Abstract
Type-1 IFN (interferon)-associated innate immune response is increasingly getting attention in neurodegenerative and metabolic diseases like type 2 diabetes (T2DM). However, its significance in T2DM/lipotoxicity-induced neuroglia changes and cognitive impairment is missing. The present study aims to evaluate the involvement of cGAS (cyclic GMP-AMP synthase)-STING (stimulator of interferon gene), IRF3 (interferon regulatory factor-3), TBK (TANK binding kinase)-mediated Type-1 IFN response in the diabetic brain, and lipotoxicity (palmitate-bovine serum albumin conjugate/PA-BSA)-induced changes in cells (neuro2a and BV2). T2DM was induced in C57/BL6 mice by feeding on a high-fat diet (HFD, 60% Kcal) for 16 weeks and injecting a single dose of streptozotocin (100 mg/kg, i.p) in the 12th week. Plasma biochemical parameter analysis, neurobehavioral assessment, protein expression, and quantitative polymerase chain reaction study were carried out to decipher the hypothesis. T2DM-associated metabolic and lipotoxic stress led to mitochondrial impairment causing leakage of mtDNA to the cytoplasm further commencing cGAS activation and its downstream signaling. The diseased hippocampus and cortex showed decreased expression of synaptophysin (p < 0.01) and PSD-95 (p < 0.01, p < 0.05) with increased expression of cGAS (p < 0.001), p-STING (p < 0.001), p-STAT1 (signal transducer and activator of transcription) (p < 0.01), and IFN-β (p < 0.001) compared to normal control. The IFN-β/p-STAT1-mediated microglia activation was executed employing a conditioned media approach. C-176, a selective STING inhibitor, alleviated cGAS/p-STING/IFN-β expression and proinflammatory microglia/M1-associated markers (CD16 expression, CXCL10, TNF-α, IL-1β mRNA fold change) in the diabetic brain. The present study suggests Type-1IFN response may result in neuroglia dyshomeostasis affecting normal brain function. Alleviating STING signaling has the potential to protect T2DM-associated central ailment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive neuroscience association with diabetes is becoming a matter of great concern based on the ever-growing clinical, epidemiological, and experimental evidence since the 1980s. Metabolic syndrome is a collective term for disorders, related to an increased risk of cardiovascular disease further advancing to type 2 diabetes (T2DM) [1]. According to various trial studies, diabetic subjects presented different extents of dementia and are more prone to Alzheimer’s disease (AD) or vascular dementia (VD) [2, 3]. Various population-based trial studies in their respective geographical area have shown the prevalence of cognitive decline with reduced performance in multiple domains among diabetic subjects [4, 5, 67]. The diabetes-associated cognitive changes were related to poorer visuospatial construction, planning, visual memory, and speed [8, 9]. Moreover, in trial studies, besides type 1 and type 2 DM subjects, longitudinal behavioral and structural neuroimaging studies have also shown neuroanatomical, volumetric changes in the high-fat diet (HFD) fed obese mice. Obesity was reported to be significantly affecting memory-related functions [10, 11]. Type-1 interferon response (Type-1 IFN) is reported in obesity, palmitic acid (PA)-induced nonalcoholic fatty liver to nonalcoholic steatohepatitis (NASH), DM-induced delayed wound healing, angiogenesis suppression, endothelial dysfunction causing blood–brain barrier (BBB) damage, and neurodegenerative disorders like multiple sclerosis (MS), Huntington’s disease (HT), AD, and Parkinson’s disease (PD) as well [12,13,14,15,16,17,18,19,20,21]. DNA damage induces Type-1 IFN response and other immune-regulatory cytokine secretions [22, 23]. A cytosolic DNA sensing pathway has emerged as the major link between DNA damage and immunity. DNA normally resides in the nucleus and mitochondria; hence, its presence in the cytoplasm serves as a danger-associated molecular pattern (DAMP) to trigger immune responses. Cyclic guanosine monophosphate (GMP)–adenosine monophosphate (AMP) synthase (cGAS) is the sensor that detects DNA as a DAMP and induces Type-1 IFN response. DNA damage releases both single-stranded (ss) and double-stranded (ds) DNA. The ssDNA binding to cGAS causes a minor Type-1 IFN response compared to dsDNA. The binding of DAMPS to cGAS brings conformational change which converts GTP and ATP to cyclic GMP-AMP (cGAMP). The cGAMP, a high-affinity ligand for the STING (stimulator of interferon gene) brings out the conformational change, further the phosphorylated STING translocates from endoplasmic reticulum (ER) membrane to the Golgi apparatus nearing the nucleus. The p-STING further recruits TANK binding protein (TBK) and IFN regulatory factor 3 (IRF3) via a phosphorylation-dependent mechanism. The nuclear translocation of IRF3 led to the transcription of ISG (interferon-stimulated gene) causing IFN-β production [20, 22, 24,25,26]. TBK also acts as the Tau-interacting partner further accelerating the neuronal damage causing AD and related tauopathy [27]. The p-STING also leads to the phosphorylation of NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) causing the transcription of various other cytokines [28]. DNA damage underpinning the neurological disorder was previously reported in the context of Aicardi-Goutières syndrome (AGS). Chronic IFN signaling due to a genetic deficiency of a gene involved in nucleic acid metabolism causes AGS, which is a prime example of an inheritable neurological disease that causes microcephaly, intellectual retardation, and childhood death. The TREX (gene encodes a nuclear protein with 3′ exonuclease activity)-deficient mice showing accelerated aging is also due to the accumulation of damaged DNA causing the increased Type-1 IFN response [29,30,31].
