Abstract
The lens is an avascular organ that transmits and focuses light images onto the retina. Intercellular gap junction channels, formed by at least three different connexin protein subunits, α1 (connexin43 or Gja1), α3 (connexin46 or Gja3) and α8 (connexin50 or Gja8), are utilized to transport metabolites, ions and water in the lens. In combination with physiological and biochemical analyses, recent genetic studies have significantly improved our understanding about the roles of diverse gap junction channels formed by α3 and α8 connexin subunits during lens development and cataract formation. These studies have demonstrated that α3 connexin is essential for lens transparency while α8 connexin is important for lens growth and transparency. Diverse gap junction channels formed by α3 and α8 subunits are important for the differentiation, elongation and maturation of lens fiber cells. Aberrant gap junction communication, caused by alterations of channel assembly, channel gating or channel conductance, can lead to different types of cataracts. These findings provide some molecular insights for essential roles of connexins and gap junctions in lens formation and the establishment and maintenance of lifelong lens transparency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intercellular gap junction channels provide pathways for metabolic and electrical coupling between cells in excitatory and nonexcitatory tissues. Gap junction channels consist of connexin protein subunits. Most of cells utilize more than one type of connexin subunits to form gap junction channels, and different cells also express various types of connexin subunits from a gene family that has more than 20 members. One of the main subjects in the gap junction research is to understand why and how different connexin isoforms are utilized in cells for the physiological needs of a given organ. The eye lens is one of the simplest developmental models to understand cell proliferation, differentiation and maturation in vivo. The transparent lens is also a unique aging model for investigating cell physiology and protein chemistry. Multiple isoforms of the connexin gene family are expressed in the lens. In addition, mouse lens and human lens share many similar features in anatomy, physiology, biochemistry, development and disease (such as cataract). Therefore, we select the mouse lens as our model system and investigate the function of gap junction channels by using a modern genetic approach in combination with techniques of molecular and cellular biology, electrophysiology and biochemistry.
Functional Differences between α3 and α8 Connexins in Lens Development
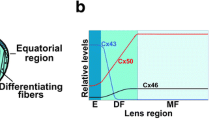
A mature lens is composed of elongated lens fiber cells covered with a monolayer of epithelial cells on the anterior hemisphere. The lens is formed through several sequential events: (1) the lens vesicle forms at an early embryonic stage, (2) posterior lens vesicle cells elongate to become lens primary fibers that fill in the lumen and (3) surface epithelial cells at the lens equator differentiate and elongate to become lens secondary fiber cells (Bassnett, 2004; Piatigorsky, 1981). Interior fiber cells, including both primary and secondary fiber cells, undergo a maturation process to eliminate all intracellular organelles, thereby minimizing light scattering and ensuring lens transparency. The lens continues to grow throughout life as new secondary fiber cells form from equatorial epithelial cells.
Lens cells communicate through intercellular gap junction channels formed by at least three different connexin proteins, α1 (Cx43), α3 (Cx46) and α8 (Cx50), encoded by the Gja1, Gja3 and Gja8 genes, respectively (Goodenough, 1992). Connexin α1 is mainly expressed in lens epithelial cells (Beyer et al., 1989), α3 is predominantly expressed in lens fiber cells and α8 is expressed in both lens epithelial and fiber cells (Rong et al., 2002). Connexin proteins have four transmembrane domains with three intracellular regions (the N terminus, a cytoplasmic loop and the C terminus) and two extracellular loops (E1 and E2) (Yeager & Nicholson, 2000). Six connexin protein subunits oligomerize to form one connexon. A gap junction channel is formed by the docking of extracellular loops of two opposing connexons (hemichannels) in the plasma membrane. Hundreds of gap junction channels come together to form gap junctions that are morphologically defined as specialized punctate “plaques” of cell-to-cell contacts or pentalamellar structures, detected by thin-section transmission electron microscopic examination. These channels, with pore diameters of about 10-15 angstroms, provide a pathway for the direct exchange of small molecules between adjacent cells (Fleishman et al., 2004; Unger et al., 1999).
