Abstract
Connexin 46 (Cx46) is important for gap junction channels formation which plays crucial role in the preservation of lens homeostasis and transparency. Previously, we have identified a missense mutation (p.V44M) of Cx46 in a congenital cataract family. This study aims at dissecting the potential pathogenesis of the causative mutant of cataract. Plasmids carrying wild-type (wt) and mutant (V44M) of Cx46 were constructed and expressed in Hela cells respectively. Western blotting and fluorescence microscopy were applied to analyse the expression and subcellular localization of recombinant proteins, respectively. Scrape loading dye transfer experiment was performed to detect the transfer capability of gap junction channels among cells expressed V44M mutant. The results demonstrated that in transfected Hela cells, both wt-Cx46 and Cx46 V44M were localized abundantly in the plasma membrane. No significant difference was found between the protein expressions of the two types of Cx46. The fluorescent localization assay revealed the plaque formation, significantly reduced in the cells expressing Cx46 V44M. Immunoblotting analysis demonstrated that formation of Triton X-100 insoluble complex decreased obviously in mutant Cx46. Additionally, the scrape-loading dye-transfer experiment showed a lower dye diffusion distance of Cx46 V44M cells, which indicates that the gap junction intercellular communication activity was aberrant. Human Cx46 V44M mutant causing cataracts result in abnormally decreased formation of gap junction plaques and impaired gap junction channel function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gap-junctions are order-packing cell-to-cell channels that facilitate the exchange of small metabolites, such as water, ions and secondary messengers (Goodenough 1992; Kumar and Gilula 1996; Mathias et al. 1997). In the lens, gap-junctions are comprised of at least three gap junction proteins a1, a3 and a8 (Beyer et al. 1987; Kistler and Bullivant 1988; Valdimarsson et al. 1991; White et al. 1992). Each gap-junction channel consists of two hemichannels (also named connexons), and each connexon is composed of six transmembrane protein subunits called connexins (Berthoud and Beyer 2009). All connexins have well conserved transmembrane domains and extracellular loops. In contrast, the intracellular loops and C termini of connexins are different.
Studies of connexins show that channel gating and permeability could be affected by alterations of charged residues of connexins (Verselis et al. 1994; Purnick et al. 2000; Tong and Ebihara 2006). Connexin mutations have been associated with various pathologies including cataracts, deafness, skin diseases, X-linked Charcot—Marie—tooth disease and oculodentodigital dysplasia.
Distinct gap junctions developed between fibre cells are different from that between epithelial cells (Rae and Kuszak 1983; Miller 1986). The primary lens fibres consist of a3 and a8 connexins, which are coexpressed in the same gap junction plaque in the cytomembrane (Gong et al. 1997; Benedetti et al. 2000). Cx43 is mainly identified in the anterior epithelial cells. The expression of Cx43 is downregulated and replaced by Cx50 and Cx46 as epithelial cells gradually differentiate into lens fibres (Gong et al. 2007). Cx46 plays an important role in coupling of fibre cells, particularly in mature fibres. Mutations in the GJA3, encoding Cx46, showed a direct association with human congenital cataracts (Jiang 2010; Mathias et al. 2010; Liu et al. 2011). Moreover, deletion of GJA3 gene results in severe nuclear cataracts in mice (Gong et al. 1997). Studies revealed that 20 cataract-associated mutations of Cx46 are primarily located in the extracellular loop and transmembrane domains (Bennett and Shiels 2011).
Previously, we identified a heterozygous \(130\hbox {G}{>}\hbox {A}\) mutation in the Cx46 gene (GJA3) associated with congenital nuclear cataract in a Chinese family (Zhou et al. 2010). This mutation causes the replacement of highly conserved valine at position 44 with methionine (V44M), indicating the essentiality of amino acid valine for connexin function. However, no systematic studies have been reported on the disease-linked substitution. Hence, analysing the molecular consequence of the identified mutation may focus on the pathogenesis of inherited cataracts, as well as diseases relating with this substitution in any other connexins.
