Abstract
Genetic diversity during prebreeding or postbreeding programme, is the key pillar to characterize the valuable traits and gene of interest. Whereas, superior or inferior heterotic performance of \(\hbox {F}_{1}\) depend on the diverse nature of their pedigree. Therefore, the aim of this study was to see the diversity between the interspecific crosses and effect of heterosis, and inheritance for the morphological traits and ToLCV resistance. All the 24 \(\hbox {F}_{1}\) interspecific crosses were classified into four clusters on the basis of morphological traits as well as simple sequence repeat (SSR) markers. Among the \(\hbox {F}_{1}\) hybrids, 23 were grouped into clusters II, III and IV with different phylogeny, while \(\hbox {PBC} \times \) EC 521080 was individual with cluster I. On the basis of visual observation of fruit colour, deep red and green colours in the crosses of S. pimpinellifolium (EC 521080) and S. habrochaites (EC 520061) exhibited dominant effects. In context of heterosis breeding, the crosses which were made using Solanum pimpinellifolium (EC 521080), S. chmielewskii (EC 520049) and S. cerasiforme (EC 528372) were better for yield capacity and the crosses of S. habrochaites (EC 520061) exhibited low and negative heterosis for ToLCV resistance. The \(\hbox {F}_{2}\) progenies were segregated in various Mendelian ratio as follows 3:1, 1:2:1, 1:3, 13:3, 15:1, 12:3:1 and 9:6:1 for ToLCV disease reaction of incidence, plant growth habit and fruit colour appearance, respectively. Therefore, these interspecific crosses can be utilized for developing high yield, impressive fruit colour combiners and resistant hybrids/varieties of tomato.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most of the commercial tomato cultivars (Solanum lycopersicum L.) have been reported for susceptible to tomato leaf curl virus (ToLCV) around the tomato growing countries (Muniyappa et al. 2002; Anbinder et al. 2009; Borah and Dasgupta 2012; Singh et al. 2015a). Whereas, a number of resistance sources are available with vigorous morphological traits in many wild relatives of tomato (Banerjee and Kalloo 1987a; Pico et al. 1998; Vidavski et al. 2008; Singh et al. 2015a). Previously, it was studied that the tomatoes have a limited genetic diversity (Miller and Tanksley 1990) but this crop has commendable adaptability for selection and breeding programmes under both field and glasshouse conditions (Singh et al. 2015a, b, c). The study of diversity by using morphological traits is an easy way for identifying the close relationships within tomato pedigrees (Banerjee and Kalloo 1987a, b; Singh et al. 2014). The genetic diversity between parental lines is usually considered as an important factor to maximize the chances of heterotic performance (Kaur et al. 2007). Molecular diversity between wild and cultivar accessions is evolved either complex or intensive condition during hybridization (Grandillo et al. 2011) because the molecular markers allowed for making high density genetic maps of the tomato genome (Tanksley et al. 1992). The simple sequence repeats (SSRs) or microsatellite markers are considered in various studied due to their high reproducibility, multiallelic, codominance and wide-genome coverage (Frary et al. 2005). The crosses derived from diverse parents produced diverse and useful progenies (Singh et al. 2014, 2015b). However, the presence of genetic variation among cultivated tomato (intraspecific) and between wild species (interspecific) is potential to produce new breeding materials (Grandillo et al. 2011).
A deep red colour is essential for obtaining standard value added tomato products like ketchup, puree, sauce etc. in processing industry. The deep red colour gives a good visual appearance under glasshouse conditions where darkness is maintained or sun light is lacking (Britton 1998). The chlorophyll (green) colour changed into carotene (yellow, red or orange) colour of tomato fruits due to production of small amount of ethylene during ripeness and degradation of chlorophyll pigments (Kader 1996). During maturation of the tomato friuts, the concentration of carotenoids and lycopene increased around 10 to 14 folds (Fraser et al. 1994). The mature ripe tomatoes containing high lycopene (80–90%) of the total pigments has rich antioxidant potential for health benefits (Rao and Agarwal 2000).
During the past several decades, wild tomato species have been utilized extensively in traditional breeding programmes and the major breeding efforts in tomatoes have been directed towards higher degree of disease resistance against ToLCV. Heterosis involves genome wide dominance pair and inheritance model such as locus-specific over dominance (Lippman and Zamir 2007). Heterosis breeding is considered as a function of heterozygosity and a good approach to examine better yield traits as well as resistance capacity during interspecific hybridization (Singh et al. 2014). The segregation between plant populations is a way to know the genetics of traits and it is a major breeding tool for improving the attainable productivity of crops (Singh et al. 2015a, b). The objective of this study was to elucidation of the genetic diversity between interspecific crosses to know the heterosis and inheritance pattern for morphological traits and resistance to ToLCV.