It has been hypothesized that lens homeostasis is maintained by a circulation current propagated through gap junction channels in the lens (Mathias, Rae & Baldo, 1997). Genetic studies indicate that α3 connexin is essential for maintaining lens transparency. The lens of α3−/− knockout mice grows normally but displays dense opacity in the center, called a “nuclear cataract” (Gong et al., 1997). The nuclear cataract in α3−/− lenses is associated with an elevation of intracellular calcium concentration and the subsequent increase of calcium-dependent protein degradation in lens fiber cells (Baruch et al., 2001; Gao et al., 2004). Connexin α8 has been shown to be important for lens growth and transparency (Rong et al., 2002; Sellitto, Li & White, 2004; White, Goodenough & Paul, 1998). Lenses of α8−/− knockout mice are smaller (about 60% the wet lens weight of wild-type lenses) and have mild nuclear cataracts. The smaller lens in α8−/− knockouts is associated with delayed fiber cell maturation and reduced epithelial cell proliferation. The mechanism for how the loss of α8 connexin leads to small lenses remains unknown. Lenses of knockin α3 (50KI46/50KI46) mice, where α3 connexin is expressed under the endogenous α8 connexin locus, are transparent but smaller than wild-type lenses (White, 2002). These data suggest that α3 connexin cannot substitute for α8 connexin in lens growth but can prevent lens opacity caused by a lack of α8 connexin. Lens phenotypes of different knockout and knockin mice are summarized in Figure 1. Electrophysiological studies of intact lenses confirm that α3 connexin is essential for the coupling of interior fiber cells while α8 connexin is needed for the coupling of both peripheral and interior fiber cells (Baldo et al., 2001; Gong et al., 1998; Martinez-Wittinghan et al., 2004).
Lens phenotypes of different connexin knockout and knockin mice. The wild-type (α3+/+α8+/+) lens is a normal control. Connexin α3 (Cx46) knockout (α3−/−α8+/+) lenses develop nuclear cataracts, and connexin α8 (Cx50) knockout (α3+/+α8−/−) lenses are smaller with mild nuclear cataracts. Double knockout (α3−/−α8−/−) lenses have severe cataracts with degenerated inner fibers, and α3 knockin (ki/ki) with or without endogenous α3 (Cx46) (α3+/+α8 ki/ki or α3−/−α8ki/ki) lenses are clear but smaller than wild-type lenses
Connexin Point Mutations Affect Diverse Gap Junction Channels to Cause Different Types of Cataracts
Coexpression of α3 and α8 connexin subunits allows the formation of diverse gap junction channels, including homomeric channels (consisting of one type of subunit), heteromeric channels (consisting of two types of subunits), homotypic channels (docking of the same type of hemichannels) or heterotypic channels (docking of two different types of hemichannels) (Kumar & Gilula, 1996). Diverse gap junction channels display different electrophysiological properties in vitro (White et al., 1994). However, it has been difficult to investigate the distinct functions of diverse gap junction channels during lens development.
Mutations of α3 (Gja3) and α8 (Gja8) connexin genes are one of the common causes for different types of inherited cataracts in humans (Addison et al., 2006; Hansen et al., 2006; Vanita et al., 2006). Interestingly, we have found that mice with heterozygous and homozygous α8 connexin point mutations display different types of cataracts, such as nuclear cataracts, cortical cataracts or lens posterior rupture (Chang et al., 2002; Xia et al., 2006c). The presence or absence of α3 connexin alters lens phenotypes caused by α8 point mutations. Thus, we hypothesize that different types of cataracts are caused by altered intercellular communication mediated by diverse gap junction channels consisting of mutant and wild-type connexin subunits in the lens. Electrophysiological studies of the α8-G22R mutation at the N-terminal region have confirmed that mutant subunits alter the gating and conductance of gap junction channels in vitro (Xia et al., 2006b). In addition, an elevated level of wild-type α3 connexin can facilitate the stability or formation of gap junction channels that contain α8 mutant subunits in vivo (Xia et al., 2006b).
