Abstract
Background and aims
Rifaximin has been recommended as a prophylactic drug for hepatic encephalopathy (HE) and spontaneous bacterial peritonitis (SBP). This study aims to explore whether low-dose rifaximin can prevent overall complications and prolong survival in cirrhotic patients.
Methods
In this multi-centre randomized open-labelled prospective study, 200 patients with decompensated cirrhosis were randomly assigned at a ratio of 1:1. Patients in rifaximin group were administered 400 mg rifaximin twice daily for 6 months, and all other therapeutic strategies were kept unchanged in both groups as long as possible. The primary efficacy endpoints were the incidence of overall complications and liver transplantation-free survival. The secondary endspoints were the incidence of each major cirrhosis-related complication, as well as the Child–Pugh score and class.
Results
The major baseline characteristics were similar in the two groups except for HE. The cumulative incidence and frequency of overall complications were significantly lower in rifaximin group than in the control group (p < 0.001). Though liver transplantation-free survival was not significantly different between the two groups, subgroup analysis showed rifaximin markedly prolonged liver transplantation-free survival in patients with Child–Pugh score ≥ 9 (p = 0.007). Moreover, rifaximin markedly reduced the episodes of ascites exacerbation (p < 0.001), HE (p < 0.001) and gastric variceal bleeding (EGVB, p = 0.031). The incidence of adverse events was similar in the two groups.
Conclusion
Low-dose rifaximin significantly decreases the occurrence of overall complications, leading to prolonged survival in patients with advanced stages of cirrhosis in this trail. Further study should be carried out to compare the effect of this low-dose rifaximin with normal dose (1200 mg/day) rifaximin in preventing cirrhosis-related complications.
Clinical trial number
NCT02190357
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver cirrhosis, resulting from various chronic liver lesions, is characterized as a disease with typical pathologic manifestations of hepatocyte necrosis and fibrogenesis. Due to impaired liver function and portal hypertension, decompensated liver cirrhosis can lead to a series of complications, such as ascites, spontaneous bacterial peritonitis (SBP), esophageal and gastric variceal bleeding (EGVB), hepatic encephalopathy (HE) and hepatorenal syndrome (HRS). To date, the efficacy of current therapeutic strategies aimed at decompensated cirrhosis is limited, and the occurrence of complications is associated with a high mortality rate [1]. Thus, it is urgent to develop a novel therapy for decompensated cirrhotic patients.
Recent studies have indicated that the gut microbiota plays an important role in the development of liver cirrhosis [2, 3]. Rifaximin, an oral broad-spectrum antibiotic locally acting in the gastrointestinal (GI) tract, has been well documented to effectively alter the gut microbiota profile, meliorate intestinal endotoxemia, protect cirrhotic patients from HE and SBP and prolong survival in patients experiencing HE [4,5,6,7,8,9,10]. In addition, a randomized, double-blind clinical trial revealed that combination of lactulose plus rifaximin is more effective than lactulose alone in the treatment of OHE and reduces rates of death in patients who had a history of HE [11]. Currently, rifaximin is recommended as one of the first-line drugs for the treatment and prophylaxis of HE by the FDA. Recently, some small-sample studies and meta-analyses have demonstrated a prophylactic effect of rifaximin on acute kidney injury (AKI) and HRS [12, 13]. However, it is still not clear whether rifaximin can reduce the occurrence of overall complications and prolong survival in patients with decompensated cirrhosis.
The majority of previous studies recommended rifaximin at a dosage of 1200 or 1100 mg/day as conventional adoption in cirrhotic patients. However, a randomized control trial published in 2015 has shown that rifaximin treatment at a dosage of 550 mg once or twice daily has no significant difference in preventing HE recurrence [14]. Our previous research also showed that low-dose (800 mg/day) rifaximin treatment for two weeks could be analogous to high-dose (1200 mg/day) rifaximin to improve intestinal endotoxemia, despite a relatively short period of maintenance of curative effect [15]. Thus, we speculated that low-dose rifaximin might be applicable to the long-term treatment of patients with cirrhosis. Here, we designed a multi-centre open-labelled randomized prospective study to evaluate the efficacy and safety of long-term administration of low-dose (800 mg/day) rifaximin in preventing complications and prolonging survival in patients with decompensated liver cirrhosis.
