Abstract
Primary cardiac synovial sarcoma is a rare entity, arising from the pericardium or the chambers of the heart. It presents in the 4th decade of life with a striking male predisposition. We describe an unusual case of a 22-year-old female who presented with complaints of dyspnoea on exertion, palpitations, and chest pain. Trans-thoracic echocardiography was suggestive of a cystic pericardial mass with pericardial effusion anterior and lateral to the right ventricle. Computed tomography scan (CT scan) revealed thick-walled predominantly cystic lesion over the left ventricle with gross pericardial effusion with internal septations. These findings were suggestive of an infected pericardial cyst. Upon surgery, an adherent mass in the pericardial cavity was found which was not separable from the right heart structures, the great vessels, and the left ventricle. Biopsy was taken, histopathology was suggestive of spindle cell neoplasm, and an immunohistochemistry analysis revealed Transducin-like enhancer of split 1 (TLE 1)–positive malignant spindle cell tumour likely synovial sarcoma. After surgery, the patient received serial adjuvant chemo-radiation therapy. The synovial sarcoma masqueraded as effusive constrictive pericarditis, due to which it eluded preoperative diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac tumours are rare entities. Seventy-five percent of tumours are benign and the overall incidence is 0.15% [1]. Primary malignancies of the heart are rarer, with a prevalence between 0.001 and 0.03%. Sarcomas are the commonest malignant tumours of the heart including myosarcoma, liposarcoma, angiosarcoma, fibrosarcoma, leiomyosarcoma, osteosarcoma, synovial sarcoma, rhabdomyosarcoma, neurofibrosarcoma, malignant fibrous histiocytoma, and undifferentiated sarcoma. Incidence of primary synovial sarcoma from the heart is reported to be around 3% and around 5% from the pericardium. With a predilection to arise from the right-sided chambers, synovial sarcomas often elude diagnosis until overt dyspnoea develops [2, 3]. The following case report describes how the synovial sarcoma masqueraded as effusive constrictive pericarditis.
Case report
A 22-year-old female presented with a diagnosis of effusive constrictive pericarditis with a pericardial mass. She had dyspnoea on exertion, palpitations, and chest pain for 2 years. Systemic examination findings noted a normal heart rate, blood pressure, and respiratory rate; the jugular vein was distended. Heart sounds were muffled. She was taking beta-blockers and diuretics.
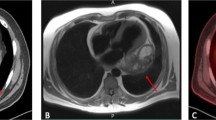
Chest X-ray demonstrated widened cardiac silhouette with mediastinal widening and consolidation in the lower lobe of the left lung. Computed tomography (CT) reported a gross pericardial effusion with internal septations, and calcification at the aortopulmonary window with compression of the left lower lobe bronchus resulting in consolidation/collapse of basal segment of the left lower lobe. A thick-walled predominantly cystic lesion was noted over the left ventricle with an ill-defined stalk extending to the aortic and pulmonary roots measuring approx. 15 cm × 12 cm. The cystic lesion had tiny calcifications as well. Multiple lymph nodes were noted in the right brachiocephalic, pre-vascular, para-tracheal, and subcarinal regions, with the largest size of 10 mm. Left-sided pleural effusion and hepatomegaly were also noticeable on the computed tomography scan (CT scan). These radiological findings were suggestive of an infected pericardial cyst or a cold abscess (Fig. 1a).
(a) Chest X-ray and computed tomographic images demonstrating enlarged cardiac silhouette and mediastinal widening with left lower lobe consolidation. CT demonstrating massive pericardial effusion (labelled — e) and a cystic mass adjacent to the left ventricle (yellow arrow). (b) Trans-thoracic echocardiography confirming presence of cystic mass posterolateral to the left ventricle (yellow arrow) with pericardial effusion (labelled — e)
Trans-thoracic echocardiography (TTE) was suggestive of a cystic pericardial mass around 4.5 × 6.9 cm lateral to and compressing the left ventricle, with pericardial effusion of 0.8 cm anterior to and 1.1 cm lateral to the right ventricle. Inferior vena cava was distended 2.2 cm with < 20% inspiratory collapse. Septal bounce and annulus reversus were present. These findings were consistent with the diagnosis of effusive constrictive pericarditis with pericardial cyst (Fig. 1b). The patient was planned for elective pericardiectomy under general anaesthesia. Upon sternotomy, a thickened pericardium with about 2 L of pericardial effusion was found. A solid globular mass of around 25 cm × 20 cm was noted within the pericardial cavity, which was completely adhered with the anterior and lateral surfaces of the heart including the right and left ventricles, the right atrium, the great vessels, and the superior vena cava up to the level of the innominate vein were fixed with the mass; the right border of the mass was not separable from the pericardium as well. A small portion of the right ventricle was visible inferior to the mass.