Materials and methods
Site-directed mutagenesis and plasmid construction
The human Cx46 expression vector was obtained from OriGene (Rockville, USA). The expression vector for Cx46 V44M mutant was constructed by using site-directed mutagenesis with the following oligonucleotides: sense primer, \(5^{\prime }\)-CGGAGGACATGTGGGGCGATGAG-\(3^{\prime }\); antisense primer, \(5^{\prime }\)-GCCCCACATGTCCTCCGCCGCG-\(3^{\prime }\). The mutation was confirmed with DNA sequencing.
To create Cx46-GFP fusion proteins, the vectors were amplified by PCR with a pair of primers (sense primer, \(5^{\prime }\)-CCGCTCGAGATGGGCGACTGGAGCTTTCTGG-\(3^{\prime }\); antisense primer, \(5^{\prime }\)-CCCAAGCTTGATGGCCAAGTCCTCCGGTCTGG-\(3^{\prime }\)). Then products were cloned into XhoI and HindIII digested GFP vector.
Cell culture and transfection
Hela cells were maintained in Iscove’s modified Dulbecco’s medium (DMEM, GIBCO) supplemented with 10% fetal bovine serum (FBS, GIBCO), 100 mg/mL penicillin and 100 mg/mL streptomycin in a humidified atmosphere containing 5% \(\hbox {CO}_{2 }\) at \(37{^{\circ }}\hbox {C}\). Transfection was carried out using lipofectamine 2000 (Invitrogen Corporation, Carlsbad, USA).
Western blotting
Total proteins were extracted from Hela cells containing wt-Cx46 (wild type), Cx46 V44M (mutant) and GFP vector plasmids, respectively. The protocol for western blotting was described previously (Ren et al. 2009). Equal amounts (20–30 \(\mu \hbox {g}\)) of protein was loaded into the wells along with molecular weight marker. After electrophoresis (1–2 h at 100 V), a polyvinylidene fluoride (PVDF) membrane was used to transfer the protein. After blocking the membrane for 1 h at room temperature using blocking buffer. The membranes were incubated with anti-\(\upbeta \)-actin antibody (Sigma) and anti-Cx46 antibody (Abcam), respectively. The membrane was washed thrice using TBST, 5 min each. Then the membrane was incubated with the recommended dilution of conjugated secondary antibody in blocking buffer at room temperature for 1 h. The signals were visualized by using the chemiluminescent substrate method with the SuperSignal West Pico kit provided by Pierce.
Subcellular localization and gap junction plaques observation
Hela cells were seeded into six-well issue culture plates 24 h before transfection at \(\sim \)70% confluency. The wild-type and mutant Cx46-GFP-fusion expression constructs and GFP vectors were transfected using lipofectamine 2000, according to the manufacturer’s instructions (Invitrogen, Carlsbad, USA). After 48 h of transfection, localization of wild-type and mutant Cx46-GFP-fusion protein in transfected cells was viewed by fluorescent microscopy (Nikon 80i). The empty vector pGFP-N1 was transfected as a control cells were also analysed by Nikon fluorescence microscopy. Fluorescence was observed with excitation filters of 460–490 nm and emission filters of 510 nm.
Triton X-100 fraction isolation assay
Transfected cells were cultured on 60-mm dishes at over 90% confluency. Triton X-100 fraction isolation assay was performed with modified method described previously (Devi et al. 2005). Briefly, cells were collected and lysed with cell lysis buffer for 30 min and then centrifuged at 140,000 g at \(4{^{\circ }}\hbox {C}\). The pellet was resuspended with 1% Triton X-100 in lysis buffer and incubated for 30 min. A quarter of the lysate was kept on ice as whole lysate samples, three quarters of lysate samples were centrifuged at 140,000 g at \(4{^{\circ }}\hbox {C}\) for another 30 min. The supernatant was collected and labelled as Triton X-100-soluble fractions. The pellet was resuspended in lysis buffer with 1% SDS as Triton X-100-insoluble fractions. Cell lysates were loaded on a 10% SDS acrylamide gel and analysed by western blotting. The intensities of bands were analysed using densitometry by NIH Image J software.
Scrape loading dye transfer assay
The scrape-loading procedure was a modification of that one described by Sato et al. (2002) and Yum et al. (2007). Hela cells transfected with wt-Cx46 and Cx46 V44M were plated on 35 mm dishes to reach over 95% confluency. The medium was changed to HBSS plus the fluorescent dye Alexa Fluor 350 (Thermo Fisher Scientific, Waltham, USA), and a scalpel blade was used to make many parallel lines on the dish. The cells were incubated for 15 min at room temperature in the dye solution and then rinsed thrice with HBSS. The cells were then fixed with 1 mL of 4% formalin and photographed using confocal microscope.
