Abstract
Assembly of diphenylphosphate (dpp) with Cu(II) salts in combination with the different N-donor linkers, e.g., pentamethyldiethylenetriamine (pmdeta), bis-(3-aminopropyl)amine (bapa) and 4-Picolene (4-pic), yielded three new metal-organic coordination complexes, namely {[Cu(dpp)(pmdeta)] ⋅ClO4.H2O}2 (1), {[{[Cu(dpp)(bapa)H2O] ⋅ClO4} (2) and [Cu(dpp)2(4-pic)2]2 (3) by stirring the constituent reactants at room temperature. Complexes 1–3 were characterized by single crystal X-ray diffraction analysis and were further characterized by elemental analysis, infrared spectroscopy (IR) and powder X-ray diffraction (PXRD) studies. Compound 1 exhibits a dimeric Cu(II) complex which forms a 1D supramolecular chain along the crystallographic c-axis by means of intermolecular π…π interactions. Compounds 2 and 3 form a monomeric and dimeric complex of Cu(II) respectively, which are further extended into a supramolecular 2D structure via C-H.. π interactions for 2 and a 3D structure for 3 with the help of both intermolecular C-H.. π and π…π interactions for 3. In addition, the solid state UV-Vis spectra of compounds 1-3 and free dpp ligand have been investigated at room temperature.

Three Cu(II) complexes based on diphenylphosphate (dpp) and different N-donor linkers, e.g., pentamethyldiethylenetriamine (pmdeta), bis-(3-aminopropyl)amine (bapa) and 4-Picolene (4-pic) were successfully synthesized and characterized. These complexes show fascinating supramolecular 1D, 2D and 3D structures by means of intermolecular π…π and C-H…π interactions additively or individually.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metal-organic coordination compounds, in which the metal centres are interconnected by organic ligands exhibiting a variety of infinite supramolecular networks have become a fascinating area of chemical research.[1,2]The chemistry of such compounds are of interest to the synthetic chemist because of their intriguing supramolecular architecture and also due to their potential use in catalysis,[3,4] gas adsorption,[5‐8]magnetism,[9,10]ion exchange,[11,12]conductance,[13,14] and photo luminescence.[15‐17]The metal nodes and the N-donor ligands are combined to such complexes, due to their inherent capability to create self-assembled structures.[18,19] The desired structural motifs of the complexes have been achieved mostly by the judicious choice of the organic linkers.[20‐23]Besides the active role of organic linkers, there are many other essential factors such as the metal ions,[24] counter anions,[25] reaction temperature,[26,27] pH of the medium,[28,29]and solvents[30,31]that also play a vital role to create the structural diversities observed in such complexes. Recently, the charged linkers have also been extensively used as co-ligands along with the N,N′-donor ligands for the extension of the polymeric structure of metal-organic coordination complexes with fascinating topology. In this context, O-donors or mixed N/O donors ligands such as succinate, gluterate, pydinedicarboxylate, nicotinate, isonicotinate, etc.,[32‐34]have been widely used. The use of these different carboxylates with different sizes and shapes and its diverse coordination modes have been well-established to create the diversity in the structures of the complexes as well as in their supramolecular structures.