Methods
See Supporting Materials for detailed methods.
Study patients
Eligibility criteria were age ranging from 18 to 75, with a clinical diagnosis of decompensated liver cirrhosis on the basis of typical clinical manifestations, laboratory tests, imaging appearances and/or representative pathology results of liver biopsy. Decompensation of the disease was defined by a Child–Pugh score of more than 7 lasting for at least 1 month or at least having an episode of severe complications, including ascites, SBP, EGVB and HE. All patients were willing to be enrolled and had signed the informed consent.
The major exclusion criteria included the following: (1) episodes of overt HE, EGVB or SBP within 1 month before the screening visit; (2) continuous antibiotic use for more than 3 days within 2 weeks prior to enrolment; (3) hepatitis B virus (HBV) DNA ≥ 500 copy/mL; (4) an intent to change the antiviral therapy during the course of the study or receipt of standard antiviral treatment for hepatitis B or hepatitis C for less than 6 months; (5) unwilling to stop alcohol abuse after inclusion (≥ 20 g/day for women or ≥ 40 g/day for men); (6) severe jaundice (serum total bilirubin level ≥ 170 μmol per litre); (7) obvious renal dysfunction (serum creatinine ≥ 1.2-fold of upper normal limits); (8) severe electrolyte abnormality (serum sodium level < 125 mmol per litre); (9) life-threatening leucocytopenia (white blood cell count < 1 × 109 per litre); (10) HIV seropositivity; and (11) poorly controlled hypertension, diabetes mellitus or other severe heart and respiratory diseases. Patients were also excluded if they were diagnosed or suspected to have malignant diseases, including primary or secondary liver cancer.
Study design and procedures
This was an investigator-initiated open-labelled study. All authors vouch for the completeness and veracity of the data as well as data analyses.
After a screening visit, the eligible individuals were randomly allocated into a rifaximin group and a control group with a randomized block digital table in a ratio of 1:1. Patients in the rifaximin group were administered 400 mg rifaximin twice daily based on conventional therapy for 6 months, and patients in the control group were administered only conventional therapy. During the entire study period, all other therapeutic strategies, such as antiviral agents, non-selective beta-blockers, liver protectants, and diuretics, were kept unchanged in both the groups as long as possible.
Efficacy and safety assessment
A complete assessment was performed in all patients at the screening visit and the end of the treatment phase, including a detailed medical history recording, physical examination, ultrasound or CT and venous blood sample collection. To ensure safety, all patients underwent routine blood tests and investigation of symptoms and adverse events at the end of 1 week after drug administration.
The primary endpoints were the incidence of overall complications resulting from decompensated liver cirrhosis and liver transplantation-free survival during the 6-month treatment phase. The investigated complications consisted of HE, ascites, SBP and other cirrhosis-related infections, EGVB, HRS and primary hepatic cancer (PHC).
The key secondary endpoint was the incidence of each major cirrhosis-related complication. The diagnosis of these complications and their severity assessment were in accordance with the related guidelines [16,17,18]. Ascites improvement was defined as a reduction in ascitic volume of at least 1 grade or a stable decrease with a dose of diuretic. In contrast, ascitic exacerbation was considered if the ascitic volume increased by at least 1 grade or the diuretic demand was evidently increased. The other secondary endpoints included Child–Pugh score, Child–Pugh class, and liver function reflected by biochemical examination, ammonia level, prothrombin time (PT) and international normalized ratio (INR).
Safety assessments consisted of monitoring adverse events, vital signs from physical examinations, and results of clinical laboratory testing. Severe adverse events were defined as those leading to hospitalization, prolonged hospitalization, disability, impact on work capacity, endangered life, or death.
Statistical analysis
The sample size was determined based on the hypothesis that 35% of the patients in the rifaximin group and 60% of patients in the placebo group would have episodes of at least one complication, and 5% of the patients in the rifaximin group and 22% of patients in the placebo group would die or undergo liver transplantation during the 6-month treatment phase with a significance level of 2.5%, respectively (α = 0.025). With these assumptions, a sample size of 94 patients in each group would provide ≥ 90% power to detect statistically significant treatment differences. Considering the cases of missing follow-up or withdrawal, the estimated sample size in each group was 100 patients ultimately. All analyses were stratified by the analysis centres.