Materials and methods
Genetic materials and development of crosses
A set of 24 interspecific crosses were generated in Line \(\times \) tester mating design (figure 1) developed by crossing with four cultivars of S. lycopersicum, namely Punjab Chhuhara (PBC), Kashi Vishesh (H-86), Hissar Anmole (H-24) and Kashi Anupam (DVRT-2) and six accessions of wild species, namely EC 521080 (S. pimpinellifolium), EC 520061 (S. habrochaites), EC 520049 (S. chmielewskii), EC 528372 (S. cerasiforme), WIR 5032 (S. chilense) and WIR 3957 (S. arcanum). The four cultivars were susceptible to ToLCV along with better fruit size and fruit weight, while, six wild accessions were resistant to ToLCV with vigorous growth and bearing more number of fruits (Singh et al. 2014). The experiment was conducted at the ICAR-Indian Institute of Vegetable Research, Varanasi, India, situated at \(83^{\circ }00'\hbox {E}\), \(25^{\circ }19'\hbox {N}\) latitude and 128.93 metres above sea level (masl) during rainy season (March) as well as winter (September) season of 2006 and 2007 (Singh et al. 2014). The \(\hbox {F}_{1}\)’s were preserved to generate \(\hbox {F}_{2}\) progenies. Thirty-four genotypes (24 \(\hbox {F}_{1}\hbox {s} + 10\) parents) and \(24 \,\hbox {F}_{2}\) segregating population of tomato were evaluated in three replications with completely random block design under field and glasshouse conditions (mass and cage inoculation) during September 2008. During field trails, all the 10 parents and 24 \(\hbox {F}_{1}\) crosses were planted with 60 plants (20 plants in a replication) and \(24 \,\hbox {F}_{2}\) segregating population were planted with 180 plants of each (60 plants in a replication) at a determined spacing of 45 cm (plant to plant) and 60 cm (row to row). However, during glasshouse experimentation all the parents and \(\hbox {F}_{1}\) crosses planted with 30 plants of each (10 in a replication) and all the \(\hbox {F}_{2}\) segregating population planted with 90 plants of each (30 plants in a replication). Repeated planting of susceptible tomato variety, Punjab Chhuhara between the alternate rows of trials. The buildup of vector population (whitefly; Bemisia tabaci) was already maintained in insect proof glasshouse of ICAR-IIVR, Varanasi and allowed by avoiding spray of insecticides for insect vector control (Singh et al. 2015a).
Severity of ToLCV disease scoring
Natural screening completed under open field conditions at the favourable environment for rapid multiplication of whiteflies. Twenty-one days old seedlings were transplanted in the field, and observations were recorded on 30, 60 and 90 days after transplanting during each season of experiment. Artificial inoculation was done in an insect proof glasshouse by both mass and cage inoculation techniques. Seeds of tomato genotypes (parents, \(\hbox {F}_{1}\)’s and \(\hbox {F}_{2}\)’s) were sown in plastic tray. Ten days old seedlings were transplanted in plastic pots in insect proof glasshouse for mass and cage inoculation. Seedlings were inoculated with whiteflies and observations were recorded at 15, 30, 45 and 60 days after transplantation as per the procedure of Banerjee and Kalloo (1987a, b). The symptom severity was recorded at six point (0–5) scale and the coefficient of infection (CI), per cent disease incidence (PDI) and response value (RV) were calculated according to procedure of Singh et al. (2015a, b).
Genomic DNA extraction and gel electrophoresis
Genomic DNA was extracted from young fresh leaves of all \(\hbox {F}_{1}\) crosses at the seedling stage by using the modified DNA cetyltrimethyl ammonium bromide (CTAB) method developed by Doyle and Doyle (1990). DNA purification, DNA quantification, gel electrophoresis, gel documentation and PCR optimization were followed by same method of Singh et al. (2014).
Hybrid seed purity testing, microsatellite genotyping, scoring of alleles and cluster analysis
For hybrid seed purity testing, 11 polymorphic SSRs primers were selected from previous study of 10 parental genetic diversity (Singh et al. 2014). Whereas, 80 SSR primers or microsatellite primer pairs (including 11 polymorphic SSRs) were selected from publicly available databases (e.g., http://www.sgn.cornell.edu/, http://hornbill.cspp.latrobe.edu.au/ssrdiscovery.html) for the genotyping assays among \(\hbox {F}_{1}\) crosses. The SSR gel images were scanned using the gel doc 2000 Bio-Rad system and all the genotypes were scored for the presence and absence of the SSR band using quantity one software ver. 4.0.1 (Bio-Rad, Hercules, USA). The scoring of alleles was entered into a binary matrix as discrete variables, e.g. 1 for presence and 0 for absence of the character and this data matrix was subjected to further analyses. Similar scoring was used for phenotypic characters and pooled the data. The software NTSYS-pcver. 2.11a, an advanced version of 2.02 (Rohlf 1994) was used to calculate the pair-wise differences matrix and plot. Cluster analysis was based on similarity matrix obtained with the unweighted pair-group method using arithmetic averages (UPGMA) (Sneath and Sokal 1973; Singh et al. 2014).
Data observation and heterosis analysis
For the assessment of colour characteristics of tomato fruits of parents and hybrids, the latest Royal Horticultural Society (RHS) colour chart were used during both field and glasshouse conditions. For the study of heterosis, six traits of parents and hybrids such as percentage of coefficient of infection (CI), plant height (PH), number of fruits per plant (NOFPP), fruit set per cent (FSP), average fruit weight (AFW) and fruit yield per plant (FYPP) were assayed for both field and glasshouse planted \(\hbox {F}_{1}\)’s. The heterosis over better parents (heterobeltiosis) was calculated by the formula:
Where, Dii is heterobeltiosis, i.e. heterosis over better parent and BP is mean performance of better parent in the respected cross combination.
Inheritance analysis
Expected Mendelian genetic ratio of ToLCV disease incidence, plant growth habit and colour effect of fruits were analysed as per visual observation of number of segregation between plant populations of \(\hbox {F}_{2}\) progenies and using chi-square analyses followed by Singh et al. (2015a, b).
Results
Colour appearance of fruits in parents and their \(\hbox {F}_{1}\)’s
Of the 34 genotypes, 10 parents were categorized in six different colour groups e.g., deep red (PBC, EC 521080 and EC 528372), light red (H-24, DVRT-2 and WIR 5032), pinkish red (H-86), green (EC 520061), yellow (520049) and orange (WIR 3957). Whereas, in their 24 interspecific crosses, the wild parental colours were expressed as dominant genetic effect (figure 1). It was also recorded that the tomatoes were deep red in colour under glasshouse conditions as compared to field conditions. The crosses of EC 520061 and EC 521080 expressed green and deep red colour, respectively. However, crosses of EC 520049 manifested as yellow (\(\hbox {PBC} \times \) EC 520049 and \(\hbox {DVRT-2} \times \) EC 520049) and reddish yellow (\(\hbox {H-86} \times \) EC 520049 and \(\hbox {H-24} \times \) EC 520049) colour. The crosses of EC 520072 and WIR 5032 showed only deep red and light red colours. The cross combinations made by WIR 3957 were light red (\(\hbox {H-86} \times \)WIR 3957 and \(\hbox {H-24} \times \)WIR 3957) and orange (\(\hbox {PBC} \times \)WIR 3957 and \(\hbox {DVRT-2} \times \)WIR 3957) in colour (figure 1).
