Abstract
Density functional theory (DFT)-based simulations have been performed to provide electronic structure property correlation based reasoning for conceptualizing the effect of encapsulated methane molecule on the formation of methane hydrate cages, the role of methanol and ethylene glycol as inhibitor and the role of tetra-hydro-furan (THF) and cyclopentane as promoter of methane hydrate. Geometry optimization of 512 cage, 51262 cage and 51264 cage with and without encapsulated methane and the cluster of 512 cage with ethylene glycol, methanol, cyclopentane have been performed by density functional theory using ωB97X-D/6-31+ +G(d,p) method. Methane hydrate formation inhibition by methanol and ethylene glycol as well as methane hydrate stabilization by cyclopentane and tetrahydrofuran are critically analysed based on the interaction energy, free energy change, dipole moment and infrared frequency calculation. Calculation of free energy change for formation of methane hydrate with/without reagents at various temperature and pressure using optimized structure is reported here. It is observed that hydrogen bond between water molecules of clathrate 512 cages become stronger in the presence of cyclopentane and tetrahydrofuran but weaker/broken in the presence of ethylene glycol and methanol. Simulated results correspond well with experimental findings and can be useful for designing new inhibitor and promoter molecules for gas hydrate formation.

DFT studies have been performed to show the effect of some inhibitor and promoter molecules on pentagonal dodecahedron methane hydrate cage (1CH4@512) structures and electronic properties. It illustrates scientifically the role of promoter (e.g., cyclopentane) and inhibitor (e.g., methanol) on stability and formation possibility of (1CH4@512).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Methane hydrates are non-stoichiometric clathrate made of hydrogen bonded network of water molecules encapsulating methane gas as guest. These complex crystalline structures are stable at high pressure and low temperature.[1–3] Prolific deposits of gas hydrates in marine sediments and below permafrost regions are considered as future energy source.[3–5] Methane hydrates are also identified as the source for atmospheric methane gas in Mars.[6] Methane hydrates are found to have three common hydrate unit structures (i.e., structure-I, structure-II and structure-H).[7, 8] Unit cell of structure-I consist of two pentagonal dodecahedron (512) cages and six hexagonal truncated trapezohedron (51262) cages. Structure-II unit cell has sixteen 512 cages and eight 51264 cages and structure-H unit cell has three 512 cages, two 435663 cages, and one 51268 cage. The gas hydrate has become an important research topic worldwide and lot of experimental and theoretical studies have been performed in last two decades. Laboratory scale gas hydrates preparation,[9–13] thermodynamic stability analysis and phase diagram study of clathrate hydrate,[14–18] structural and physical properties of gas hydrate[7, 8, 19] and study of cage occupancy by guest molecules in clathrate cages[20] have been performed by several researchers. Theoretical study of methane hydrate cage structure,[21] vibration of methane molecule in clathrate cage,[22] diffusion and absorption of different guest molecules in various clathrate cages[23] and molecular dynamics study of nucleation of methane hydrate[24–26] have been reported in the literature.