Efficacy data were analysed for the intention-to-treat (ITT) population, which included patients who received at least one dose of the study medication and underwent one follow-up. Safety was determined in all the enrolled individuals. Continuous parameters were expressed as the mean ± standard deviation, while categorical variables were expressed as numbers and percentages or frequencies. The associations between categorical parameters were determined using two-tailed Fisher’s exact tests. Normally distributed continuous parameters were compared using Student’s t-tests, and non-normally distributed continuous parameters were compared using the Mann–Whitney U test. The frequency of complications was analysed by the Mann–Whitney U test. Kaplan–Meier methods were used to analyse liver transplantation-free survival and estimate the proportions of patients experiencing complications at successive time points during the study. Cox proportional hazards models were used to compare the time to a breakthrough episode of complications between the two groups with a 2-sided test. In addition, due to the unbalanced baseline history of HE, the comparison of the OHE episodes between the two groups was analyzed by the adjusted logistic regression method. The previous history of HE was taken as the covariate, then the incidence of OHE after treatment was compared between the two groups. Statistical analyses were performed using SPSS 22.0 (IBM, Armonk, NY, USA). p < 0.05 was considered statistically significant.
Results
Study patients
A total of 265 patients with decompensated liver cirrhosis were screened, and 200 individuals were ultimately enrolled and randomly assigned in 8 investigative centres from September 2014 to November 2017 (Fig. 1). Finally, 195 patients who received at least one dose of the study drug and underwent at least one follow-up were included in the ITT and safety populations. As shown in Table 1 and Supplementary Table 1, the dominant baseline characteristics interrelated with cirrhosis were similar in the two groups, and there was no significant difference in the occurrence of overall complications at baseline except for HE.
Rifaximin prevented the overall complications in patients with decompensated cirrhosis
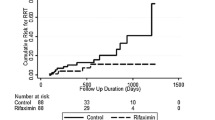
In total, 23 patients experienced 44 complications in the rifaximin group during the entire 6-month treatment phase, while 54 patients experienced 117 complications in the control group. The proportion of patients with episodes of complications during the treatment phase was significantly lower in the rifaximin group than in the control group (23.71% vs. 55.10%, p < 0.001). As shown in Fig. 2a and Supplementary Table 2, 6 months of rifaximin administration significantly decreased the cumulative incidence of overall complications (p < 0.001) and their frequency (p < 0.001).
Analysis for the effect of rifaximin on overall complications. a Kaplan–Meier estimates of non-complicated survival in the intention-to-treat population. b Subgroup analysis for the effect of rifaximin on overall complications. Hazard ratios for the risk of a breakthrough episode of complications during the 6-month treatment period are shown for the rifaximin group compared with the control group, for various subgroups. p values were calculated by means of the log-rank test. Symbols represent patients for whom data were censored. ALD autoimmune liver disease, MELD model for end-stage liver disease, HE hepatic encephalopathy, EGVB oesophageal and gastric variceal bleeding SBP spontaneous bacterial peritonitis
To select the most appropriate population for rifaximin administration, we performed further subgroup analyses according to baseline characteristics. As shown in Fig. 2b, rifaximin reduced the risk of episodes of overall complications regardless of sex, etiology of cirrhosis, Child–Pugh class or MELD score. Interestingly, it seems that the age and history of complications at baseline had an impact on the protective effect of rifaximin. Patients aged ≥ 65 years did not benefit from rifaximin treatment. Rifaximin administration led to an evident reduction in the incidence of overall complications in patients experiencing ascites, while no obvious change was found in individuals without a history of ascites. Rifaximin treatment also decreased the risk of the breakthrough of complications in patients without HE and SBP episodes. In addition, the incidence of complications decreased after rifaximin delivery regardless of whether EGVB episodes occurred. However, our data failed to reveal any prophylactic effect of rifaximin on overall complications in patients with a history of HE or SBP.