Hybrid seed purity testing and genetic diversity
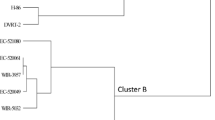
All the 11 polymorphic SSR markers have been identified during genetic diversity of 10 parents, gave positive results during testing of hybrid seed purity for 24 \(\hbox {F}_{1}\)’s (figure 1). The genetic diversity was studied between a set of 24 \(\hbox {F}_{1}\) hybrids by using genotypic and phenotypic scoring data. Among the 80 SSR markers, only 18 (22.5%) SSR markers were polymorphic (table 1) and 35 (43.75%) indicated monomorphic banding pattern. However, four SSRs, namely SSR73, SSR117, SSR218 and SSR304 exhibited highest 100% polymorphic per cent with polymorphic information content (PIC) of the markers range from 0.10 to 0.33. A dendrogram was generated with four major clusters I, II, III and IV by using genotypic and phenotypic scoring data (figure 2). Cluster I consisted of only one \(\hbox {F}_{1}(\hbox {PBC} \times \)EC 521080) with a major genetic distance from other hybrids by 0.40 coefficient value. Cluster I included 11 hybrids in which \(\hbox {H-86} \times \)WIR 5032 and \(\hbox {H-24} \times \)EC 520049 were very close with a coefficient value 0.03. \(\hbox {PBC} \times \)EC 520061 and \(\hbox {DVRT-2} \times \)EC 520061 were followed by two pairs comprising of \(\hbox {H-86} \times \)EC 521080 and \(\hbox {H-24} \times \)EC 521080 as well as \(\hbox {PBC} \times \)WIR 5032 and \(\hbox {DVRT-2} \times \)WIR 5032 with the range of coefficient value from 0.03 to 0.17. Cluster III had eight hybrids in which the pairs of \(\hbox {PBC} \times \)EC 520049 and \(\hbox {DVRT-2} \times \)EC 520049, along with the pair of \(\hbox {PBC} \times \)WIR 3957 and \(\hbox {DVRT-2} \times \)WIR 3957 were very close to each other with the coefficient range 0.05 to 0.07. Cluster IV included four hybrids, and was differentiated in two individual pairs of \(\hbox {H-86} \times \)EC 520061 and \(\hbox {H-24} \times \)EC 520061 with the coefficient value ranged from 0.03 to 0.28 and \(\hbox {H-86} \times \)EC 528372 and \(\hbox {H-24} \times \)EC 528372 within a coefficient range of 0.09 to 0.11 (figure 2).
Heterosis over better parents or heterobeltiosis
Analysis of variance for various characteristics evaluated in two environments (field and glasshouse) showed the significant differences among the genotypes, parents, lines, testers, line versus tester, \(\hbox {line} \times \)tester and parent versus hybrid for all the traits. While, PH and NOFPP were non significant for lines under field and glasshouse, FSP and AFW were non significant for tester under field condition (table 2). All the traits showed significant differences between parents and hybrids at \(P < 0.05\) and \(P < 0.01\). The significance of average heterosis for CI, PH, NOFPP, FSP, AFW and FYPP were variable across the two environments.
Extent of heterosis was studied in two environments (field and glasshouse) for ToLCV disease (CI) along with five eco-horticultural yield traits e.g., PH, NOFPP, FSP, AFW and FYPP (table 3). The highest and negative heterosis was recorded in field as compared to glasshouse conditions for each horticultural trait except AFW but expression of heterosis for ToLCV (CI) was low and negatively significant in glasshouse. In case of ToLCV (CI) the maximum and negative heterosis was recorded in \(\hbox {PBC} \times \hbox {EC}\, 520061\) (\(-83.96\) and \(-88.97\)) followed by \(\hbox {DVRT-2} \times \hbox {EC}\, 520061\), \(\hbox {H-24} \times \hbox {EC}\, 520061\) and \(\hbox {H-86} \times \hbox {EC}\, 520061\) under both field and glasshouse conditions. The heterosis range was \(-5.02\) (\(\hbox {H-86} \times \hbox {EC}\, 521080\)) to \(-83.96\) (\(\hbox {PBC} \times \hbox {EC}\, 520061\)) in field and from \(-4.00\) (\(\hbox {H-86} \times \hbox {EC}\, 520049\)) to \(-88.97\) (\(\hbox {PBC} \times \hbox {EC}\, 520061\)) in glasshouse conditions for ToLCV (CI). For PH, according to mean value the crosses of EC 520061 exhibited maximum value in field. The minimum and maximum range of heterosis was \(-39.07\) (\(\hbox {PBC} \times \hbox {EC}\, 528372\)) to 59.74 (\(\hbox {H-24} \times \hbox {WIR}\) 5032) in field and from \(-37.36\) (\(\hbox {DVRT-2} \times \hbox {EC}\, 520061\)) to 22.54 (\(\hbox {H-86} \times \hbox {WIR}\) 5032) in glasshouse conditions for PH. In case of NOFPP, maximum number of fruit was recorded in the crosses of EC 521080 followed by the crosses of EC 520061, EC 520049 and EC 520072, whereas, maximum heterosis was recorded in the crosses of \(\hbox {DVRT-2} \times \hbox {EC}\, 528372\) (18.34) and \(\hbox {DVRT-2} \times \hbox {EC}\, 528372\) (13.31) in field and glasshouse conditions, respectively. In case of fruit set per cent (FSP) maximum heterosis was found in the crosses of \(\hbox {H-24} \times \hbox {EC}\, 521080\) (13.57) in field and \(\hbox {H-86} \times \hbox {EC}\, 528372\) (49.69) in glasshouse conditions. For AFW, the maximum mean value of fruit weight was recorded in crosses of EC 520072 followed by the crosses of WIR 5032 and WIR 3957, while the minimum and maximum heterosis range was \(-96.90\) (\(\hbox {DVRT-2} \times \hbox {EC}\, 520061\)) to \(-54.78\) (\(\hbox {H-24} \times \hbox {EC}\, 528372\)) in field conditions and from \(-96.19\) (\(\hbox {DVRT-2} \times \hbox {EC}\, 520061\)) to \(-65.43\) (\(\hbox {PBC} \times \hbox {EC}\, 528372\)) in glasshouse conditions. However, as per mean value of FYPP, the crosses made in H-86 and DVRT-2 expressed high yield but in case of heterosis the cross combination \(\hbox {PBC} \times \hbox {EC}\) 520072 (63.46) and \(\hbox {PBC} \times \hbox {EC}\, 521080\) (42.17) recorded highest heterosis in field and glasshouse conditions, respectively (table 3).