Promoter molecules increase the gas hydrate formation rate as well as stability, and consequently help in the storage and transportation of natural gas using hydrate technology. The experimental studies on evaluating the role of different gas hydrate promoters like, micellar surfactant solutions with cyclopentane,[27] sodium dodecyl sulphate,[28] various alkanes, alkenes, alkynes, cycloalkanes or cycloalkene,[29, 30] tetra-n-butylammonium bromide,[31] potato starch,[32] tetra-n-butyl ammonium chloride,[33] mixture of sodium dodecyl sulphate and tetrahydrofuran[34] have been carried out by various researchers. On the other hand, formation of clathrate hydrate in oil pipeline is of great concern for petroleum industry as it plugs the oil flow.[35] Elimination of hydrate plug formation problem can be achieved either by adjusting gas hydrate phase equilibrium boundary by thermodynamic inhibitors[36, 37] or by lingering of gas hydrate nucleation using kinetic inhibitors[36, 38] and anti-agglomerates.[36] The role of methanol, ethylene glycol and NaCl as a thermodynamic inhibitor for gas hydrate formation has been studied by Lee et al.[39] Quantum chemical calculation based investigation using density functional theory (DFT), has been applied effectively to analyse the role of gas hydrate inhibitors. A theoretical study of hydrogen bond formation in different polyethylene glycol + water complex, dipropylene glycol + water complex[40, 41] and trymethylene glycol + water complex[42] using Hatree–Fock (HF) method, second order Møller– Plesset perturbation theory (MP2) and DFT using 6- 31+ +G(d,p) basis set have been performed. Density functional theory-based studies of methane hydrate pentagonal dodecahedron (512) cage in the presence of methanol and chitosan have been performed to elucidate the role as methane hydrate inhibitors (methanol and chitosan).[43, 44] There it has been revealed that the presence of methanol distorts the 512 methane hydrate cage and the presence of chitosan reduce the strength of the hydrogen bonded water network of 512 cage nearest to them. Detail theoretical study of inhibitors + clathrate cage configuration and promoter + clathrate cage configuration considering calculated free energy change of cluster formation in various temperatures and pressures, using ab initio method has not been performed so far. The objective of this work is to study the effect of ethylene glycol (EG), methanol, cyclopentane and tetra-hydro furan (THF) on pentagonal dodeca hedron methane hydrate cage formation and stability in terms of free energy change, interaction energy, dipole moment, red shift and intensity of IR spectra values. Based on optimized structures, calculation of change in free energy for formation of methane hydrate with/without reagents at various temperature and pressure reported here is first of its kind. We propose selection criteria to identify methane hydrate cage inhibitor and promoters based on DFT calculation. This work would be helpful in understanding the electronic structure-property correlation based insight on inhibition effect by ethylene glycol and methanol, and stabilization performance by cyclopentane and tetra-hydro furan of methane hydrate.
2 Computational details
Geometry optimization and frequency calculation of 512, 51262 and 51264 hydrate cages with and without encapsulated methane and the cluster of 512 cage with ethylene glycol, methanol, cyclopentane and solvated in tetra-hydro furan have been performed by density functional theory[45, 46] using ωB97X-D/6-31+ +G(d,p) method. ωB97X-D[47] is long range corrected hybrid density functional having empirical atom–atom dispersion correction term. ωB97X-D functional has been chosen for simulating hydrate clathrate system here as it is better suited for describing non-bonded interaction. All the DFT calculations have been carried out by using Gaussian 09 software package.[48] Visualization of molecules have been carried out in Discovery Studio v3.1 of Accelrys Software Inc. Calculated vibrational frequency is scaled using scaling factor 0.975.[49]
Interaction energy (ΔE) for cluster formation has been determined using equation (1),
where, E Cluster and E Components are optimized energy of cluster and individual components, respectively. Basis set superposition error (BSSE) correction to the interaction energy has not been carried out as it is computationally prohibitive for the large system size studied here. Moreover, BSSE is expected to be very less for calculation using extended basis set including diffuse and polarization function like 6-31G+ +(d,p). With larger basis set, the BSSE corrected and uncorrected values of structural parameters are generally found to be almost same. A cluster is more stable if its interaction energy is more negative compared to other clusters. Free energy at various temperature and pressure is calculated using ‘freqcheck’ utility of Gaussian 09. Free energy changes for cluster formation with and without the presence of inhibitor and promoter molecule are calculated using equation (2),
where, G Cluster and G Components are free energy of cluster and individual components, respectively.
3 Result and discussion
Optimized structures and contours of calculated free energy change (ΔG, kcal/mol) of formation of 1CH4@512, 1CH4@51262 and 1CH4@51264 methane hydrate cage with respect to various temperature and pressure using ωB97X-D/6-31+ +G(d,p) have been shown in figure 1. It is observed that at 298 K, 1CH4@512 (figure 1a), 1CH4@51262 (figure 1b) and 1CH4@51264 (figure 1c) hydrate cage formation are favourable i.e., showing negative free energy change values at ≥ 8 atm., ≥ 9 atm. and ≥ 20 atm. pressure, respectively. It is also observed that 1CH4@512 cage and 1CH4@51262 cage formations are favourable process at 265 K or lower temperature for one atmospheric pressure but the formation of 1CH4@51264 cage is only possible at 265 K for 3 atm. or higher pressure. The formation of 1CH4@512 and 1CH4@51262 hydrate cages are more favourable in comparison with 1CH4@51262 cage formation, therefore higher pressure is required to form 1CH4@51264 cage compared to form 1CH4@512 cage and 1CH4@51262 cage at same temperature.