Statistical analysis
The student t-test was used to calculate the statistical significance of the experimental data. The significance level was set as *\(P < 0.05\); **\(P < 0.01\); error bars denote standard deviations.
Results
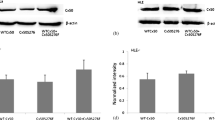
To determine if the p.V44M mutation affected the expression level of Cx46 protein in the plasma membrane, Western blot was performed using an anti-Cx46 antibody. The GFP expression plasmid was used as a negative control. The test results showed no obvious difference in the expression level of Cx46 V44M compared with the wt-Cx46 protein of the cell lysates (figure 1a). This means that the expression level and instability of the protein were not greatly altered by introducing the mutation.
To examine the subcellular locations of wt-Cx46 and Cx46 V44M, the GFP-tagged Cx46 constructs were transfected separately into Hela cells and analysed by fluorescence microscopy. As shown in figure 2, GFP as a control was located in the nucleus and cytoplasm, while both wt-Cx46 and Cx46 V44M were localized in the plasma membrane of culture cells. These images mainly illustrated that the mutation in the Cx46 did not alter their plasma localization.
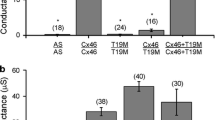
Although, both wt-Cx46 and Cx46 V44M were mainly localized in the plasma membrane of culture cells, the ability to form gap junction plaques of wt-Cx46 and Cx46 V44M was different. As shown in figure 3a, cells transfected with wt-Cx46 showed strong staining at gap junction plaques under fluorescence microscopy. In contrast, Cx46 V44M cells showed lower level of gap junction plaques between adjacent cells (figure 3a). To quantify the gap junction plaque content in the wt-Cx46 and Cx46 mutant cells, Triton X-100 fraction isolation assay was used in our study. Previous studies have found that connexin assembled into gap junction plaques is not soluble in 1% Triton X-100 (Du et al. 2013). Our results showed that cells transfected with wt-Cx46 and V44M mutant were divided into Triton X-100-soluble and Triton X-100-insoluble fractions, respectively. Western blots demonstrated that Cx46 V44M showed lower levels of protein in the Triton X-100-insoluble junctional plaque fractions than wt-Cx46. This was best illustrated by the ratio of Triton X-100-insoluble to total Cx46 protein (figure 3b). As shown in figure 3b, the insoluble/total Cx46 protein ratio reached a value of 21.55%, compared with 39.43% of the wt-Cx46 group (figure 3c). These declines indicate that Cx46 V44M reduces the formation of gap junction plaques. Further, our studies revealed that the mutant Cx46 failed to form Cx46 oligomers, which may result in functional Cx46 gap junction plaque disruption, and impaired intercellular communication.
Expression level of wt-Cx46 and Cx46 p.V44M mutant proteins by Western blot. (a) Hela cells were transfected with wt-Cx46 and Cx46 V44M expression plasmids, respectively and western blotting was performed with Cx46 antibody. (b) No notable difference was found in quantitative protein expression level between wt-Cx46 and V44M. The quantitative analysis of protein expression level between wt-Cx46 and V44M was done using Image-Pro Plus data analysis software. *\(P < 0.05\); **\(P < 0.01\) versus the wt-Cx46 group.
Localization of GFP, wt-Cx46 and Cx46 V44M in Hela cells. Cx46-GFP expression constructs containing wild-type or mutant were transfected into Hela cells using lipofectamine 2000. Localization of wild-type and mutant Cx46 GFP-fusion proteins in transfected Hela cells was viewed using a fluorescent microscope. The GFP empty vector was used as a control. (a) GFP empty vector, (b) wt-Cx46 and (c) p.V44M mutant. Original magnification was \(200\times \).
