Miseta A, Csutora P. Relationship between the occurrence of cysteine in proteins and the complexity of organisms. Mol Biol Evol. 2000;17:1232–9.
Nakagawa Y. Effects of dicoumarol on cytotoxicity caused by tert-butylhydroquinone in isolated rat hepatocytes. Toxicol Lett. 1996;84:63–8.
Nakagawa Y, Nakajima K, Moore G, Moldeus P. On the mechanisms of 3-tert-butyl-4-hydroxyanisole-and its metabolites-induced cytotoxicities in isolated rat hepatocytes. Eur J Pharmacol. 1994;270:341–8.
Nakamura Y, Kumagai T, Yoshida C, Naito Y, Miyamoto M, Ohigashi H, et al. Pivotal role of electrophilicity in glutathione S-transferase induction by tert-butylhydroquinone. Biochemistry. 2003;42:4300–9.
Nakaso K, Yano H, Fukuhara Y, Takeshima T, Wada-Isoe K, Nakashima K. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett. 2003;546:181–4.
Okubo T, Yokoyama Y, Kano K, Kano I. Cell death induced by the phenolic antioxidant tert-butylhydroquinone and its metabolite tert-butylquinone in human monocytic leukemia U937 cells. Food Chem Toxicol. 2003;41:679–88.
Peters MM, Rivera MI, Jones TW, Monks TJ, Lau SS. Glutathione conjugates of tert-butyl-hydroquinone, a metabolite of the urinary tract tumor promoter 3-tert-butyl-hydroxyanisole, are toxic to kidney and bladder. Cancer Res. 1996;56:1006–11.
Petzer JP, Navamal M, Johnson JK, Kwak MK, Kensler TW, Fishbein JC. Phase 2 enzyme induction by the major metabolite of oltipraz. Chem Res Toxicol. 2003;16:1463–9.
Pinkus R, Weiner LM, Daniel V. Role of oxidants and antioxidants in the induction of AP-1, NF-kappaB, and glutathione S-transferase gene expression. J Biol Chem. 1996;271:13422–9.
Rhee SG, Kang SW, Chang TS, Jeong W, Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 2001;52:35–41.
Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–52.
Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991;266:11632–9.
Schilderman PA, van Maanen JM, Smeets EJ, ten Hoor F, Kleinjans JC. Oxygen radical formation during prostaglandin H synthase-mediated biotransformation of butylated hydroxyanisole. Carcinogenesis. 1993;14:347–53.
Solis WA, Dalton TP, Dieter MZ, Freshwater S, Harrer JM, He L, et al. Glutamate-cysteine ligase modifier subunit: mouse Gclm gene structure and regulation by agents that cause oxidative stress. Biochem Pharmacol. 2002;63:1739–54.
Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27:612–6.
Watson WH, Pohl J, Montfort WR, Stuchlik O, Reed MS, Powis G, et al. Redox potential of human thioredoxin 1 and identification of a second dithiol/disulfide motif. J Biol Chem. 2003;278:33408–15.
Acknowledgements
This work was supported by the Emory University Department of Pediatrics and the Emory Children's Center Pilot Grants Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Imhoff, B.R., Hansen, J.M. Tert-butylhydroquinone induces mitochondrial oxidative stress causing Nrf2 activation. Cell Biol Toxicol 26, 541–551 (2010). https://doi.org/10.1007/s10565-010-9162-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-010-9162-6