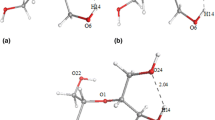
Deformed 1CH4@512 cage structure in the presence of methanol and corresponding contours of calculated free energy change (ΔG, kcal/mol) at various temperature and pressure using ωB97X-D/6-31+ +G (d,p) have been shown in figure 2a. At 298 K methanol found to hinder the formation of well built 1CH4@512 cage structure even at higher pressure (≥ 8 atm). It is also observed that 1CH4@512 cage structure is broken in the presence of methanol (figure 2a) even in the low temperature-high pressure region where well build 1CH4@512 cage formation is favourable without the presence of methanol as evident in figure 1a. Hydrogen bond formation between methanol and water molecules of 1CH4@512 cage and subsequent breaking of hydrogen bonded 1CH4@512 cage structure and negative free energy of formation of distorted 1CH4@512 cage structure at high pressure and low temperature strongly indicate methanol should be thermodynamic inhibitor. It has also been experimentally found that methanol is an effective gas hydrate inhibitor.[36, 40]
It is found that ethylene glycol form hydrogen bonded cluster with 1CH4@512 cage and thereby weaken the original hydrogen bonded 1CH4@512 cage structure (evident from IR red shift values in table 1). This weakened 1CH4@512 cage structure in the presence of EG is also found to be stable at high pressure and low temperature region from free energy change diagram (figure 2b). These suggest that EG should act as an effective inhibitor for 1CH4@512 hydrate formation and the same has been observed experimentally.[36, 37, 40] Optimized structures of 1CH4@512 cage solvated by THF taking polarizable continuum model using the integral equation formalism variant (IEFPCM) with ωB97X-D/6-31+ +G (d,p) and the contour of ΔG of formation at various temperature and pressure have been presented in figure 3a. THF solvation does not distort the 1CH4@512 cage structure and the formation of 1CH4@512 cage, solvated with THF, is more favourable than the formation of 1CH4@512 cage in vacuum as evident from ΔG values in figure 1a and figure 3a. ΔG values of the formation of 1CH4@512 cage in THF solution are found to be negative at all the pressure and temperature combinations studies (1–20 atm. and 263–298 K). These findings indicate the role of THF as methane hydrate promoter as supported by experimental and thermodynamic studies.[34]
Optimized structure and contour of free energy change (ΔG, kcal/mol) of formation of 1CH4@512 cage in the presence cyclopentane at various temperature and pressure using ωB97X-D/6-31+ +G(d,p) are shown in figure 3b. It is observed that cyclopentane, unlike methanol/EG, does not disturb 1CH4@512 cage structure and ΔG values of formation of unperturbed 1CH4@512 cage structure in the presence cyclopentane are found to be negative at high pressure and low temperature regions denoted by blue and sky blue colour in figure 3b. These outcome also suggest cyclopentane should be an effective promoter for gas hydrate formation and the same has been reported in literature.[27, 29]
Calculated interaction energies and dipole moments of 1CH4@512 cage, 1CH4@51262 cage, 1CH4@512 cage in THF solution, 1CH4@512 cage in water solution, 1CH4@512 cage + methanol, 1CH4@512 cage + cyclopentane and 1CH4@512 cage + EG using ωB97X-D/6-31G+ +(d,p) have been plotted in figure 4. The dipole moment value of 1CH4@51262 cage is found to be lesser than that of 1CH4@512 and 1CH4@51264 cages. This can be attributed to the more symmetrical structure of 1CH4@51262 cage compared to other two methane hydrate cages. It has been found that CH4 molecule remains closed to one side of the optimized 1CH4@51262 cage with minimum O–HM (HM being H of CH4) distance of 2.83 Å, where as 1CH4@512 and 1CH4@51264 cages are found to be optimized with CH4 molecule at the centre, with minimum O–HM distance of 3.63 Å and 3.76 Å, respectively. Consequently attractive van-der Walls interaction is more in 1CH4@51262 cage compared to 1CH4@512 and 1CH4@51264 cages. Thus, the interaction energy value of 1CH4@51262 cage is also found to be more negative in comparison with that of 1CH4@512 cage and 1CH4@51264 cage. It implies that 1CH4@51262 