Scrape loading dye transfer assay is a well-known classical method for detection of gap junction channels between the cells. In this experiment, we used Alexa fluor 350 hydrazide as a tracer. As shown in figure 4a, intercellular gap junction communication between transfected Hela cells expressing Cx46 V44M had significantly lower dye diffusion distance than control groups. We quantified the extent of dye transfer by measuring the distance from the scrape line to the blue fluorescence point, the dye diffusion distance of Cx46 V44M cells was about 45% less than wt-Cx46 cells (figure 4b). This result indicated that the transfer capacity was significantly reduced in the Cx46 mutant cells.
Cx46 V44M is inefficient at gap junction plaques formation. (a) Fluorescent microscopy showed the gap junction plaques formation from Hela cells which were transfected with GFP, wild-type human Cx46 and V44M mutant plasmids, irrespectively. The left panel was GFP, the middle panel was wild-type human Cx46 and the right panel was V44M mutant. Original magnification was \(200\times \). (b) Solubility of wt-Cx46 and Cx46 V44M in Triton X-100 solution. Hela cells expressing wt-Cx46 or Cx46 V44M were treated by the Triton X-100 soluble or insoluble pellet was tested by Western blot with anti-Cx46 antibody. (c) The ratio of Triton X-100 insoluble fraction to total Cx46 protein was quantified using Image-Pro Plus data analysis software. *\(P < 0.05\); **\(P < 0.01\) versus the wt-Cx46 group, \(n = 3\).
Results of scrape-loading experiments in transfected cells expressed Cx46 V44M. (a) The fluorescence micrographs show the diffusion of Alexa fluor\(^\circledR \) 350 hydrazide (blue) in cells transfected with wt-Cx46 and Cx46 mutants. (b) Quantitative evaluation of the dye diffusion distance was performed to reveal the gap junction function. The columns represent the relative ratio of dye transfer distance between cells transfected with wt-Cx46 and Cx46 V44M. The dye diffusion distance was measured for the indicated dyes, by acquiring from five randomly selected fields from different plates of cells, and are shown as means ± SD. *\(P < 0.05\); **\(P < 0.01\) versus the wt-Cx46 group. Scale \(\hbox {bar} = 100\, \mu \hbox {m}\).
Discussion
The lens, being an avascular system, develops a complicated cell–cell communication network including gap junction channels to maintain lens homeostasis. Gap junctions in lens are mainly composed of three members of the connexins (Cx43, Cx46 and Cx50) as channel proteins. These channels facilitate intercellular communication between cells (Mathias et al. 2010). The intercellular communication is essential for numerous cellular processes, including proliferation, differentiation and development (Loewenstein 1979). Any mutations in connexins causing degradation or decline in their expression, improper assemblage, mis-trafficking or inability to form the functional gap junctions may impair communication between neighbouring cells. These disruptions of gap junction complexes can alter cell–cell communication and cause a range of various diseases, such as congenital cataract, tumour, cardiovascular disease and deafness (Loewenstein and Kanno 1966; Gros and Jongsma 1996; Gong et al. 1997; Rivedal and Opsahl 2001; Miura et al. 2004; Dalamon et al. 2016). Until now, about 20 human and mouse genes, respectively, encoding for connexin have been identified (Oyamada et al. 2005). Cx46 encoded by GJA3 is abundant in terminally differentiated lens fibres (Paul et al. 1991). Hitherto, studies have identified at least 19 different heterozygous coding mutations in GJA3 associated with autosomal dominant cataracts (Mackay et al. 1999; Rees et al. 2000; Jiang et al. 2003; Bennett et al. 2004; Burdon et al. 2004; Devi et al. 2005; Hansen et al. 2009). In mouse, the knockout of Gja3 developed nuclear cataract at about 12 days of age (Gong et al. 1997, 1998; Baruch et al. 2001). Previously, we have identified a novel missense mutation (c.130G>A) in the connexin 46 gene (GJA3) leading to congenital nuclear cataract in a three-generation Chinese family (Zhou et al. 2010).