The mass was non-resectable due to extensive involvement. Hence, a biopsy was taken and further procedure was abandoned (Fig. 2). The patient did well in the immediate postoperative period and was discharged after 5 days postoperatively. Metastatic workup in the form of chest and spine X-ray, ultrasonography of the whole abdomen and pelvis, and CT of the head (CT scan) were done which revealed no primary source or any systemic metastasis.
Intraoperative image of the tumour (marked with double arrows) which was fixed to the great vessels, superior vena cava, right atrium, right ventricle, and left ventricle. RV, free portion of the right ventricle visible below the mass; asterisk symbol indicates biopsy site; P, parietal pericardium, which was densely adherent with the mass
The histopathology showed spindle to ovoid cells in short storiform and ill-formed fascicles. The cells displayed pleomorphism with vesicular chromatin and scant cytoplasm with atypical mitotic figures seen in 2 to 3 cells per 10 high-power fields, which was suggestive of a spindle cell neoplasm. Immunohistochemistry revealed positive stain for nuclear protein Ki 67, B-cell lymphoma-2 (BCL-2), Transducin-like enhancer of split 1 (TLE-1), and vimentin, suggesting that the tumour was a malignant spindle cell tumour, likely synovial sarcoma (Fig. 3). Molecular analysis could not be completed in our case due to fixative technique.
As the tumour was non-resectable, palliative chemotherapy was planned after thoroughly discussing the case with the patient and her family. She is undergoing adjuvant chemotherapy using vincristine, adriamycin, and cyclophosphamide (VAC) regimen. She received 2 mg of vincristine as the total dose per course, adriamycin at 70 mg/m2, and cyclophosphamide at 600 mg/m2, administered as intravenous bolus infusion. The patient is scheduled to receive six courses of adjuvant chemotherapy at 21-day intervals, followed by radiation therapy.
This patient is being followed up at our cardiothoracic outpatient clinic every 3 months. After chemo-radiation therapy, her surgical options will be re-evaluated.
Discussion
Primary cardiac synovial sarcomas are an exceedingly rare entity, with very few cases described in literature. These tumours have a strong male predilection with a male to female ratio of 2.5–3:1. Most synovial sarcomas arise in the extremities and are notable among children and young adults; however, cardiac synovial sarcomas have been reported mostly in the 3rd or 4th decade of life. Synovial sarcomas frequently arise from the right-sided heart structures twice as common as the left and the atrium is also the most frequently involved structure [4]. Dyspnoea is the commonest presentation, whereas chest pain and other cardiorespiratory symptoms are less frequently seen.
Several non-cardiorespiratory and non-specific symptoms have also been reported with less frequency. Physical signs of heart failure, such as distended jugular vein, ascites, pedal oedema, tachypnoea, and a hyperdynamic precordium, are notable findings. Patients can also present with tamponade physiology, added heart sounds, pulsus paradoxus, tachycardia, and hyper- or hypotension. Laboratory parameters are usually non-specific with elevations in liver enzymes, lactate dehydrogenase, adenosine deaminase, C-reactive protein, and erythrocyte sedimentation rate being noted in previous patient series. TTE and plain or contrast-enhanced CT scan are mostly utilised for imaging cardiac tumours; however, TTE alone is less useful in cases with large pericardial effusions. Cardiac magnetic resonance imaging (MRI) has an added advantage over CT scan in clarity of tumour extent as well as for pericardial thickening [5].
Histologically, synovial sarcomas are characterised as either monophasic or biphasic based on cellular morphology and types. The translocation t(X;18)(p11;q11), associated with the formation of fusion oncogene SYT-SSX1 or SYT-SSX2, is exclusively seen with this tumour. Positive immunohistochemical markers include vimentin, B-cell lymphoma-2 (Bcl-2), nuclear protein Ki-67, epithelial membrane antigen (EMA), and anti-cytokeratin monoclonal antibodies (AE1/AE3) [6]. Transducin-like enhancer of split 1 (TLE-1) is a relatively new immune histochemical marker that is considered very sensitive and specific for cardiac synovial sarcomas, performing better than other known immunohistochemical markers and correlates well with t(x;18) mutation and can significantly aid in the pathologic diagnosis [7].
While surgical excision is considered standard treatment, massive tumours present challenge for reconstruction of the cardiac structures involved as well as incomplete resection due to extensive involvement. Local recurrence and distant metastasis were the chief adverse events in patients undergoing surgical resection with adjuvant chemo-radiation therapy (CRT) and a survival of 59% at 1 year and 29.9% at 5 years was reported. The role of adjuvant chemotherapy is still under evaluation with VAC regimen. This combination is the most frequently used chemotherapeutic agents. In view of the intraoperative findings and the chemotherapy regimen the patient is on that includes adriamycin, an echocardiogram will be performed at the end of each treatment cycle to reassess the functional status of the heart.