Rifaximin improved ascites and reduced the risk of the episode of HE and EGVB
As shown in Table 2, rifaximin administration obviously reduced the risk of ascites exacerbation or appearance and augmented the proportion of patients with ascites improvement or disappeared (p < 0.001). Rifaximin administration also significantly repressed the proportion of patients experiencing the episode of HE after adjustment for the history of HE (p < 0.001). In addition, compared with that in the control group, the proportion of patients with EGVB breakthrough was markedly reduced in the rifaximin group (7.22% vs. 19.39%, p = 0.019). There was a downward trend of SBP episode in the treatment group in comparison with that in the control group (9.18% vs. 2.06%, p = 0.058). Log-rank analysis showed similar results (Supplementary Fig. 1). Nevertheless, our observation did not reveal a protective effect of rifaximin against other complications, including portal vein thrombosis, other infections, AKI and HRS and PHC.
Rifaximin prolonged liver transplantation-free survival in patients with poor liver function.
Four patients in the rifaximin group and 6 individuals in the control group died within the 6-month treatment period. All deaths in both the groups were cirrhosis-related (Supplementary Table 3). In addition, 3 patients in the control group but none in the treatment group received liver transplantation. As shown in Fig. 3a, liver transplantation-free survival was not significantly different between the two groups (p = 0.180). However, log-rank analysis in subgroups showed that rifaximin treatment for 6 months markedly prolonged liver transplantation-free survival in patients with Child–Pugh score ≥ 9 (p = 0.007, Fig. 3b) or Child–Pugh class C (p = 0.003, Fig. 3c). Similar to the whole enrolled populations, there was no difference of baseline characteristics except for HE history between the two groups in patients with Child–Pugh score ≥ 9 (Supplementary Table 4). No difference of baseline characteristics was observed between the two groups in patients with Child–Pugh class C (Supplementary Table 5).
Kaplan–Meier estimates of overall liver transplantation-free survival in the intention-to-treat population. a Kaplan–Meier estimates of overall liver transplantation-free survival in the intention-to-treat population, according to study group. b Results of subgroup analyses. Kaplan–Meier estimates of liver transplantation-free survival in patients with Child–Pugh score ≥ 9. c Results of subgroup analyses. Kaplan–Meier estimates of liver transplantation-free survival in patients with Child–Pugh class C
Rifaximin ameliorated the Child–Pugh score after 6 months treatment
Serological parameters and Child–Pugh score and class were examined at various time points (Supplementary Table 6). There was no significant difference in any of the serological indicators between the two groups at either visit (p > 0.05). However, current observation indicated that the average Child–Pugh score was markedly reduced from 7.78 ± 2.14 to 7.06 ± 2.18 in the rifaximin group (p < 0.001), while it was slightly elevated in the control group (p = 0.041) (Supplementary Fig. 2a). In parallel, the percentage of patients with Child–Pugh class A was significantly increased and that with Child–Pugh class C was obviously decreased upon rifaximin treatment (Supplementary Fig. 2b).
Safety
There was no significant difference in the overall incidence of adverse events (73.20% vs. 60.20%, p = 0.129) or serious adverse events (9.28% vs.11.34%, p = 0.814) between the rifaximin and control groups (Table 3, Supplementary Table 7). The most frequently reported adverse events in the rifaximin group included cough, upper gastrointestinal ulcer, uric acid elevation, trauma, and duodenitis. The majority of adverse events were ameliorated during the study without special therapy.
Rifaximin was discontinued in 5 patients due to adverse events, including constipation (n = 1), obvious abdominal distention (n = 1), nausea (n = 1), edema in lower limbs (n = 1), and neutropenia (n = 1). All the aberrant symptoms, body signs and laboratory examination results returned to their baseline levels 2 weeks after drug withdrawal without specific treatment.
It is reported that the systemic exposure of rifaximin was markedly elevated in patients with severe hepatic impairment. We then further analyzed the adverse events among patients with different Child–Pugh class. The results showed that the gastrointestinal adverse effect, including constipation and diarrhea were more common in the Child–Pugh C patients receiving rifaximin compared with the control group. All of these symptoms were tolerated and none of the patients discontinued rifaximin treatment (Supplementary Table 8). Moreover, there was no difference in the incidence of serious adverse events between the two groups with Child–Pugh C.
Discussion
Cirrhosis-related complications are the principal causes of death in patients with end-stage liver diseases. Preventing the occurrence of complications will notably improve the outcomes and quality of life in cirrhotic patients. In this report, we demonstrated that long-term administration of low-dose (800 mg/day) rifaximin prevents the complications in decompensated cirrhotic patients with good safety and tolerability. Most intriguingly, our results revealed that rifaximin treatment prolongs survival in cirrhotic patients with Child–Pugh score ≥ 9 or Child–Pugh class C.