Moreover, our genetic studies of the α8-S50P point mutation, in extracellular loop 1, further support this hypothesis (Fig. 2) (Xia et al., 2006c). Connexin α8-S50P mutant subunits require the presence of wild-type α8 subunits to specifically inhibit the elongation of primary fiber cells in the embryonic lens, but this combination does not affect the elongation of secondary fiber cells. In contrast, the α8-S50P mutant subunits need wild-type α3 subunits to disrupt the differentiation and elongation of secondary lens fibers, but this combination does not affect the primary fiber cells. In addition, without wild-type α3 and α8 connexin subunits, the mutant α8-S50P subunit itself is a loss-of-function mutant. The α8-S50P mutant mice without α3 and α8 connexins have an identical lens phenotype to α3/α8 double homozygous knockout lenses (Xia et al., 2006a).
Different types of cataracts caused by mixing mutant and wild-type connexins. The α8-S50P mutation is located in extracellular loop 1. The drawing shows the development of a wild-type lens (α8-WT + α3-WT, black arrows), α8-S50P heterozygous mutant lens (α8-S50P + α8-WT + α3-WT, blue arrows), α8-S50P heterozygous mutant lens without wild-type α3 (α8-S50P + α8-WT - α3-WT, red arrows), α8-S50P heterozygous mutant lens without wild-type α8 (α8-S50P + α3-WT - α8-WT, green arrows) and α8-S50P heterozygous mutant lens without wild-type α3 and α8 (α8-S50P - α8-WT - α3-WT, pink arrows)
In summary, our work reveals that diverse gap junctions mediate distinct mechanisms to control the formation of lens primary and secondary fiber cells. This explains why and how various cataracts can result from altered diverse gap junctions during lens development. Ion concentration, synthesis of crystallin proteins and cell volume regulators are speculated to be involved in the regulation of fiber cell elongation. However, mechanisms that drive the elongation of lens fiber cells remain unknown. Future studies will be directed to elucidate the mechanism for how altered gap junction communication inhibits fiber cell elongation.
References
Addison PK, Berry V, Holden KR, Espinal D, Rivera B, Su H, Srivastava AK, Bhattacharya SS (2006) A novel mutation in the connexin 46 gene (GJA3) causes autosomal dominant zonular pulverulent cataract in a Hispanic family. Mol Vis 12:791–795
Baldo GJ, Gong X, Martinez-Wittinghan FJ, Kumar NM, Gilula NB, Mathias RT (2001) Gap junctional coupling in lenses from alpha(8) connexin knockout mice. J Gen Physiol 118:447–456
Baruch A, Greenbaum D, Levy ET, Nielsen PA, Gilula NB, Kumar NM, Bogyo M (2001) Defining a link between gap junction communication, proteolysis, and cataract formation. J Biol Chem 276:28999–29006
Bassnett S (2004) Lens fiber differentiation. In: Lovicu FJ, Robinson ML (eds), Development of the Ocular Lens. Cambridge: Cambridge University Press, pp 214–244
Beyer EC, Kistler J, Paul DL, Goodenough DA (1989) Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol 108:595–605
Chang B, Wang X, Hawes NL, Ojakian R, Davisson MT, Lo WK, Gong X (2002) A Gja8 (Cx50) point mutation causes an alteration of alpha 3 connexin (Cx46) in semi-dominant cataracts of Lop10 mice. Hum Mol Genet 11:507–513
Fleishman SJ, Unger VM, Yeager M, Ben-Tal N (2004) A Calpha model for the transmembrane alpha helices of gap junction intercellular channels. Mol Cell 15:879–888
Gao J, Sun X, Martinez-Wittinghan FJ, Gong X, White TW, Mathias RT (2004) Connections between connexins, calcium, and cataracts in the lens. J Gen Physiol 124:289–300