This V44M mutation of Cx46 is located in the extracellular loop domain, a common site of human connexin mutations associated with disease. Connexins are synthesized in the endoplasmic reticulum (ER) and then transported to the Golgi complex (Musil and Goodenough 1991; Falk et al. 1994). Studies on Cx46 carrying the fs380 mutation (Cx46fs380) showed that mutant connexins were retained within the ER, ERGIC and/or Golgi apparatus possibly because of misfolding and/or incomplete/improper oligomerization of connexins, which contains an abnormal C-terminal sequence (Koval et al. 1997; Arora et al. 2008). The normal C-terminal region is essential for connexin trafficking to the membrane and the formation of gap junction plaques (Schlingmann et al. 2013). Our data showed that the V44M mutation located in extracellular loop did not alter their plasma localization and the expression level or instability of the Cx46 protein. Studies on Cx46 revealed that mutations in the NH2 terminus of Cx46 (e.g. Cx46D3Y, Cx46N63S and Cx46L11S) or N188T mutation in the intracellular loop of Cx46 were also able to oligomerize and traffic to the plasma membrane properly in transfected Hela cells of Cx46 (Beyer et al. 2013).
In this study, we have revealed that substitution of the conserved valine-44 causes a specific range of alterations of connexin behaviour. Studies showed that most of the mutations among the extracellular loop or the first/second transmembrane domains of Cx46 are associated with gap junction formation (Schadzek et al. 2016). Likewise, V44M, localized in the first extracellular loop of Cx46 (Zhou et al. 2010), displays impaired formation of gap junction plaque, gap junction assembly and aberrant gap junctional channel activities, indicating that this residue is critical for physiological and biological functions in lens fibre cell. Poor formation of gap junction plaques in Cx46 mutants is likely responsible for aberrant intercellular communication in V44M. Studies have reported that inability gap junction function attributes to ineffective formation of gap junction plaques, which is resulted from mis-trafficking of the connexin mutants that cause cataracts or other disease (Pal et al. 2000; VanSlyke et al. 2000; Forge et al. 2003; Arora et al. 2008; Du et al. 2013). Hence, the invalid assemblage of mutant protein into gap junction plaques appears to be the most likely explication. However, V44M showed decreased formation of gap junction plaques, but it can still traffic to the plasma membrane.
Cx46 is an indispensible fibre cell gap junctional component. The regulation of cell–cell communication depends essentially on the accumulation of Cx46 into gap junctional plaques under physiological conditions (Zhang et al. 2014). Cx46 gap junction channels can be isolated in detergent-resistant junctional plaque fraction (Musil and Goodenough 1991; Solan and Lampe 2007, 2009). We detect levels of Triton X-100 detergent resistant forms of Cx46 V44M and wt-Cx46, to test the possibility that the reduced gap junctional coupling in Cx46 V44M cells was resulted from reduced formation of junctional plaques. Consistent with the fluorescence microscopy results, the mutant demonstrated lower levels of Triton X-100-insoluble protein in the junctional plaque fraction than wild-type Cx46. These results indicated that Cx46 V44M will cripple the formation of gap junction plaques. Moreover, it implies that the minimal gap junctional coupling is possibly as a result of the failure to assemble into stable gap junction structures.
We showed that Cx46 V44M had lower dye coupling as compared to wild-type Cx46. In agreement with our result, Cx43 mutations G138R, G143S and I130T are reduced in transfer of injected dye (Dobrowolski et al. 2007). Likewise our result demonstrated that Cx46 V44M mutation reduces gap junctional dye transfer. The V44M mutant is able to assemble on cell–cell interfaces, which excludes the possibility that the impaired gap junctional coupling is due to abnormal delivery of Cx46 to junctional plaques. Since Cx46 V44M mutant forms smaller and less junctional plaques than wt-Cx46, this is possibly owing to the decreased formation of junctional plaques or because of the mutant somehow may have weaker interaction with those from neighbouring cells as well.
The gap junction plaques defects in Cx46 function caused by the V44M mutation may contribute to the development of cataracts in people expressing the lens characterized as a central nuclear opacity (Zhou et al. 2010). All the affected people in our family showed the same clinical presentation. It is believed that the functional absence of Cx46 alters the internal circulation system on which the avascular lens relies to maintain metabolic homeostasis, function and transparency. Owing to the dye transfer results showed that the transfer capacity of dye was significantly reduced in the Cx46 V44M mutant cells, expression of mutant in the lens nucleus is expected to cause a reduction in the gap junction-mediated flux of water, ions and other solutes to the surface of the lens. These alterations could lead to the loss of metabolic homeostasis, lower transparency of lens and nuclear cataract formation. Hereby, formation of fewer gap junction plaques was most likely the reason why nuclear cataract was developed in our family.