cage is more stable compared to 1CH4@512 and 1CH4@51264 cages. Presence of THF solution increases stability of 1CH4@512 cage as the interaction energy for 1CH4@512 cage formation in THF solution is found to be more negative compared to 1CH4@512 cage formation in vacuum. As the dipole moment value of 1CH4@512 cage is also found to be increased in THF solution, the directionality and consequently strength of hydrogen bonds of 1CH4@512 cage structure increase in THF solution. In the presence of cyclopentane, the 1CH4@512 cage structure remain intact and the interaction energy of 1CH4@512 cage formation (in the presence of cyclopentane) is found to be more negative compared 1CH4@512 cage formation in vacuum. Thus it can be further inferred from the interaction energy and dipole moment that THF and cyclopentane are effective promoters for methane hydrate. 1CH4@512 cage also become stable and have stronger hydrogen bonded network in water solution as evident in figure 4. The negative interaction energy value of the distorted 1CH4@512 cage and methanol cluster ensures the feasibility of distortion of 1CH4@512 cage in the presence of methanol. The presence of methanol also lowers the dipole moment of 1CH4@512 cage structure and consequently reduces the strength of hydrogen bonded network of 1CH4@512 cage. The interaction energy of hydrogen bonded cluster of 1CH4@512 cage and ethylene glycol is found to be negative but the presence of ethylene glycol does not alter the dipole moment value of 1CH4@512 cage in vacuum. These results suggest that methanol would be much more effective methane hydrate inhibitor compared to ethylene glycol.
Calculated vibrational frequencies (infra red) of O–H stretching in water, methanol, water dimer, 1CH4@512 cage, 1CH4@51262 cage, 1CH4@51264 cage, 1CH4@512 cage in THF solution, 1CH4@512 cage in water solution, 1CH4@512 + methanol and 1CH4@512cage + EG clusters are summarized along with some experimental values in table 1. The calculated scaled vibrational frequency values of O–H stretching of water molecule, water dimer and 1CH4@512 cage corresponds well with experimental values.[52, 50] The red shifts of O–H vibrational frequency are the result of hyper-conjugation interaction for conventional hydrogen bond formation and higher red shift values indicate stronger hydrogen bonding tendency. The calculated red shift of O–H stretching of water molecule for different methane hydrate cages are of following order: water dimer < 1CH4@512 cage < 1CH4@51262 cage < 1CH4@51264 cage, as the increasing number of water molecules in a cluster increases hydrogen bond cooperativity effect. It is observed that the red shifts of O–H stretching of water molecule in 1CH4@512 cage in THF solution, 1CH4@512 cage in water solution, 1CH4@512 cage + cyclopentane cluster are higher than that of 1CH4@512 cage. It is also found that the red shifts of O–H stretching of water molecule in 1CH4@512 cage + methanol and 1CH4@512cage + EG clusters are less than that of 1CH4@512 cage. Calculated IR intensities of O–H stretching of water molecule are in order of 1CH4@512 cage + EG (696.91 km-mol − 1) < 1CH4@512 cage + methanol cluster (807.5 km-mol\(^{-1})<\) 1CH4@512 cage (1286.6 km-mol − 1) < 1CH4@512 cage in THF sol (1725.69 km-mol − 1) < 1CH4@512 cage in water sol (3522.03 km-mol − 1) as evident in table 1. The rise in IR intensity of O–H stretching of water molecule in 1CH4@512 cage in THF and water solution can be for the increased ionic character of O–H normal mode according to the view point of the proposition of Barrow.[51] Calculated higher red shift and intensity of O–H stretching of water molecule of 1CH4@512 hydrate cage in the presence of THF and cyclopentane indicate higher tendency of hydrogen bonded 1CH4@512 hydrate cage formation. On the contrary, lower values of calculated red shift and intensity of O–H stretching of water molecule of 1CH4@512 hydrate cage in the presence of methanol and EG demonstrate lower tendency of hydrogen bonded 1CH4@512 hydrate cage formation. These findings also further illustrate the roles of methanol and ethylene glycol as inhibitor, THF and cyclopentane as promoter for methane hydrate formation.