The recommended dose of rifaximin in cirrhotic patients was 1100–1200 mg/day [19,20,21,22,23,24]. However, our previous study and a randomized control trial revealed the possibility of maintenance therapy with low-dose rifaximin in cirrhotic patients [14, 15]. We found that low-dose (800 mg/day) rifaximin treatment for two weeks significantly reduced the serum endotoxin concentration in cirrhotic patients, and the reduction was similar to that with 1200 mg/d rifaximin treatment. In the current study, low-dose rifaximin showed a substantial protective effect in patients with decompensated liver cirrhosis. This dosage will certainly reduce the medical burden for the patients, improve compliance, and possibly further decrease the potential side effect in long-term therapy.
To date, few studies have investigated the efficacy of rifaximin on overall complications of liver cirrhosis and patient survival [20, 23]. A study containing 23 patients with decompensated alcoholic cirrhosis who had improved liver hemodynamics with 28-day rifaximin treatment revealed that rifaximin at a daily dose of 1200 mg reduced the complications of portal hypertension and improved the five-year cumulative probability of survival [20]. A post hoc analysis found that rifaximin treatment for 6 months decreased the incidence of cirrhosis-related complications in patients with MELD scores ≥ 12 and INR ≥ 1.2 when preventing the recurrence of overt HE [21]. Our findings provide reliable evidence of the effect of low-dose rifaximin on the prevention of overall complications through a large-scale prospective randomized controlled study. The reduction in episodes of complications was independent of sex, etiology, Child–Pugh class and MELD score. The results that rifaximin had no prophylactic effect on the complications episodes in patients older than 65 years, without a history of ascites, with a history of either HE or SBP may be partly explained by the fact that only a small number of patients were enrolled in these groups. An interesting result of this study was that rifaximin only improved survival in patients with relatively severe liver injury (Child–Pugh score ≥ 9 or Child–Pugh class C). Currently, the underlining mechanism for rifaximin in controlling cirrhotic complications is not fully clarified. It is known that gut microbiota plays an important role in the development of liver cirrhosis. The intestinal microecology imbalance not only leads to the aggravation of microcirculation disturbance and immune dysfunction, promote the release of pro-inflammatory factors, resulting to the deterioration liver injury, is also a key mediator of the pathogenesis and severity of portal hypertension. Thus, we speculated that the implicated mechanisms for rifaximin in controlling complications of cirrhosis may be due to the improvement of the gut microbiota profile and reduction of intestinal endotoxemia.
Rifaximin has been well documented to be capable of preventing episodes of SBP and HE at doses of 1200 or 1100 mg/day [7, 9, 22,23,24]. Similar to the results of these studies, our current data also showed that rifaximin exerted a favourable effect on the prophylaxis of HE at a low dose. Although there was only a downward trend in the incidence of SBP episodes after rifaximin treatment, the p value was 0.058, which was close to a significant difference. The small number of patients with SBP breakthrough might be attributed to the relatively inadequate difference. In addition, a significant reduction of EGVB episodes by rifaximin was observed in our study, which was analogous to the reports by Vlachogiannakos et al. [20] and Lim et al. [25]. Although the efficacy of rifaximin against HE and SBP is convincing, whether rifaximin could meliorate ascites has not been reported. Herein, we found that rifaximin significantly mitigated ascites in cirrhotic patients. This result provided a clear indication for the application of rifaximin in cirrhotic patients with ascites. Nevertheless, due to the extremely small number of cases with AKI or HRS, our study failed to reveal the prophylactic effect of rifaximin on AKI or HRS.
It has been proven that intestinal endotoxemia plays a critical role in hepatocarcinogenesis. Administration of antibiotics can dramatically mitigate endotoxemia and prevent tumor formation in the liver [26]. However, our observation failed to reveal the inhibition of rifaximin on the initiation of PHC. This may be due to the short observation period, and it is hard to examine the preventative effect on tumors in such a short time. Further large-scale and long-term clinical trials need to be carried out to identify the prolonged utility of rifaximin on PHC prevention.