In conclusion, our data show that disease-associated Cx46 mutation, V44M, causes decreased formation of gap junction plaques, minimal gap junctional coupling and effects on transfected Hela cells, which may account for the molecular mechanisms underlying cataracts in this Cx46 V44M family.
References
Arora A., Minogue P. J., Liu X., Addison P. K., Russel-Eggitt I., Webster A. R. et al. 2008 A novel connexin50 mutation associated with congenital nuclear pulverulent cataracts. J. Med. Genet. 45, 155–160.
Baruch A., Greenbaum D., Levy E. T., Nielsen P. A., Gilula N. B., Kumar N. M. et al. 2001 Defining a link between gap junction communication, proteolysis, and cataract formation. J. Biol. Chem. 276, 28999–29006.
Benedetti E. L., Dunia I., Recouvreur M., Nicolas P., Kumar N. M. and Bloemendal H. 2000 Structural organization of gap junctions as revealed by freeze-fracture and SDS fracture-labeling. Eur. J. Cell Biol. 79, 575–582.
Bennett T. M. and Shiels A. 2011 A recurrent missense mutation in GJA3 associated with autosomal dominant cataract linked to chromosome 13q. Mol. Vis. 17, 2255–2262.
Bennett T. M., Mackay D. S., Knopf H. L. and Shiels A. 2004 A novel missense mutation in the gene for gap-junction protein alpha3 (GJA3) associated with autosomal dominant “nuclear punctate” cataracts linked to chromosome 13q. Mol. Vis. 10, 376–382.
Berthoud V. M. and Beyer E. C. 2009 Oxidative stress, lens gap junctions, and cataracts. Antioxid. Redox Signal. 11, 339–353.
Beyer E. C., Paul D. L. and Goodenough D. A. 1987 Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J. Cell Biol. 105, 2621–2629.
Beyer E. C., Ebihara L. and Berthoud V. M. 2013 Connexin mutants and cataracts. Front. Pharmacol. 4, 43.
Burdon K. P., Wirth M. G., Mackey D. A., Russell-Eggitt I. M., Craig J. E., Elder J. E. et al. 2004 A novel mutation in the Connexin 46 gene causes autosomal dominant congenital cataract with incomplete penetrance. J. Med. Genet. 41, e106.
Dalamon V. K., Buonfiglio P., Larralde M., Craig P., Lotersztein V., Choate K. et al. 2016 Connexin 26 (GJB2) mutation in an Argentinean patient with keratitis-ichthyosis-deafness (KID) syndrome: a case report. BMC Med. Genet. 17, 37.
Devi R. R., Reena C. and Vijayalakshmi P. 2005 Novel mutations in GJA3 associated with autosomal dominant congenital cataract in the Indian population. Mol. Vis. 11, 846–852.
Dobrowolski R., Sommershof A. and Willecke K. 2007 Some oculodentodigital dysplasia-associated Cx43 mutations cause increased hemichannel activity in addition to deficient gap junction channels. J. Membr. Biol. 219, 9–17.
Du G., Yang Y., Zhang Y., Sun T., Liu W., Wang Y. et al. 2013 Thrombocytosis and immunohistochemical expression of connexin 43 at diagnosis predict survival in advanced non-small-cell lung cancer treated with cisplatin-based chemotherapy. Cancer Chemother. Pharmacol. 71, 893–904.
Falk M. M., Kumar N. M. and Gilula N. B. 1994 Membrane insertion of gap junction connexins: polytopic channel forming membrane proteins. J. Cell Biol. 127, 343–355.
Forge A., Marziano N. K., Casalotti S. O., Becker D. L. and Jagger D. 2003 The inner ear contains heteromeric channels composed of cx26 and cx30 and deafness-related mutations in cx26 have a dominant negative effect on cx30. Cell Commun. Adhes. 10, 341–346.
Gong X., Li E., Klier G., Huang Q., Wu Y., Lei H. et al. 1997 Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell 91, 833–843.
Gong X., Baldo G. J., Kumar N. M., Gilula N. B. and Mathias R. T. 1998 Gap junctional coupling in lenses lacking alpha3 connexin. Proc. Natl. Acad. Sci. USA 95, 15303–15308.