Mendelian ratio in segregating population
For ToLCV disease incidence among the \(24 \,\hbox {F}_{2}\) progeny, 10 crosses were segregated in 3 : 1 (resistant : susceptible) and six crosses in 1 : 2 : 1 (resistant : moderately resistant : susceptible) expected ratio. The four crosses of EC 520061 (\(\hbox {PBC} \times \hbox {EC}\, 520061\), \(\hbox {DVRT-2} \times \hbox {EC}\, 520061\), \(\hbox {H-86} \times \hbox {EC}\, 520061\) and \(\hbox {H-24} \times \hbox {EC}\, 520061\)) segregated in 13 : 3 expected Mendelian ratio and remaining four crosses, namely \(\hbox {H-24} \times \hbox {EC}\, 528372\), \(\hbox {H-24} \times \hbox {WIR} \,3957\), \(\hbox {H-86} \times \hbox {EC}\, 528372\) and \(\hbox {H-86} \times \hbox {WIR}\, 3957\) segregated in 1 : 3 Mendelian ratio for ToLCV disease incidence (table 4). However, in case of plant growth habit, 12 crosses of EC 528372, WIR 5032 and WIR 3957 segregated in 1 : 3 (indeterminate : determinate) expected genetic ratio, and the crosses of EC 520061, EC 521080 and EC 520049 segregated in 3 : 1 (indeterminate : determinate), 1 : 2 : 1 (indeterminate : semi-indeterminate : determinate) and 9 : 6 : 1 (indeterminate : semi-indeterminate : determinate) expected ratio, respectively. In case of colour effects, of the 24 crosses, 12 crosses of EC 521080, EC 528372 and WIR 5032 could not be identified for their colour segregation. Four crosses of EC 520061 (\(\hbox {PBC} \times \hbox {EC}\, 520061\), \(\hbox {DVRT-2} \times \hbox {EC}\, 520061\), \(\hbox {H-86} \times \hbox {EC}\, 520061\) and \(\hbox {H-24} \times \hbox {EC}\, 520061\)) segregated in 15 : 1 (green : red) Mendelian ratio. Whereas, four crosses, namely \(\hbox {PBC} \times \hbox {EC}\, 520049\) (yellow : red), \(\hbox {DVRT-2} \times \hbox {EC}\, 520049\) (yellow : red), \(\hbox {PBC} \times \hbox {WIR}\, 3957\) (orange : red) and \(\hbox {DVRT-2} \times \hbox {WIR}\, 3957\) (orange : red) segregated in 3 :1 genetic ratio, and two crosses \(\hbox {H-86} \times \hbox {EC}\, 520049\) (yellow : orange : red), \(\hbox {H-24} \times \hbox {EC}\, 520049\) (yellow : orange : red) segregated in 12 : 3 : 1 expected genetic ratio. Remaining two crosses, \(\hbox {H-86} \times \hbox {WIR}\, 3957\) (orange : red : yellow) and \(\hbox {H-24} \times \hbox {WIR}\, 3957\) (orange : red : yellow) segregated in 9 : 6 : 1 expected Mendelian ratio (table 4).
Discussion
Fruit colour of tomato is an attractive trait for visualization in diverse tomato genotypes. Red, pink, orange and yellow colour fruits indicated richness of lycopene and carotene content and are preferred for edible purposes. In the present study, green colour of EC 520061, deep red colour of EC 521080 and EC 528372 and light red colour of WIR 5032 masked their colour effects on their hybrids, which may be due to dominant morphology of S. habrochaites, S. pimpinellifolium, S. ceraseforme and S. chilense, and exhibited additive gene effects (Banerjee and Kalloo 1989; Singh et al. 2014). Earlier, it has been reported that during the fruit ripening a small amount of ethylene was produced which started the degradation in chlorophyll pigments and induced carotene pigment, and this chlorophyll or green colour changed into yellow, red and orange colours (Kader 1996). In the present study, the parents were in different colour pigments and they showed dominant over their hybrids. Similarly, in an earlier study, the pod colours of okra hybrids were same as their parents due to the dominant genetic effect of colour (Solankey et al. 2013). It was also noticed that the deep colour were got in glasshouse than field conditions. The accumulation of red colour indicated a high lycopene in tomatoes which may be due to the nature of cultivars or environment. Sun light is also important during development of fruits and increases carotenoid concentration in tomatoes but in relatively darker conditions fruits colour appeared as deep red (Giuliano et al. 1993; Britton 1998). However, the cross combinations of EC 520049 (yellow and reddish yellow) and WIR 3957 (orange and light red) expressed their colour effect in 1:1 ratio which showed intermediate colour. The yellow (high carotene) and orange (low carotene) colour of hybrids showed the dominant effect of their parents EC 520049 and WIR 3957, whereas the intermediate colour (reddish yellow and light red) of hybrids indicated a medium carotene content and partial dominance of the parents. It has been reported that chlorophyll produce green colour and familiar red and yellow colours are produced by carotenoids, lycopene and \(\beta \)-carotene in tomato (Lumpkin 2005). Hybrid seed purity testing is required for assessing the identity of parents and this confirmed the crossing and hybridity between two parents (Liu et al. 2008). However, the \(\hbox {F}_{1}\) hybrid exhibited both the alleles of the parents confirming the heterozygosity condition of the hybrid (Peralta et al. 2007). In present study, a total of 11 parental polymorphic SSRs were used to hybrid seed purity test, and found the purity between hybrids. The result was showed the conformity and proof for genetic purity of parents which were used during crossing programme (Singh et al. 2014). A previous study also supported our finding and they found similar results with 95.1% hybridity in \(\hbox {F}_{1}\) crosses of tomato by using 321 different molecular markers (Liu et al. 2008).