[1,2]On the contrary, the use of phosphate in the fabrication of such supramolecular architecture are not so common but it has the similar prospect when it can be utilized in combination with suitable organic groups. In this context, we have used diphenylphosphate (dpp) with different N-donor ligands to propagate the acquired knowledge from carboxylate to phosphate. Moreover, the two phenyl groups can be utilized for creating π…π interactions in the solid state structure for the stabilization of the extended structure of the complexes. Here, we have obtained three new Cu(II) complexes namely {[Cu(dpp)(pmdeta)].ClO4.H2O}2 (1), {[Cu(dpp)(bapa) H2O] ⋅ClO4} (2) and [Cu(dpp)2(4-pic)2]2 (3) [where pmdeta = pentamethyldiethylenetriamine, bapa = bis-(3-aminopropyl)amine and 4-pic = 4-Picolene]. All the complexes exhibit supuramolecular[1,2]self-assembly through π…π and C-H …π interactions with the phenyl ring of dpp ligand which acts as a bridging as well as a monodentate ligand in the resulting architecture. It is worth to mention that the preparation of phosphate based materials are very important to the synthetic chemist because of their potential use in proton conduction,[35,36]ion exchange,[11,12]and catalysis.[3,4]Moreover, the metal phosphates are also essential in designing optical materials such as phosphors,[37] nonlinear optical materials[38,39]and laser optics materials[40] because the phosphate anions do not absorb in the UV-visible light. On the other hand, the phosphodiester has been also used to make several important materials e.g., the preparation of organo-phosphorus polymers,[41,42]insecticides,[43] reagents in solvent extraction of heavy metal ions,[44] etc., but their use in the design of metal-organic coordination compounds was very limited. Though there are several reports on substituted metal-phosphate[45‐47]complexes in literature, the effect of substituents on phosphate in the resulting solid state structure of the complexes have not been explored scrupulously. Hence, with the aim of exploring the role of dpp in the design of framework, here we report the syntheses, characterization and single crystal X-ray structures of three diphenyl phosphate based Cu(II) complexes with the variation of N-donor ligands (Scheme 1).
2 Experimental
2.1 Materials
High purity copper(II) perchlorate, copper(II) nitrate trihydrate, diphenyl phosphate (dpp), pentamethyldiethylenetriamine (pmdeta), bis-(3-aminopropyl)amine (bapa) and 4-Picolene (4-pic), were also obtained from Sigma-Aldrich Chemical Co. Inc. All other chemicals, including solvents were of AR grade and used as received.
2.2 Physical measurements
The elemental analyses (carbon, hydrogen, and nitrogen) of compounds 1–3 were carried out on a Perkin–Elmer 240C elemental analyzer. The Infrared spectra were obtained using KBr pellets on a Perkin–Elmer Spectrum BX-II IR spectrometer in the 4400–400 cm−1 region. The X-ray powder diffraction (PXRD) patterns of the bulk samples were recorded in a Bruker D8 Discover instrument using Cu-K α radiation (λ = 1.5418 Å) at room temperature; whereas the single crystal X-ray diffraction analysis was performed at room temperature using Bruker APEX II diffractometer, equipped with a normal focus, sealed tube X-ray source with graphite monochromated Mo-K α radiation (λ = 0.71073 Å). UV-Vis spectra were recorded on a Perkin–Elmer Lambda 35 UV-vis spectrophotometer.
2.3 Syntheses
2.3.1 Synthesis of {[Cu(dpp)(pmdeta)].ClO4.H 2O}2 (1)
1 mmol of Cu(ClO4)2 .6H2O (i.e., 0.3704 gm) was dissolved in 10 mL water in a 50 mL flat bottom flask. Then 1 mmol (i.e., 0.21 mL) of pentamethyldiethylenetriamine (pmdeta) was dissolved in 10 mL MeOH in a beaker. This solution was added to the flat-bottom flask. After that the above mixture was stirred for 30 min when a deep blue color appeared. Then 1 mmol (i.e., 0.250 gm) aqueous solution of diphenyl phosphate (dpp) was added to the deep blue solution and the resultant mixture was stirred for 4 h. The resulting blue solution was filtered and filtrate was kept in desk. After seven days block shaped blue crystals suitable for X-ray diffraction analysis were obtained in the reaction beaker. The crystals were separated and washed with methanol–water (1:1) mixture and dried under air (Yield: 60%). Anal. Calc.(%) for C21 H 35 N 3 O 9PClCu: C, 41.80; H, 5.85; N, 6.96. Found(%): C, 41.84; H, 5.81; N, 6.92. IR spectra (cm−1): ν(CH-Ar), 3061–2940; ν (C =C), 1608–1457; ν(P =O), 1320–1140; ν(P-O), 996–905 and ν(Ar-O), 1242–1110.