Gong X., Cheng C. and Xia C. H. 2007 Connexins in lens development and cataractogenesis. J. Membr. Biol. 218, 9–12.
Goodenough D. A. 1992 The crystalline lens. A system networked by gap junctional intercellular communication. Semin. Cell Biol. 3, 49–58.
Gros D. B. and Jongsma H. J. 1996 Connexins in mammalian heart function. BioEssays 18, 719–730.
Hansen L., Mikkelsen A., Nurnberg P., Nurnberg G., Anjum I., Eiberg H. et al. 2009 Comprehensive mutational screening in a cohort of Danish families with hereditary congenital cataract. Invest. Ophthalmol. Vis. Sci. 50, 3291–3303.
Jiang H., Jin Y., Bu L., Zhang W., Liu J., Cui B. et al. 2003 A novel mutation in GJA3 (connexin46) for autosomal dominant congenital nuclear pulverulent cataract. Mol. Vis. 9, 579–583.
Jiang J. X. 2010 Gap junctions or hemichannel-dependent and independent roles of connexins in cataractogenesis and lens development. Curr. Mol. Med. 10, 851–863.
Kistler J. and Bullivant S. 1988 The gap junction proteins: vive la difference! BioEssays 9, 167–168.
Koval M., Harley J. E., Hick E. and Steinberg T. H. 1997 Connexin46 is retained as monomers in a trans-golgi compartment of osteoblastic cells. J. Cell Biol. 137, 847–857.
Kumar N. M. and Gilula N. B. 1996 The gap junction communication channel. Cell 84, 381–388.
Liu J., Xu J., Gu S., Nicholson B. J. and Jiang J. X. 2011 Aquaporin 0 enhances gap junction coupling via its cell adhesion function and interaction with connexin 50. J. Cell Sci. 124, 198–206.
Loewenstein W. R. 1979 Junctional intercellular communication and the control of growth. Biochim. Biophys. Acta 560, 1–65.
Loewenstein W. R. and Kanno Y. 1966 Intercellular communication and the control of tissue growth: lack of communication between cancer cells. Nature 209, 1248–1249.
Mackay D., Ionides A., Kibar Z., Rouleau G., Berry V., Moore A. et al. 1999 Connexin46 mutations in autosomal dominant congenital cataract. Am. J. Hum. Genet. 64, 1357–1364.
Mathias R. T., Rae J. L. and Baldo G. J. 1997 Physiological properties of the normal lens. Physiol. Rev. 77, 21–50.
Mathias R. T., White T. W. and Gong X. 2010 Lens gap junctions in growth, differentiation, and homeostasis. Physiol. Rev. 90, 179–206.
Miller T. M. 1986 Goodenough DA. Evidence for two physiologically distinct gap junctions expressed by the chick lens epithelial cell. J. Cell Biol. 102, 194–199.
Miura T., Ohnuma Y., Kuno A., Tanno M., Ichikawa Y., Nakamura Y. et al. 2004 Protective role of gap junctions in preconditioning against myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 286, 214–221.
Musil L. S. and Goodenough D. A. 1991 Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J. Cell Biol. 115, 1357–1374.
Oyamada M., Oyamada Y. and Takamatsu T. 2005 Regulation of connexin expression. Biochim. Biophys. Acta 1719, 6–23.
Pal J. D., Liu X., Mackay D., Shiels A., Berthoud V. M., Beyer E. C. et al. 2000 Connexin46 mutations linked to congenital cataract show loss of gap junction channel function. Am. J. Physiol. Cell Physiol. 279, 596–602.
Paul D. L., Ebihara L., Takemoto L. J., Swenson K. I. and Goodenough D. A. 1991 Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J. Cell Biol. 115, 1077–1089.
Purnick P. E., Oh S., Abrams C. K., Verselis V. K. and Bargiello T. A. 2000 Reversal of the gating polarity of gap junctions by negative charge substitutions in the N-terminus of connexin 32. Biophys. J. 79, 2403–2415.
Rae J. L. and Kuszak J. R. 1983 The electrical coupling of epithelium and fibers in the frog lens. Exp. Eye Res. 36, 317–326.
Rees M. I., Watts P., Fenton I., Clarke A., Snell R. G., Owen M. J. et al. 2000 Further evidence of autosomal dominant congenital zonular pulverulent cataracts linked to 13q11 (CZP3) and a novel mutation in connexin 46 (GJA3). Hum. Genet. 106, 206–209.