Gong X, Baldo GJ, Kumar NM, Gilula NB, Mathias RT (1998) Gap junctional coupling in lenses lacking alpha3 connexin. Proc Natl Acad Sci USA 95:15303–15308
Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, Kumar NM, Horwitz J, Gilula NB (1997) Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell 91:833–843
Goodenough DA (1992) The crystalline lens. A system networked by gap junctional intercellular communication. Semin Cell Biol 3:49–58
Hansen L, Yao W, Eiberg H, Funding M, Riise R, Kjaer KW, Hejtmancik JF, Rosenberg T (2006) The congenital “ant-egg” cataract phenotype is caused by a missense mutation in connexin46. Mol Vis 12:1033–1039
Kumar NM, Gilula NB (1996) The gap junction communication channel. Cell 84:381–388
Martinez-Wittinghan FJ, Sellitto C, White TW, Mathias RT, Paul D, Goodenough DA (2004) Lens gap junctional coupling is modulated by connexin identity and the locus of gene expression. Invest Ophthalmol Vis Sci 45:3629–3637
Mathias RT, Rae JL, Baldo GJ (1997) Physiological properties of the normal lens. Physiol Rev 77:21–50
Piatigorsky J (1981) Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation 19:134–153
Rong P, Wang X, Niesman I, Wu Y, Benedetti LE, Dunia I, Levy E, Gong X (2002) Disruption of Gja8 (alpha8 connexin) in mice leads to microphthalmia associated with retardation of lens growth and lens fiber maturation. Development 129:167–174
Sellitto C, Li L, White TW (2004) Connexin50 is essential for normal postnatal lens cell proliferation. Invest Ophthalmol Vis Sci 45:3196–3202
Unger VM, Kumar NM, Gilula NB, Yeager M (1999) Three-dimensional structure of a recombinant gap junction membrane channel. Science 283:1176–1180
Vanita V, Hennies HC, Singh D, Nurnberg P, Sperling K, Singh JR (2006) A novel mutation in GJA8 associated with autosomal dominant congenital cataract in a family of Indian origin. Mol Vis 12:1217–1222
White TW (2002) Unique and redundant connexin contributions to lens development. Science 295:319–320
White TW, Bruzzone R, Wolfram S, Paul DL, Goodenough DA (1994) Selective interactions among the multiple connexin proteins expressed in the vertebrate lens: the second extracellular domain is a determinant of compatibility between connexins. J Cell Biol 125:879–892
White TW, Goodenough DA, Paul DL (1998) Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J Cell Biol 143:815–825
Xia CH, Cheng C, Huang Q, Cheung D, Li L, Dunia I, Benedetti LE, Horwitz J, Gong X (2006a) Absence of alpha3 (Cx46) and alpha8 (Cx50) connexins leads to cataracts by affecting lens inner fiber cells. Exp Eye Res 83:688–696
Xia CH, Cheung D, DeRosa AM, Chang B, Lo WK, White TW, Gong X (2006b) Knockin a3 (Cx46) connexin prevents severe cataracts caused by an a8 (Cx50)–G22R mutation. J Cell Sci (in press)
Xia CH, Liu H, Cheung D, Cheng C, Wang E, Du X, Beutler B, Lo WK, Gong X (2006c) Diverse gap junctions modulate distinct mechanisms for fiber cell formation during lens development and cataractogenesis. Development 133:2033–2040
Yeager M, Nicholson BJ (2000) Structure and biochemistry of gap junction. Adv Mol Cell Biol 30:31–98
Acknowledgements
This work was funded by research grant EY013849 (to X. G.) from the National Eye Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gong, X., Cheng, C. & Xia, Ch. Connexins in Lens Development and Cataractogenesis. J Membrane Biol 218, 9–12 (2007). https://doi.org/10.1007/s00232-007-9033-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-007-9033-0