2.3.2 Synthesis of {[Cu(dpp)(bapa)H2O].ClO4} (2)
This compound was synthesized by following the same procedure as that of 1, but using bis-(3-aminopropyl) amine (bapa) (1 mmol, 0.14 mL) instead of pentamethyldiethylenetriamine (pmdeta) (1 mmol, 0.21 mL). After ten days, block shaped blue crystals suitable for X-ray diffraction analysis were obtained in the reaction beaker. The crystals were separated and washed with methanol–water (1:1) mixture and dried under air (Yield: 72%). Anal. Calc.(%) for C18 H 24 N 3 O 9PClCu: C, 38.86; H, 4.35; N, 7.55. Found(%): C, 38.83; H, 4.39; N, 7.51. IR spectra (cm−1): ν(CH-Ar), 3420–2945; ν(N-H, amine), 3331; ν(C =C), 1599–1419; ν(C-H), 2895; ν(P =O), 1320-1140; ν(P-O), 996–905 and ν(Ar-O), 1242–1110.
2.3.3 Synthesis of [Cu(dpp)2(4-pic)2]2 (3)
This compound was synthesized by following the same procedure as that of 1, but using 4-Picolene (4-pic) (2 mmol, 0.2 mL) instead of pentamethyldiethylenetriamine (pmdeta) (1 mmol, 0.21 mL). After two weeks block shaped blue crystals suitable for X-ray diffraction analysis were obtained in the reaction beaker. The crystals were separated and washed with methanol-water (1:1) mixture and dried under air (Yield: 72%). Anal. Calc.(%) for C36 H 34 N 2 O 8 P 2Cu: C, 57.80; H, 4.58; N, 3.74. Found(%): C, 57.83; H, 4.55; N, 3.71. IR spectra (cm−1): ν(CH-Ar), 3410–2930; ν(C =N), 1599; ν(C =C), 1599–1419; ν(C-H), 2945, ν(P =O), 1320–1140; ν(P-O), 996–905 and ν(Ar-O), 1242–1110.
The bulk products of complexes 1–3 have been synthesized by direct mixing of the corresponding aqueous-methanolic ligands mixture with the aqueous solution of Cu(II) salt in equal-molar ratio. After 12 hours, the bulk products are separated from the solution and washed with H2O-MeOH solution repeatedly. Then the purity of the bulk products was verified by PXRD, which gave identical peak positions with their simulated PXRD patterns. The purity of the bulk compounds were also confirmed by the results of elemental analysis and IR spectra as well, which were found to be in accordance with the data obtained for the single crystals.
2.4 Crystallographic data collection and refinement
The single crystals of compounds 1, 2 and 3 were mounted on the tips of glass fibers with commercially available glue. X-ray data collection of all three single crystals were performed at room temperature using Bruker APEX II diffractometer, equipped with a normal focus, sealed tube X-ray source with graphite monochromated Mo-K α radiation (λ = 0.71073 Å). The data were integrated using SAINT[48] program and the absorption corrections were made with SADABS.[49] All the structures were solved by SHELXS-97[50] using Patterson method and followed by successive Fourier and difference Fourier synthesis. Full matrix least-squares refinements were performed on F2 using SHELXL-97[51] with anisotropic displacement parameters for all non-hydrogen atoms. All the hydrogen atoms were fixed geometrically by HFIX command and placed in ideal positions in case of both structures. Calculations were carried out using SHELXS-97,[50] SHELXL-97[51] PLATON v1.15,[52] ORTEP-3v2,[53] WinGX system Ver-1.80.[54] Data collection and structure refinement parameters and crystallographic data for all the complexes are given in Table 1.