Ren G., Zhang G., Dong Z., Liu Z., Li L., Feng Y. et al. 2009 Recruitment of HDAC4 by transcription factor YY1 represses HOXB13 to affect cell growth in AR-negative prostate cancers. Int. J. Biochem. Biol. 41, 1094–1101.
Rivedal E. and Opsahl H. 2001 Role of PKC and MAP kinase in EGF- and TPA-induced connexin43 phosphorylation and inhibition of gap junction intercellular communication in rat liver epithelial cells. Carcinogenesis 22, 1543–1550.
Sato T., Haimovici R., Kao R., Li A. F. and Roy S. 2002 Downregulation of connexin 43 expression by high glucose reduces gap junction activity in microvascular endothelial cells. Diabetes 51, 1565–1571.
Schadzek P., Schlingmann B., Schaarschmidt F., Lindner J., Koval M., Heisterkamp A. et al. 2016 The cataract related mutation N188T in human connexin46 (hCx46) revealed a critical role for residue N188 in the docking process of gap junction channels. Biochim. Biophys. Acta 1858, 57–66.
Schlingmann B., Schadzek P., Hemmerling F., Schaarschmidt F., Heisterkamp A. and Ngezahayo A. 2013 The role of the C-terminus in functional expression and internalization of rat connexin46 (rCx46). J. Bioenerg. Biomembr. 45, 59–70.
Solan J. L. and Lampe P. D. 2007 Key connexin 43 phosphorylation events regulate the gap junction life cycle. J. Membr. Biol. 217, 35–41.
Solan J. L. and Lampe P. D. 2009 Connexin43 phosphorylation: structural changes and biological effects. Biochem. J. 419, 261–272.
Tong J. J. and Ebihara L. 2006 Structural determinants for the differences in voltage gating of chicken Cx56 and Cx45 gap-junctional hemichannels. Biophys. J. 91, 2142–2154.
Valdimarsson G., De Sousa P. A., Beyer E. C., Paul D. L. and Kidder G. M. 1991 Zygotic expression of the connexin43 gene supplies subunits for gap junction assembly during mouse preimplantation development. Mol. Reprod. Dev. 30, 18–26.
VanSlyke J. K., Deschenes S. M. and Musil L. S. 2000 Intracellular transport, assembly, and degradation of wild-type and disease-linked mutant gap junction proteins. Mol. Biol. Cell 11, 1933–1946.
Verselis V. K., Ginter C. S. and Bargiello T. A. 1994 Opposite voltage gating polarities of two closely related connexins. Nature 368, 348–351.
White T. W., Bruzzone R., Goodenough D. A. and Paul D. L. 1992 Mouse Cx50, a functional member of the connexin family of gap junction proteins, is the lens fiber protein MP70. Mol. Biol. Cell. 3, 711–720.
Yum S. W., Zhang J., Valiunas V., Kanaporis G., Brink P. R., White T. W. et al. 2007 Human connexin26 and connexin30 form functional heteromeric and heterotypic channels. Am. J. Physiol. Cell Physiol. 293, 1032–1048.
Zhang Z., Huang Y., Xie H., Pan J., Liu F., Li X. et al. 2014 Benzalkonium chloride suppresses rabbit corneal endothelium intercellular gap junction communication. PLoS One 9, e109708.
Zhou Z., Hu S., Wang B., Zhou N., Zhou S., Ma X. et al. 2010 Mutation analysis of congenital cataract in a Chinese family identified a novel missense mutation in the connexin 46 gene (GJA3). Mol. Vis. 16, 713–719.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (grant no. 31601116); the China Postdoctoral Science Foundation (grant no. 2016M601447) and the Natural Science Foundation of Heilongjiang Province of China (grant no. QC2014C120).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Corresponding editor: Kunal Ray
Lijuan Chen, Dongmei Su, Shanshan Hu and Xu Ma contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, L., Su, D., Li, S. et al. The connexin 46 mutant (V44M) impairs gap junction function causing congenital cataract. J Genet 96, 969–976 (2017). https://doi.org/10.1007/s12041-017-0861-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12041-017-0861-0