Conservational exchange and sustainable use of genetic resources is an essential tool for future food security but successful conservation of any given gene pool is dependent on their diversity and distribution in a selected community (Singh et al. 2014, 2015a, c). Creation of genetic variation and selection of suitable genotypes is one of the common way that can assist in crop improvement and may be easier to enhance the exploitation of the genotypes with molecular markers (Frary et al. 2005). Recently, molecular analysis of the structure of a large set of accessions of wild S. pimpinellifolium, cherry tomato and cultivated accessions showed that domesticated and wild tomatoes have evolved as a species complex with intensive hybridization (Peralta et al. 2007). In present study, only 18 (22.5%) SSR markers showed polymorphic banding pattern. It has been illuminated that the SSRs have low level of polymorphism. While, SSRs are useful as molecular markers because their development is inexpensive, and they are useful for identification of functional diversity, natural diversity or germplasm collections (Varshney et al. 2005). Earlier, SSR marker was used in a study and got 10 polymorphic of 50 used (Frary et al. 2005). In tomato, more than 600 EST-derived SSR markers have been identified and made available for genome research through Solanaceae Genome Network (SGN) and these SSR markers were shown applicable for genetic diversity studies of tomato (Frary et al. 2005). The S. lycopersicum accounts for only about 5% of the total genetic variability in the tomato gene pool, but wild species of tomato have rich source of genetic variability for many important agro-horticultural traits (Peralta et al. 2007). In this study, it was found that genetic variations existed between interspecific crosses due to the contrast in taxonomical characters. In the result of present study \(\hbox {PBC} \times \hbox {EC}\, 521080\) exhibited a high level of genetic diversity from other hybrids. It was observed separately in a cluster which may be due to its diverse morphological characters because in another study its parent EC 521080 (S. pimpinellifolium) was also seen individually in a cluster (Singh et al. 2014). Earlier, it has been studied that the cultivated tomato has been changed from the accessions of S. pimpinellifolium due to large genetic diversity (Peralta et al. 2007). It was also recorded that most of the crosses clustered in common pairs, namely ‘\(\hbox {PBC} \times \hbox {EC}\, 520061\) and \(\hbox {DVRT-2} \times \hbox {EC}\, 520061\)’, ‘\(\hbox {H-24} \times \hbox {EC}\, 521080\) and \(\hbox {H-86} \times \hbox {EC}\, 521080\)’, ‘\(\hbox {H-86} \times \hbox {EC}\, 521080\) and \(\hbox {H-24} \times \hbox {EC}\, 521080\)’, ‘\(\hbox {PBC} \times \hbox {WIR}\) 5032 and \(\hbox {DVRT-2} \times \hbox {WIR}\) 5032’, ‘\(\hbox {PBC} \times \hbox {EC}\, 520049\) and \(\hbox {DVRT-2} \times \hbox {EC}\, 520049\)’ and ‘\(\hbox {PBC} \times \hbox {WIR }3957\) and \(\hbox {DVRT-2} \times \hbox {WIR-}\, 3957\)’, ‘\(\hbox {PBC} \times \hbox {EC}\, 520061\) and \(\hbox {DVRT-2} \times \hbox {EC}\, 520061\)’, ‘\(\hbox {H-86} \times \hbox {EC}\, 528372\) and \(\hbox {DVRT-2} \times \hbox {EC}\, 528372\)’ made by similar parent. They indicated their similar genetic characters or dominant taxonomical phylogeny of their wild parents (Kalloo and Banerjee 1990). An interesting fact was that the crosses of EC 520061 clustered in two groups of different clusters, namely the pairs of \(\hbox {PBC} \times \hbox {EC}\, 520061\) and \(\hbox {DVRT-2} \times \hbox {EC}\, 520061\) and \(\hbox {H-24} \times \hbox {EC}\, 520061\) and \(\hbox {H-86} \times \hbox {EC}\, 520061\) were in different clusters. This may be due to strong genetic link with S. habrochaites (EC 520061) because H-24 and H-86 both were derived by S. habrochaites f. glaboratum (Banerjee and Kalloo 1989; Singh et al. 2015b).