4 Conclusions
Density functional theory-based calculation using ωB97X-D/6-31+ +G (d,p) have been performed to explain the effect of encapsulated methane molecule on the formation of methane hydrate cages, the role of methanol and ethylene glycol as inhibitor and the role of tetra-hydro-furan (THF) and cyclopentane as promoter for methane hydrate formation. Methane molecule provide better stability for 512 cage and 51262 cage in comparison with 51264 cage therefore higher pressure is required to form 1CH4@51264 cage compared to 1CH4@512 cage and 1CH4@51262 cage at same temperature. The distortion of 1CH4@512 cage structure in the presence of methanol and the weakening of 1CH4@512 cage structure in the presence of ethylene glycol have been favourable according to calculated ΔG contour, dipole moment and interaction energy values. The formation of 1CH4@512 cage is found to be more favourable in THF solution compared to the formation of 1CH4@512 cage in vacuum as per calculated ΔG values. IR red shift values and intensities have been identified as important parameters for understanding the reason behind the role methanol and ethylene glycol as inhibitor and the role of tetra-hydro-furan (THF) and cyclopentane as promoter of methane hydrate. This study clearly demonstrates the structure-property correlation for methane hydrate formation in the presence of promoter and inhibitor molecules and the same can be used for designing better promoter and inhibitor molecules.
References
Demirbas A 2010 Methane gas hydrate (London, UK: Springer)
Koh C A, Sum A K and Sloan E D 2009 J. Appl. Phys. 106 061101
Loveday J S, Nelmes R J, Guthrie M, Belmonte S A, Allan D R, Klug D D, Tse J S and Handa Y P 2001 Nature 410 661
Collett T S 2002 AAPG Bull. 86(11) 1971
Collett T S and Lee M W 2000 Annals N. Y. Acad. Sci. 912 51
Chastain B K and Chevrier V 2007 Planet. Space Sci. 55 1246
Sloan Jr. E D 2003 Nature 426 353
Sum A K, Koh C A and Sloan E D 2009 Ind. Eng. Chem. Res. 48 7457
Skovborg P, Rasmussen H J, NG P and Mohn U 1993 Chem. Eng. Sci. 48(3) 445
Stern L A, Kirby S H, Circone S and Durham W B 2004 Am. Mineral. 89 1162
Waite W F, Helgerud M B, Nur A, Pinkston J C, Stern L A, Kirby S H and Durham W B 1999 ‘Laboratory measurements of compressional and shear wave speeds through methane hydrate’, 3rd International Conference on Gas Hydrates (Salt Lake City, UT)
Du Frane W L, Stern L A, Weitemeyer K A, Constable S, Pinkston J C and Roberts J J 2011 ‘Electrical conductivity of laboratory-synthesized methane hydrate’, Proceedings of the 7th International Conference on Gas Hydrates (ICGH), 17–21
Gupta A, Lachance J, Sloan Jr. E D and Koh C A 2008 Chem. Eng. Sci. 63 5848
Tanaka H and Kiyohara K 1993 J. Chem. Phys. 98(5) 4098
Patchkovskii S and Tse J S 2003 Proc. Natl. Acad. Sci. USA 100(25) 14645
Yang S O, Cho S H, Lee H and Lee C S 2001 Fluid Phase Equilibr. 185 53
Ballard A L and Sloan Jr. E D 2001 Chem. Eng. Sci. 56 6883