3 Results and discussion
3.1 Synthesis
Complexes 1–3 have been synthesized using the aqueous solutions of Cu(ClO4)2⋅6H2O and diphenyl phosphate (dpp) along with the methanolic solutions of different N-donor neutral ligands (i.e., pmdeta, bapa and 4-pic) with stirring the reaction mixture at room temperature (Scheme 1), wherein dpp acts as a mono anionic co-ligand for the formation of neutral complexes.
3.2 Infrared spectra
The IR spectra of complexes 1–3 discussed in the synthesis part of experimental Section exhibit sharp and strong peaks ranging from 1320–1140 cm−1 due to the stretching of the P =O moiety of diphenyl phosphate, which is strong in IR but medium in Raman. The P-O-Ar exhibits two different characteristic bands, O-Ar and P-O which shows the absorption bands at 1242–1110 cm−1 and 996–905 cm−1 respectively. The absence of any absorption bands at around 2725 cm−1 for O-H bond of diphenyl phosphate confirms that it adopts a completely deprotonated monoanionic form which coordinates with the metal centres.
3.3 Crystal structure description of the complexes
3.3.1 Structural description of {[Cu(dpp)(pmdeta)] ⋅ClO\(_{4}^{\mathrm {.}}\) H2O}2 (1)
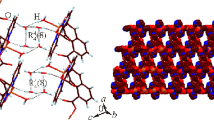
Compound 1 crystallizes in the triclinic P-1 space group with Z = 1. The structure analysis reveals the formation of a dimeric complex of Cu(II) with the combination of diphenyl phosphate and pmdeta. The asymmetric unit of 1 contains one crystallographically independent Cu(II) atom, one chelating pmdeta ligand, one diphenyl phosphate (dpp), one perchlorate counter ion and one lattice water forming a distorted trigonal bipyramid geometry with CuN3 O 2 coordination environment (Figure 1). The equatorial positions around the Cu(II) atoms are occupied by two oxygen atoms (O1 and O2a where: a = 1−x,2−y,1−z) of two diphenyl phosphate forming a bridge in between two Cu(II) centers and N3 atom of chelating N-donor ligand. The Cu-O bond distances range from 1.9714(17) to 2.1568(18) Å and Cu-N bond distance is 2.051(2) in basal plane (Table 2). Two nitrogen atoms (N1 and N2) of the chelating ligand are in the axial position with Cu-N distance varying from 2.052(2) to 2.052(2) Å (Table 2). In the solid state structure, each Cu(II) dimeric units are organized in such a way so that one of the phenyl rings of dpp ligands from each dimeric units are held together by intermolecular π…π interactions. This intermolecular π…π interactions are responsible for the formation of supramolecular 1D chain along crystallographic c-axis (Figure 2 and Table 3). Moreover, there are intramolecular C-H …π interactions (Figure 2 and Table 3) which is also responsible for the stability of the solid state structure.
3.3.2 Structural descriptions of {[Cu(dpp)(bapa)H2O]. ClO4} (2)
Compound 2 crystallizes in the Orthorhombic P212121 space group with Z = 4. The structure analysis reveals the formation of a monomeric complex of Cu(II) with the combination of diphenyl phosphate and chelating N-donor bis-(3-aminopropyl)amine (bapa) ligand. The asymmetric unit of 2 contains one crystallographically independent Cu(II) atom, one chelating bapa, one dpp, one perchlorate counter ion and one coordinated water forming a distorted octahedral geometry with CuN3 O 3 coordination environment (Figure 3). The equatorial positions around the Cu(II) atoms are occupied by three oxygen atoms (O1, O2a and O1w; where: a = −1/2+x, 1 /2 −y, 2 −z.) of two diphenyl phosphate and one coordinated water molecule respectively and N2 atom of chelating bis-(3-aminopropyl)-amine (bapa) ligand. The Cu-O bond distances range from 2.052(3) to 2.639(3) Å and Cu-N bond distance is 2.042(4) in basal plane (Table 4). Two nitrogen atoms (N1 and N3) of the chelating ligand are in the axial position with Cu-N distance values lying between 1.989(4) to 2.001(4) (Table 4). In the crystal packing, the monomeric Cu(II) units are organized in a manner such that the phenyl rings of dpp is held by the intermolecular C-H …π interaction. These non-covalent interactions link the monomeric fragments to give rise to a supramolecular 2D arrangement in solid state (Figure 4 and Table 5).