Rifaximin is recognized as a drug with favourable safety. The major reported severe side effects were neutropenia and toxic epidermal necrolysis, which were resolved after symptomatic treatments [27, 28]. In our research, adverse effects and severe adverse effects were similar in the two groups. It has been documented that systemic exposure of rifaximin was markedly elevated in patients with severe hepatic impairment, which raised the concerns about the use of rifaximin in patients with severe liver disease. In this trial, the gastrointestinal adverse effects were more common in Child–Pugh C patients treated with rifaximin, but all of them were tolerated. More importantly, incidence of serious adverse events was not significantly different between the two groups. These data confirm the safety of long-term administration of rifaximin in decompensated cirrhotic patients.
There were some limitations to this study. First, the distribution of patients with a previous history of HE is different between the two groups. Secondly, we did not compare the effect of low-dose (800 mg/day) rifaximin with normal dose rifaximin (1200 mg/day) in preventing the complications of decompensated cirrhosis. A future trial will be conducted to confirm the effect of low-dose rifaximin on decompensated cirrhosis compared with normal dose rifaximin.
In conclusion, our findings indicate that low-dose rifaximin significantly decreases the occurrence of overall complications of decompensated cirrhosis and prolongs the survival in cirrhotic patients with poor liver function. Long-term treatment with low-dose rifaximin might present as a safe and effective therapeutic strategy for decompensated liver cirrhosis.
Abbreviations
- SBP:
-
Spontaneous bacterial peritonitis
- EGVB:
-
Oesophageal and gastric variceal bleeding
- HE:
-
Hepatic encephalopathy
- HRS:
-
Hepatorenal syndrome
- HCC:
-
Hepatocellular carcinoma
- GI:
-
Gastrointestinal
- AKI:
-
Acute kidney injury
- HBV:
-
Hepatitis B virus
- AIH:
-
Autoimmune hepatitis
- PBC:
-
Primary biliary cholangitis
- MELD:
-
Model For End-Stage Liver Disease
- PT:
-
Prothrombin time
- INR:
-
International normalized ratio
- ITT:
-
Intention-to-treat
- PHC:
-
Primary hepatic cancer
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- γ-GT:
-
γ-Glutamyl transferase
- ALP:
-
Alkaline phosphatase
- HVPG:
-
Hepatic venous pressure gradient
References
Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749–61.
Wiest R, Albillos A, Trauner M, et al. Targeting the gut-liver axis in liver disease. J Hepatol. 2017;67:1084–103.
Tilg H, Cani PD, Mayer EA. Gut microbiome and liver diseases. Gut. 2016;65:2035–44.
Ponziani FR, Gerardi V, Pecere S, et al. Effect of rifaximin on gut microbiota composition in advanced liver disease and its complications. World J Gastroenterol. 2015;21:12322–33.
Peleman C, Camilleri M. Rifaximin, microbiota biology, and hepatic encephalopathy. Clin Transl Gastroenterol. 2016;7:e195.
Kang DJ, Kakiyama G, Betrapally NS, et al. Rifaximin exerts beneficial effects independent of its ability to alter microbiota composition. Clin Transl Gastroenterol. 2016;7:e187.
Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–81.
Kimer N, Krag A, Møller S, et al. Systematic review with meta-analysis: the effects of rifaximin in hepatic encephalopathy. Aliment Pharmacol Ther. 2014;40:123–32.
Kamal F, Khan MA, Khan Z, et al. Rifaximin for the prevention of spontaneous bacterial peritonitis and hepatorenal syndrome in cirrhosis: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2017;29:1109–17.
Kang SH, Lee YB, Lee JH, et al. Rifaximin treatment is associated with reduced risk of cirrhotic complications and prolonged overall survival in patients experiencing hepatic encephalopathy. Aliment Pharmacol Ther. 2017;46:845–55.
Sharma BC, Sharma P, Lunia MK, et al. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108:1458–63.
Dong T, Aronsohn A, Gautham Reddy K, et al. Rifaximin decreases the incidence and severity of acute kidney injury and hepatorenal syndrome in cirrhosis. Dig Dis Sci. 2016;61:3621–6.
Ibrahim ES, Alsebaey A, Zaghla H, et al. Long-term rifaximin therapy as a primary prevention of hepatorenal syndrome. Eur J Gastroenterol Hepatol. 2017;29:1247–50.