Lundgaard L and Mollerup J 1992 Fluid Phase Equilibr. 76 141
Gabitto J F and Tsouris C 2010 J. Thermodyn. 2010 1 article ID 271291 1–12
Udachin K A, Ratcliffe C I and Ripmeester J A 2002 J. Supramol. Chem. 2 405
Hermida-Ramón J M, Grańa A M and Estévez C M 2007 Struct. Chem. 18 649
Tse J S 2002 J. Supramol. Chem. 2 429
Román-Pérez G, Moaied M, Soler J M and Yndurain F 2010 Phys. Rev. Lett. 105 145901
Hawtin R W, Quigley D and Rodger P M 2008 Phys. Chem. Chem. Phys. 10 4853
Jacobson L C, Hujo W and Molinero V 2010 J. Am. Chem. Soc. 132 11806
Vatamanu J and Kusalik P G 2010 Phys. Chem. Chem. Phys. 12 15065
Sun Z, Wang R, Ma R, Guo K and Fan S 2003 Energy Convers. Manag. 44 2733
Moraveji M K, Sadeghi A, Fazlali A and Davarnejad R 2010 World Appl. Sci. J. 9(10) 1121
Tohidi B, Danesh A, Todd A C, Burgass R W and Ostergaard K K 1997 Fluid Phase Equilibr. 138 241
Babaee S, Hashemi H, Javanmardi J, Eslamimanesh A and Mohammadi A H 2012 Fluid Phase Equilibr. 336 71
Belandriaa V, Mohammadia A H, Eslamimanesha A, Richon D, Sánchez-Mora M F and Galicia-Luna L 2012 Fluid Phase Equilibr. 322–323 105
Fakharian H, Ganji H, Far A N and Kameli M 2012 Fuel 94 356
Sun Z and Liu C 2012 J. Chem. Eng. Data 57 978
Torré J, Ricaurte M, Dicharry C and Broseta D 2012 Chem. Eng. Sci. 82 1
Hammerschmidt E G 1934 Ind. Eng. Chem. 26(8) 853
Igboanusi U P and Opara A C 2011 Int. J. Chem. Environ. Eng. 2(2) 131
Wu H and Englezos P 2006 J. Chem. Eng. Data 51 1811
Storr M, Taylor P C, Monfort J and Rodger P M 2004 J. Am. Chem. Soc. 126 1569
Lee J and Kang S 2011 Ind. Eng. Chem. Res. 50 8750
Pal S and Kundu T K 2013 ISRN Phys. Chem. 2013 1 Article ID 753139 1–16
Kumar R M, Baskar P, Balamurugan K, Das S and Subramanian V 2012 J. Phys. Chem. A 116 4239
Pal S and Kundu T K 2012 ISRN Phys. Chem. 2012 1
Pal S and Kundu T K 2013 J. Chem. Sci. 125(2) 379
Pal S and Kundu T K 2013 Chem. Sci. Trans. 2(2) 447
Hohenberg P 1964 Phys. Rev. 136(3B) 864
Kohn W and Sham L J 1965 Phys. Rev. 140(4A) 1133
Chai J and Head-Gordon M 2008 Phys. Chem. Chem. Phys. 10 6615
Frisch M J et al. 2010 Gaussian 09 Revision (B.01) (Gaussian Inc Wallingford CT)
Alecu I M, Zheng J, Zhao Y and Truhlar D G 2010 J. Chem. Theory Comput. 6 2872
Dartois E and Deboffle D 2008 Astron. Astrophys. 490 L19
Barrow G M 1955 J. Phys. Chem. 59 1129
Buck U and Huisken F 2000 Chem. Rev. 100(11) 3863
Acknowledgements
This work was financially supported by the Ministry of Earth Science, Govt. of India (Project No. MoES/16/48/09-RDEAS (MRDM5)). We thank Accelrys Inc. for providing free Discovery studio 3.1 visualization tool.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
PAL, S., KUNDU, T.K. DFT-based inhibitor and promoter selection criteria for pentagonal dodecahedron methane hydrate cage. J Chem Sci 125, 1259–1266 (2013). https://doi.org/10.1007/s12039-013-0470-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-013-0470-2