Improvement of tomato by exploiting traits from wild species is a slow process because of the complexity of the genes and linkage drag (Foolad 2007). However, there were several attempts reported on introgression of valuable traits from wild species but the time requirement could be a discouraging factor for plant breeders. The commercial exploitation of the phenomenon of heterosis is one of the most important contributions in plant breeding (Shankarappa et al. 2008). The extent of heterosis response of \(\hbox {F}_{1}\) hybrids largely depends on the breeding value and genetic diversity of the parents included in the cross, and on the environmental conditions under which hybrids are grown (Jordaan et al. 1999; Shankarappa et al. 2008). During the scanning of literature, it was observed that a few workers used interspecific crosses for heterosis study due to lack of market potential. Morphologically, most of the \(\hbox {F}_{1}\)s (\(S.~lycopersicum \times \) wild accessions) have large similarity to their wild parent(s) which indicated the dominance of morphological attributes of wild taxa over esculentum types (Vidavski et al. 2008; Singh et al. 2014). Another reason could be it may be due to the expression of two heterogeneous genes one favourable dominant and other unfavourable recessive alleles (AAbb/AaBb/aaBB).
In the present investigation, most of the cross combinations where S. habrochaites acc. EC 520061, was used as a male parent (with PBC, H-86, H-24 and DVRT-2) exhibited low and negative heterosis in both field and glasshouse conditions. Low and negative heterosis is an indication of least infection and is desirable traits for resistance to ToLCV disease. This may be due to presence of Ty-2 (Hanson et al. 2006) genes of TYLCV in S. habrochaites accession which have a dominant gene for ToLCV resistance. The dominant nature of heterosis for ToLCV resistance was governed by two completely dominant genes and inhibitory gene action (Banerjee and Kalloo 1987a, b; Singh et al. 2015a, d). In this study, negative heterosis was observed for various yield traits because the male wild accessions were diverse and dominant in genetic taxonomical phylogeny (Vidavski et al. 2008). Significant and high heterosis for yield traits (PH, NOFPP, FSP, AFW and FYPP) was found in field conditions than in glasshouse conditions. It indicated the importance of dominant genetic effects in the inheritance of these characteristics and effects of favourable environment. Genotypes performed differently across the two environments, field and glasshouse, these results were in agreement with the previous reports (Banerjee and Kalloo 1989; Pico et al. 1998). A large number of hybrids showed superiority over their parents for various characteristics, revealing substantial heterosis in the hybrids. Earlier, many workers have been reported high and positive heterosis over better parents for yield traits in tomato (Rajput et al. 2003; Shankarappa et al. 2008) involving \(S.~lycopersicum \times S.~lycopersicum\) as intervarietal cross combinations.
The expected genetic ratio for pheno-traits in segregating plant populations can be easily identified on the basis of visual observation (Singh et al. 2015a, d). In the present study, 10 and six crosses segregated in 3 : 1 (resistant : susceptible) and 1 : 2 : 1 (resistant : intermediate : susceptible) expected ratio for ToLCV disease incidence because these crosses expressed monogenic dominant gene effects and partial dominant gene effects of resistant capacity (Singh et al. 2015a, d). However, four crosses, namely \(\hbox {H-24} \times \hbox {EC}\, 528372\), \(\hbox {H-24} \times \hbox {WIR} \,3957\), \(\hbox {H-86} \times \hbox {EC}\, 528372\) and \(\hbox {H-86} \times \hbox {WIR}\,3957\) segregated in 1 : 3 (recessive effect) expected ratio for ToLCV disease and this may be due to the dominance of S. lycopersicum over their male parents, S. ceraseforme and S. arcanum for disease resistance. Similar study for recessive nature of crosses of S. pimpinellifolium and S. arcanum were described earlier (Fulton et al. 1997; Sharma et al. 2008). In another finding, the crosses of EC 520061 segregated in 13 : 3 (resistant : susceptible) expected ratio (a modified ratio of 9 : 3 : 3 : 1) indicating inhibitory gene effect. This finding of modified ratio of 9 : 3 : 3 : 1 had been commensurated in the crosses of S. habrochaites (Banerjee and Kalloo 1989). A number of witnesses for the resistant story of S. habrochaites had been reported (Banerjee and Kalloo 1987a, b; Singh et al. 2014, 2015a, b, d). Tomato plants may have different growth habits, including indeterminate, semideterminate and determinate type depending on the nature of crosses. In this study, of the 24 crosses 12 of S. ceraceforme, S. chilense and S. arcanum segregated in 1 : 3 (indeterminate : determinate) expected genetic ratio and showed recessive gene effects. This may be due to the additive genetic variation in the crops (Fulton et al. 1997; Castro et al. 2013; Singh et al. 2015a, d). The four crosses of S. habrochaites segregated in 3 :1 (indeterminate : determinate) genetic ratio because of their vigourous growth habits which were indicative to the monogenic dominant gene effect and has been observed in many studies (Singh et al. 2014, 2015a, b). The crosses of S. pimpinellifolium and S. chmielewskii segregated in 1 : 2 : 1 (indeterminate : semideterminate : determinate) and 9 : 6 : 1 (indeterminate : semideterminate : determinate) ratios exhibiting partial dominance and additive gene effects between the populations. The colour of the fruits got from crossing S. pimpinellifolium, S. ceraceforme, and S. chilense could not be categorized during observation. This may be due to less segregation for fruit colour in crosses since the fruit colours of their parents were visually similar to S. lycopersicum (Fulton et al. 1997; Castro et al. 2013; Singh et al. 2014, 2015a, d). However, the crosses of S. habrochaites were segregated in 15:1 (green : red) expected ratio and exhibited duplicate genetic effects, due to dominance of green fruited nature of S. habrochaites species. The dominant nature of green colour fruits of S. habrochaites had been discussed earlier (Banerjee and Kalloo 1987a, b; Singh et al. 2014, 2015a). The crosses of S. chmielewskii segregated in two phases, namely yellow : red (with PBC and DVRT-2) on 3 : 1 and yellow : orange : red (with H-86 and H-24) on 12:3:1 expected ratio, thereby exhibiting monogenic dominant and epistatic genetic effects between populations. Similarly the crosses of S. arcanum segregated on 3:1 (orange : red) with PBC and DVRT-2, while in 9:6:1 (orange : red : yellow) ratio with H-86 and H-24, thereby showing monogenic dominant and additive genetic effects of the population. The 3:1 expected ratio indicated the dominant genetic nature of male parents over S. lycopersicum. Ratio of 12:3:1 and 9:6:1 indicated the involvement of green fruited S. habrochaites f. glaboratum background in H-24 and H-86, and showed additive nature of the crosses. This has been declared that two duplicate dominant genes interacting a cumulative but unequal effect on fruits colour (Hegde 2010; Singh et al. 2015b).