Khokhar N, Qureshi MO, Ahmad S, et al. Comparison of once a day rifaximin to twice a day dosage in the prevention of recurrence of hepatic encephalopathy in patients with chronic liver disease. J Gastroenterol Hepatol. 2015;30:1420–2.
Zeng X, Tang XJ, Sheng X, et al. Does low-dose rifaximin ameliorate endotoxemia in patients with liver cirrhosis: a prospective study. J Dig Dis. 2015;16:665–74.
Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–35.
Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–35.
Angeli P, Gines P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64:531–7.
Fukui H, Saito H, Ueno Y, et al. Evidence-based clinical practice guidelines for liver cirrhosis 2015. J Gastroenterol. 2016;51:629–50.
Vlachogiannakos J, Viazis N, Vasianopoulou P, et al. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol. 2013;28:450–5.
Flamm SL, Mullen KD, Heimanson Z, et al. Rifaximin has the potential to prevent complications of cirrhosis. Therap Adv Gastroenterol. 2018;11:1756284818800307.
Bajaj JS, Barrett AC, Bortey E, et al. Prolonged remission from hepatic encephalopathy with rifaximin: results of a placebo crossover analysis. Aliment Pharmacol Ther. 2015;41:39–45.
Goyal O, Sidhu SS, Kishore H. Minimal hepatic encephalopathy in cirrhosis- how long to treat? Ann Hepatol. 2017;16:115–22.
Sidhu SS, Goyal O, Parker RA, et al. Rifaximin vs. lactulose in treatment of minimal hepatic encephalopathy. Liver Int. 2016;36:378–85.
Lim YL, Kim MY, Jang YO, et al. Rifaximin and propranolol combination therapy is more effective than propranolol monotherapy for the reduction of portal pressure: an open randomized controlled pilot study. Gut Liver. 2017;11:702–10.
Zhang HL, Yu LX, Yang W, et al. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J Hepatol. 2012;57:803–12.
Hynicka LM, Silva KN. Probable rifaximin-induced neutropenia. Am J Health Syst Pharm. 2012;69:583–6.
Patel AS, Supan EM, Ali SN. Toxic epidermal necrolysis associated with rifaximin. Am J Health Syst Pharm. 2013;70:874–6.
Acknowledgements
The authors would like to thank the patients and their families for their contribution to this study.
Funding
The study was supported by an Emerging advanced technology joint research project from Shanghai Hospital Development Center (NO SHDC12016103), a Top-Level Clinical Discipline Project from Shanghai Pudong Health Committee (NO PWYgf2018-04), a Key Projects from Shanghai Science and Technology Committee (NO 17411950800) and two grants from the National Natural Science Foundation Committee of China (NO 81530019, 81770600).
Author information
Authors and Affiliations
Contributions
XZ and W-FX designed the research and drafted the manuscript. XZ, LY, XM, J-MX, X-ZS, C-QY, XZ and N-HL presided over the enrolment and exclusion of the patients. XS, H-GX, YL, J-WZ, C-ZH, JY, T-TL, W-JM and XX followed up the patients and collected the data. P-MS and Z-LY check the data. P-QW and Y-BG established the database and analysed the data statistically.
Corresponding author
Ethics declarations
Conflict of interest
Xin Zeng, Xia Sheng, Pei-Qin Wang, Hai-Guang Xin, Yi-Bin Guo,Yong Lin, Jia-Wei Zhong, Cheng-Zhi He, Jie Yin, Tao-Tao Liu, Wei-Juan Ma, Xiao Xiao, Pei-Mei Shi, Zong-Li Yuan, Ling Yang, Xiong Ma, Jian-Ming Xu, Xi-Zhong Shen, Chang-Qing Yang, Xuan Zhu, Nong-Hua Lv and Wei-Fen Xie declares that they have no conflict of interesting.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol was reviewed and approved by the institutional review board or ethics committee at each centre.
Informed consent
Written informed consent was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zeng, X., Sheng, X., Wang, PQ. et al. Low-dose rifaximin prevents complications and improves survival in patients with decompensated liver cirrhosis. Hepatol Int 15, 155–165 (2021). https://doi.org/10.1007/s12072-020-10117-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-020-10117-y