3.3.3 Structural descriptions of [Cu(4-pic)2(dpp)2] n (3)
Compound 3 crystallizes in the triclinic P-1 space group with Z = 1. The structure analysis reveals the formation of a dimeric complex of Cu(II) with the combination of diphenyl phosphate and 4-picoline. The asymmetric unit of 3 contains one crystallographically independent Cu(II) atom, two N donor picoline ligand (4-pic), two diphenyl phosphate (dpp) forming a distorted trigonal bipyramidal geometry with CuN2 O 3 coordination environment (Figure 5). The equatorial positions around the Cu(II) atoms are occupied by three oxygen atoms (O1, O4a and O5; where: a = −x, −y, 1 −z) of three diphenyl phosphate. The Cu-O bond distances range from 1.9986(13) to 2.1639(13) Å in basal plane (Table 6). Two nitrogen atoms (N1 and N2) of two coordinating 4-picoline are in the axial positions with Cu-N distance values lying between 1.9981(15) to 2.0018(16) (Table 6). Similar to 1 and 2 the crystal packing of 3 also has the dimeric fragments of Cu(II) organized in solid state in such a way so that non-covalent interactions can be useful to enhance the dimensionality of the compounds. Here both intermolecular π…π and C-H …π interactions involving phenyl rings of diphenyl phosphate creates the supramolecular 3D structure (Figures 6 and S4 and Table 7). In addition, there are also intramolecular π…π interactions (Figures 6 and S4) involved in this structure which gives extra stabilization energy towards the solid state packing.
3.4 Powder X-ray diffraction (PXRD)
To confirm the phase purity of the bulk materials with the simulated patterns powder X-ray diffraction (PXRD) analysis was carried out for 1–3 at room temperature. The experimental PXRD patterns of 1–3 are well-matched with the simulated ones obtained from their corresponding single crystal structures (Figures S5–S7), confirming the phase purity of the compounds 1–3.
3.5 Solid-state absorption spectra
The UV-Vis absorption spectra of complexes 1–3 and dpp co-ligand were measured in the solid state at room temperature. As shown in Figure 7, dpp shows absorption maximum at 256 nm in the UV range, which corresponds to the π– π ∗ or n– π ∗ transition of the aromatic ring.[55,56]However, complexes 1, 2 and 3 exhibit absorption maxima at 322 and 700 nm, 274 and 620 nm and 256 and 729 nm respectively which are different from that of the dpp ligand, suggesting that it might have originated from an intraligand transition (ILCT) or metal to ligand charge transfer (MLCT) in the UV range and the absorption in the range 430–800 nm may be due to the visible d-d transitions.
4 Conclusions
Three diphenylphosphate (dpp) based Cu(II) complexes varying with the different N-donor linkers, e.g., pentamethyldiethylenetriamine (pmdeta), bis-(3-aminopropyl)amine (bapa) and 4-Picolene (4-pic) have been successfully synthesized and characterized. Complexes 1–3 show fascinating supramolecular 1D, 2D and 3D structures respectively by means of intermolecular π…π and C-H …π interactions additively or individually. The variation of structures have been originated due to different coordination modes of dpp co-ligand used which exhibits the bis-bridging mode for complexes 1 and 3 and mono-bridging mode for 2 respectively. In addition, the active role of the N-donor ligands used also directs the structural changes and affords new network structures. In summary, this work may give a new light to the design of diphenyl phosphate based mixed ligand metal-organic coordination complexes in solid state containing lattice perchlorate ions. These complexes maybe useful in different potential applications like proton conduction, ion exchange, sensors, etc., in future.