The present investigation concluded that most of the diverse crosses were close to each other due to similar morphology and dominant genetic effects of wild parents. Red colour of EC 521080, green colour of EC 520061 and yellow colour of EC 520049 were dominant on their crosses. All the interspecific crosses represented similar morphology of their wild parents and classified into same categories except \(\hbox {PBC} \times \hbox {EC}\, 521080\). In case of heterosis, all the \(\hbox {F}_{1}\)’s made by accession EC-520061 of S. habrochaites showed resistance as well as low and negative heterosis for ToLCV. However, for yield traits, a better heterosis was observed in the crosses of S. cerasiforme, S. chmielewskii and S. pimpinellifolium. \(\hbox {F}_{2}\) progenies expressed various additive and dominant genetic effects for ToLCV disease incidence, plant growth habit and colour effects in the ratio of 3:1 (monogenic dominant), 1:3 (recessive inheritance), 1:2:1 (partial dominance), 13:3 (inhibitory inheritance), 15:1 (duplicate factor), 12:3:1 (epistatic genetic effect) and 9:6:1 (additive effect), respectively. It is suggested that combining sources of resistance from different wild species may have the advantage of providing greater strength for desirable yield and qualities. These \(\hbox {F}_{1}\) hybrids and wild accessions can be used in resistance breeding programmes of tomatoes for enhancing the capacity of ToLCV resistance and yield traits.
References
Anbinder I., Reuveni M., Azari R., Ilan P., Nahon S., Shlomo H. et al. 2009 Molecular dissection of tomato leaf curl virus resistance in tomato line TY172 derived from Solanum peruvianum. Theor. Appl. Genet. 119, 519–530.
Banerjee M. K. and Kalloo G. 1987a Sources and inheritance of resistance leaf curl virus in lycopersicon. Theor. Appl. Genet. 73, 707–710.
Banerjee M. K. and Kalloo G. 1987b Inheritance of tomato leaf curls virus resistance in Lycopersicon hirsutum f. glabratum. Euphytica 36, 581–584.
Banerjee M. K. and Kalloo G. 1989 The inheritance of earliness and fruit weight in crosses between cultivated tomatoes and two wild species of Lycopersicon. Plant Breed. 102, 148–152.
Borah B. K. and Dasgupta I. 2012 Begomovirus research in India: a critical appraisal and the way ahead. J. Biosci. 37, 791–806.
Britton G. 1998 Overview of carotenoid biosynthesis: In Carotenoids (ed. G. Britton, S. Liaaen-Jensen and H. Pfander) vol. 3, pp. 33–147. Birkhäuser-Verlag, Basel.
de Castro A. P., Julian O. and Diez M. J. 2013 Genetic control and mapping of Solanum chilense LA1932, LA1960 and LA1971-derived resistance to tomato yellow leaf curl disease. Euphytica 190, 203–214.
Doyle J. J. and Doyle J. L. 1990 A rapid DNA isolation procedure from small quantity of fresh leaf material. Phytochem. Bull. 119, 11–15.
Frary A., Xu Y., Liu J., Mitchell S., Tedeschi E. and Tanksley S. D. 2005 Development of a set of PCR-based anchor markers encompassing the tomato genome and evaluation of their usefulness for genetics and breeding experiments. Theor. Appl. Genet. 111, 291–312.
Fraser P. D., Truesdale M. R., Bird C. R., Schuch W. and Bramley P. M. 1994 Carotenoid biosynthesis during tomato development. Plant Physiol. 105, 405–413.
Foolad M. R. 2007 Genome mapping and molecular breeding of tomato. Int. J. Plant Genom. Article ID 64358.
Fulton T. M., Nelson J. C. and Tanksley S. D. 1997 Introgression and DNA marker analysis of Lycopersicon peruvianum, a wild relative of the cultivated tomato, into Lycopersicon esculentum, followed through three successive backcross generations. Theor. Appl. Genet. 95, 895–902.
Giuliano G., Bartley G. E. and Scolnik P. A. 1993 Regulation of carotenoid biosynthesis during tomato development. Plant Cell 5, 379–387.
Grandillo S., Chetelat R., Knapp S., Spooner D., Peralta I., Cammareri M. et al. 2011 From Solanum sect. Lycopersicon. In Wild crop relatives: genomic and breeding resources. Vegetables (ed. C. Kole), 1st edition, pp. 129–215. Springer, Berlin, Heidelberg, New York.
Hanson P., Green S. K. and Kuo G. 2006 Ty-2, a gene on chromosome 11 conditioning geminivirus resistance in tomato. Rep. Tomato Genet. Coop. 56, 17–18.
Hegde V. S. 2010 Genetics of flowering time in chickpea in a semi-arid environment. Plant Breed. 129, 683–687.
Jordaan J. P., Engelbrecht S. A., Malan J. H. and Knobel H. A. 1999 Wheat and heterosis. In The genetics and exploitation of heterosis in crops (ed. J. G. Coors and S. Pandey), pp. 411–421. ASA, CSSA, and SSSA, Madison.
Kader A. A. 1996 Maturity, ripening and quality relationships of fruit-vegetables. Acta Hortic. 434, 249–255.
Kalloo G. and Banerjee M. K. 1990 Transfer of tomato leaf curl virus resistance from Lycopersicon hirsutum f. glabratum to L. esculentum. Plant Breed. 105, 156–159.