Coli et al. reported in their analysis of 55 cases that complete resection could only be performed in 23 out of the 55 candidates and a survival rate of 75% at 24 months following complete resection as compared to 55% without resection. Advanced age and absence of adjuvant CRT were found to be significantly associated with reduced survival, whereas complete surgical resection along with adjuvant CRT demonstrated a more favourable outcome. CRT in the absence of surgical resection has also shown survival benefit [8]. There are reports of adriamycin and doxorubicin showing some improvement in survival [9]. The most recent echocardiogram done 3 months after surgery following adjuvant CRT showed regression of the mass and mild tricuspid regurgitation and the patient remains symptom free and is off anti-failure drugs.
Conclusion
Primary cardiac synovial sarcomas are a rare entity. The present case eluded diagnosis preoperatively and the tumour was non-resectable. Patients with primary cardiac synovial sarcoma have an overall poor prognosis due to lack of experience from rarity of the disease, as well as the involvement of critical structures within the heart, due to which it is often impossible to fully resect the malignancy. Adjuvant chemotherapy offers a better prognosis to the overall survival. Although rare, cardiac sarcomas can be an elusive diagnosis and as such a high index of suspicion should be kept in evaluating patients with a cystic lesion in the setting of pericardial effusion and constrictive pericarditis.
Key take home message
Primary cardiac synovial sarcoma of the heart is a rare entity. Immunohistochemistry (IHC) and molecular analyses play an important role in the pathological confirmation of this tumour. Complete surgical resection may not be feasible in many cases due to its location. However, there is no standard regime, but adriamycin-based chemotherapy is commonly used and offers better prognosis and overall survival in combination with radiation therapy.
Data availability
All data compiled from departmental database.
Code availability
Software application used was Microsoft Word®.
References
Castillo JG, Silvay G. Characterization and management of cardiac tumors. Semin Cardiothorac Vasc Anesth. 2010;14:6–20.
Yokouchi Y, Hiruta N, Oharaseki T, Ihara F, Oda Y, Ito S, et al. Primary cardiac synovial sarcoma: a case report and literature review. Pathol Int. 2011;61:150–5.
Khan I, Gul S, Tufail Z, Khan K, Sharma P, Waheed A. Primary cardiac synovial sarcoma. Asian Cardiovasc Thorac Ann. 2015;23:713–5.
Varma T, Adegboyega P. Primary cardiac synovial sarcoma. Arch Pathol Lab Med. 2012;136:454–8.
Wang J-G, Li N-N. Primary cardiac synovial sarcoma. Ann Thorac Surg. 2013;95:2202–9.
Teng F, Chen D, Li Y, Fang W, Yang S, Shang J, et al. Primary cardiac synovial sarcoma: a clinicopathological, immunohistochemical, and molecular genetics study of five clinical cases. Cardiovasc Pathol. 2021;50:107286.
Knösel T, Heretsch S, Altendorf-Hofmann A, Richter P, Katenkamp K, Katenkamp D, et al. TLE1 is a robust diagnostic biomarker for synovial sarcomas and correlates with t(X;18): analysis of 319 cases. Eur J Cancer. 2010;46:1170–6.
Coli A, Cassano A, Novello M, Ranelletti FO, Lauriola L. Primary cardiac synovial sarcoma: a review correlating outcomes with surgery and adjuvant therapy. J Card Surg. 2019;34:1321–7.
Llombart-Cussac A, Pivot X, Contesso G, Rhor-Alvarado A, Delord JP, Spielmann M, et al. Adjuvant chemotherapy for primary cardiac sarcomas: the IGR experience. Br J Cancer. 1998;78:1624–8.
Acknowledgements
Patient.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was waived.
Informed consent
Written informed consent was obtained from the patient and her family regarding publishing their data and photographs.
Conflict of interest
The authors have no financial or proprietary interests in any material discussed in this article.
Statement of human and animal rights
The study was carried out on patients with written informed consent that conforms to the ethical guidelines of the “World Medical Association Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects” adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964 and amended by the 59th WMA General Assembly, Seoul, South Korea, October 2008.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, S., Rahul, K., Tewarson, V. et al. Cardiac synovial sarcoma masquerading as effusive constrictive pericarditis. Indian J Thorac Cardiovasc Surg 39, 300–304 (2023). https://doi.org/10.1007/s12055-022-01461-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-022-01461-9