References
Chen C F 2011 Chem. Commun. 47 1674
Li Y, Park T, Quansah J K and Zimmerman S C 2011 J. Am. Chem. Soc. 133 17118
Bhattacharya B, Maity D K, Pachfule P, Colacio E and Ghoshal D 2014 Inorg. Chem. Front. 1 414
Zou R Q, Sakurai H and Xu Q 2006 Angew. Chem. Int. Ed. 118 2604
Maity D K, Halder A, Bhattacharya B, Das A and Ghoshal D 2016 Cryst. Growth Des. 16 1162
Bhattacharya B, Halder A, Maity D K and Ghoshal D 2016 CrystEngComm 18 4074
Kanoo P, Ghosh A C, Cyriac S T and Maji T K 2012 Chem. Eur. J. 18 237
Panda T, Pachfule P, Chen Y, Jiang J and Banerjee R 2011 Chem. Commun. 47 2011
Dey R, Bhattacharya B, Colacio E and Ghoshal D 2013 Dalton Trans. 42 2094
Bhattacharya B, Maity D K, Mondal R, Colacio E and Ghoshal D 2015 Cryst. Growth Des. 15 4427
Maity D K, Bhattacharya B, Halder A and Ghoshal D 2015 Dalton Trans. 44 20999
Prasad T K, Hong D H and Suh M P 2010 Chem. -Eur. J. 16 14043
Yoon M, Suh K, Kim H, Kim Y, Selvapalam N and Kim K 2011 Angew. Chem. Int. Ed. 123 8016
Bhattacharya B, Layek A, Alam M M, Maity D K, Chakrabarti S, Ray P P and Ghoshal D 2014 Chem. Commun. 50 7858
Maity D K, Bhattacharya B, Mondal R and Ghoshal D 2014 CrystEngComm 16 8896
Bhattacharya B, Dey R and Ghoshal D 2013 J. Chem. Sci. 125 661
Mukherjee S, Samanta D and Mukherjee P S 2013 Cryst. Growth Des. 13 5335
Samanta D, Shanmugaraju S, Joshi S A, Patil Y P, Nethaji M and Mukherjee P S 2012 Chem. Commun. 48 2298
Zhou X P, Liu J, Zhan S Z, Yang J R, Li D, Ng K M, Sun R W Y and Che C M 2012 J. Am. Chem. Soc. 134 8042
Maity D K, Bhattacharya B, Halder A, Das A and Ghoshal D 2015 Polyhedron 102 634
Janiak C 2003 Dalton Trans. 2781
Ghosh S, Chakrabarty R and Mukherjee P S 2009 Inorg. Chem. 48 549
Xin L Y, Liu G Z and Wang L Y J 2011 Solid State Chem. 184 1387
Zhang X, Huang Y Y, Lin Q P, Zhang J and Yao Y G 2013 Dalton Trans. 42 2294
Li J R, Bu X H and Zhang R H 2004 Dalton Trans. 813
Zheng B, Dong H, Bai J F, Li Y Z, Li S H and Scheer M 2008 J. Am. Chem. Soc. 130 7778
Liu G X, Xu H, Zhou H, Nishihara S and Ren X M 2012 CrystEngComm 14 1856
Burrows N D, Hale C R H and Penn R L 2013 Cryst. Growth Des. 13 3396
Wu S T, Long L S, Huang R B and Zheng L S 2007 Cryst. Growth Des. 7 1746
Yang J, Li G D, Cao J J, Yue Q, Li G H and Chen J S 2007 Chem. Eur. J. 13 3248
Bu X H, Xie Y B, Li J R and Zhang R H 2003 Inorg. Chem. 42 7422
Prasad P A, Neeraj S, Natarajan S and Rao C N R 2000 Chem. Commun. 1251
Prior T J and Rosseinsky M J 2001 Chem. Commun. 495
Kepert C J and Rosseinsky M J 1998 Chem. Commun. 31
Sen S, Yamada T, Kitagawa H and Bharadwaj P K 2014 Cryst. Growth Des. 14 1240
Nagarkar S S, Unni S M, Sharma A, Kurungot S and Ghosh S K 2014 Angew. Chem. Int. Ed. 53 2638
D Bartolo (Ed.) 1978 In Luminescence of Inorganic Solids (New York: Plenum)