Kaur G., Bansal P., Kaur B. and Banga S. 2007 Genetic diversity and its association with heterosis in Brassica rapa. In Proceedings of the 12th International Rapeseed Congress, vol. I, pp. 144–146, 6–30 June 2007. Wuhan, China.
Lippman Z. B. and Zamir D. 2007 Heterosis: revisiting the magic. Trends Genet. 23, 60–66.
Liu L., Wang Y., Gong Y., Zhai X., Yu F. and Shen H. 2008 Genetic purity test of F1 hybrid tomato using molecular marker analysis. Acta Hortic. 771, 231–238.
Lumpkin H. 2005. A comparison of lycopene and other phytochemicals in tomatoes grown under conventional and organic management systems. Technical Bulletin No. 34. AVRDC publication number 05-623, AVRDC-The World Vegetable Center, pp. 48. Shanhua, Taiwan.
Miller J. C. and Tanksley S. D. 1990 RFLP analysis of phylogenetic relationships and genetic variation in the genus Lycopersicon. Theor. Appl. Genet. 80, 437– 448.
Muniyappa V., Padmaja A. S., Venkatesh H. M., Sharma A., Chandrasekhar S., Kulkarni R. S. et al. 2002 Tomato leaf curl virus resistance tomato lines TLB111, TLB130, and TLB182. Hortic. Sci. 37, 603–606.
Peralta I. E., Spooner D. M., Razdan M. K., Mattoo A. K. 2007 History, origin and early cultivation of tomato (Solanaceae). In Genetic improvement of solanaceous crops (Tomato), vol. 2, pp. 1–27. N. H. Science Publishers, Enfield.
Pico B., Diez M. and Nuez F. 1998 Evaluation of whitefly-mediated inoculation techniques to screen Lycopersicon esculentum and wild relatives for resistance to tomato yellow leaf curl virus. Euphytica 101, 259–271.
Rajput Y. S., Rai N., Yadav R. K. and Asati B. S. 2003 Line x Tester analysis in tomato. J. Assam Sci. Soc. 44, 32–39.
Rao A. V. and Agarwal S. 2000 Role of antioxidant lycopene in cancer and heart disease. J. Am. Coll. Nutr. 19, 563–569.
Rohlf F. J. 1994 NTSYS-pc, numerical taxonomy and multivariate analysis system version 2.2. State University, New York, New York.
Shankarappa K. S., Sriharsha R. K. T., Aswathanarayana D. S., Prameela H. A., Kulkarni R. S., Muniyappa V. et al. 2008 Development of tomato hybrids resistant to tomato leaf curl virus disease in South India. Euphytica 164, 531–539.
Sharma A., Zhang L., Ni no-Liu D., Ashrafi H., Foolad M. R. 2008 A Solanum lycopersicum \(\times \) Solanum pimpinellifolium linkage map of tomato displaying genomic locations of R-genes, RGAs, and candidate resistance/defense-response ESTs. Int. J. Plant Genomics. Article ID 926090.
Singh R. K., Rai N., Singh M., Singh S. N. and Srivastava K. 2014 Genetic analysis to identify good combiners for ToLCV resistance and yield components in tomato using inter-specific hybridization. J. Genet. 93, 623–629.
Singh R. K., Rai N., Singh M., Saha S. and Singh S. N. 2015a Detection of tomato leaf curl virus resistance and inheritance in tomato (Solanum lycopersicum L.). J. Agric. Sci. Cambridge. 153, 78–89.
Singh R. K., Rai N., Singh M., Singh S. N., Srivastava K. 2015b Selection of resistance genotypes of tomato against tomato leaf curl virus (ToLCV) disease using biochemical and physiological approaches. J. Agric. Sci. Cambridge. 153, 646 – 655.
Singh R. K., Rai N., Singh M., Singh R. and Kumar P. 2015c Effect of climate change on tomato leaf curl virus (ToLCV) disease in tomato. Ind. J. Agric. Sci. 85, 290–292.
Singh R. K., Rai N., Kumar P. and Singh A. K. 2015d Inheritance study in tomato (Solanum lycopersicum) for Tomato leaf curl virus (ToLCV) resistance. Ind. J. Agric. Sci. 85, 896–901.
Sneath P. H. and Sokal R. R. 1973 Numerical taxonomy: the principles and practice of numerical classification, 1st edition. W. H Freeman, San Francisco.
Solankey S. S., Singh A. K. and Singh R. K. 2013 Genetic expression of heterosis for yield and quality traits during different growing seasons in okra (Abelmoschus esculentus). Ind. J. Agric. Sci. 83, 815–819.
Tanksley S. D., Ganal M. W., Prince J. P., De Vicente M. C., Bainerbale M. W., Broun P. et al. 1992 High density molecular linkage maps of tomato and potato genomes. Biol. Infer. Pract. Appl. Genet. 123, 1141–1160.
Varshney R. K., Graner A. and Sorrells M. E. 2005 Genic microsatellite markers in plants: features and applications. Trends Biotechnol. 23, 48–55.
Vidavski F., Czosnek H., Gazit S., Levy D. and Lapidot M. 2008 Pyramiding of genes conferring resistance to tomato yellow leaf curl virus from different wild tomato species. Plant Breed. 127, 625–631.
Acknowledgements
We thank ICAR-IIVR, Varanasi for facilities provided during the experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: Umesh C. Lavania
Rights and permissions
About this article
Cite this article
Singh, R.K., Rai, N., Singh, A.K. et al. Elucidation of diversity among \(\hbox {F}_{1}\) hybrids to examine heterosis and genetic inheritance for horticultural traits and ToLCV resistance in tomato. J Genet 97, 67–78 (2018). https://doi.org/10.1007/s12041-018-0904-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12041-018-0904-1