Zang S, Su Y, Li Y, Zhu H and Meng Q 2006 Inorg. Chem. 45 2972
Zang S, Su Y, Li Y, Ni Z and Meng Q 2006 Inorg. Chem. 45 174
Marion J E and Weber M J 1991 Eur. J. Solid State Inorg. Chem. 28 271
Murugavel R, Choudhury A, Walawalkar M G R P and Rao C N R 2008 Chem. Rev. 108 3549
Toy D F 1976 In Phosphorus Chemistry in Everyday Living (Washington DC: American Chemical Society)
Corbridge D E C 1980 In Phosphorus: An Outline of Its Chemistry, Biochemistry and Technology (Amsterdam, Oxford, New York: Elsevier)
Phosphorus in the Environment: Its Chemistry and Biochemistry 1978, Ciba Foundation Symposium No. 57 (Amsterdam: Elsevier)
Williams N H, Lebuis A M and Chin J 1999 J. Am. Chem. Soc. 121 3341
Seo J S, Sung N D, Hynes R C and Chin J 1996 Inorg. Chem. 35 7472
Sastry M S, Kesavadast T, Rao G S and Sastry M D 1984 Proc. Indian Acad. Sci. (J. Chem. Sci.) 93 843
SMART and SAINT 1998 Bruker AXS Inc, Madison, WI
Sheldrick G M 2002 In SADABS (Version 2.03) (Germany: University of Göttingen)
Sheldrick G M 1997 SHELXS-97 Program for solution of crystal structures, University of Gottingen, Germany
Sheldrick G M 1997 SHELXL-97 Program for refinement of crystal structures, University of Gottingen, Germany
Spek A L 2009 Acta Crystallogr. D65 148
Farrugia L J 1997 J. Appl. Crystallogr. 30 565
Farrugia L J 1999 WinGX J. Appl. Crystallogr. 32 837
Ohkoshi S, Tokoro H, Hozumi T, Zhang Y, Hashimoto K, Mathonière C, Bord I, Rombaut G, Verelst M, Moulin C C and Villain F 2006 J. Am. Chem. Soc. 128 270
Wang J H, Fang Y Q, Bourget-Merle L, Polson M I J, Hanan G S, Juris A, Loiseau F and Campagna S 2006 Chem. Eur. J. 12 8539
Acknowledgements
Authors gratefully acknowledge the financial assistance given by SERB, India (Grant No. SB/S1/IC-06/2014). D.K.M. and F.H. acknowledges UGC for their research fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information (SI)
IR and PXRD patterns of complexes 1–3 related to the crystal structures are presented in supplementary section (see www.ias.ac.in/chemsci). Crystallographic data for the structural analysis have been deposited with the Cambridge Crystallographic data Centre, CCDC 1501058-1501060. Copies of the data can be obtained free of charge at www.ccdc.cam.ac.uk/conts/retrieving.html [or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK, Fax: (internet) + 44-1223-336-033; E-mail: deposit@ccdc.cam.ac.uk].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
MAITY, D.K., HAQUE, F., DUTTA, B. et al. Syntheses and characterization of three diphenyl phosphate based Cu(II) complexes and the effect of non-covalent interactions on their supramolecular framework. J Chem Sci 128, 1861–1869 (2016). https://doi.org/10.1007/s12039-016-1200-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-016-1200-3