Growing evidence demonstrates that mitochondrial dysfunction and released mtDNA itself act as the major pathological factor for neurodegenerative and metabolic syndrome [32,33,34]. One of the population-based studies on obese individuals has reported the presence of damaged mtDNA in the plasma which may cause insulin resistance or vice versa. Moreover, the mtDNA presence in the plasma is identified as one of the early events of metabolic disease which further commences many inflammatory signaling pathways [33, 35, 36]. The mtDNA-induced Type-1 IFN response promotes cellular senescence and aging, as well as autoimmune, metabolic, and degenerative diseases [33, 36,37,38]. Earlier, Type-1 IFN response involvement in glial cell reactivity, especially the microglia which plays an important role in neuroinflammation and neurodegeneration, was reported. Microglial reactivity is also IFN-β dependent. IFN-β displays both autocrine and paracrine action via the IFNAR (IFN receptor) which transduces the incoming signaling via the JAK (Janus kinase)-STAT (signal transducer and activator of transcription) pathway further inducing the transcription of various cytokine and complement genes [39,40,41]. Depending on the nature of cytokine and complement, microglia display their neuroprotective and neuroinflammatory nature and decide the lymphocyte infiltration at the site as well [14, 17].
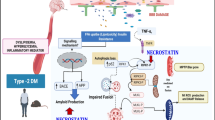
In the present work, we hypothesize that T2DM-associated peripheral metabolic stress led to synaptic changes, neuron-microglia dyshomeostasis, and neuroinflammation. T2DM causes mitochondrial dysfunction causing the release of mtDNA content in the cytoplasm (Fig. 16). Considering all cGAS/p-STING-related research underpinning metabolic stress, aging, etc., the present study first discusses T2DM-induced central changes involving CGAS, STING, TBK, and IRF3 expression in the brain, and palmitate-bovine serum albumin (PA-BSA) conjugate induced changes in neuro2a and BV2 cells. The combined use of the HFD (60% calorie from fat) and streptozotocin (STZ)-induced T2DM model in C57BL/6 mice is extensively explored and reported by the metabolic research community [42,43,44] and also standardized in our lab premise [45]. Likewise, PA-BSA conjugate was used to mimic T2DM-associated obesity at the cellular level [46,47,48]. CMA (9-Oxo-10(9H)-acridine acetic acid), a chemically synthesized molecule that is a selective STING activator, was used for comparative study at in vitro level with PA-BSA conjugate. To elucidate the IFN-β-mediated neuroglia crosstalk, IFN-β was used for BV2 cells in a comparative study with direct PA-BSA conjugate and conditioned media. Further, C-176, a synthetic molecule as a selective p-STING inhibitor, is used to explore its neuroprotective potential in vivo study [49].
Material and methods
Materials
Most of the chemicals used are of reagent grade and were procured from Sigma Aldrich (St. Louis, MO, USA), Himedia laboratories, and SRL Chemicals. HFD was obtained from VYAS Lab (Hyderabad, India). Streptozotocin was purchased from Sigma. Isoflurane was obtained from Raman 7 Weil Pvt. Ltd. (Mumbai, India). Glucose oxidase–peroxidase, total cholesterol, and triglyceride kit were purchased from Accurex (Mumbai, India). ELISA kit for TNF-α (Biolegend) and IFN-β (R&D Systems: Products & Services for Cell Biology Research) was also procured. Sodium palmitate (P9767) and BSA (A9418) were obtained from Sigma Aldrich (St. Louis, MO, USA). JC-1 (T3168) and Mitotracker (M7512) were obtained from Thermo Fisher Scientific, USA. Antibodies were obtained from Cell Signal Technology, ABCAM, Santacruz, and Millipore. C-176 (MedChem Express) and IFN-β (8234-MB-010, R&D Systems) were also procured.
Experimental design
This study was completed in four different experiments: in vivo studies in animals, and in vitro studies in neuro2a and BV2 cell lines. Study 1 consists of a preliminary study investigating the involvement of cGAS/STING-TBK-IRF3 axis-mediated Type-1 IFN response in the diabetic brain. Study 2 explores the effect of palmitate-mediated lipotoxicity on Type-1 IFN response contributing to IFN-β release in neuro2a cells. Study 3 elucidates the IFN-β-mediated neuroglia crosstalk using neuro2a and BV2 cells. Study 4 is an exploratory study investigating the neuroprotective effect of C-176 in T2DM-associated cognitive impairment.
Animal study
Diabetes induction
Animal studies were carried out as per the regulation of the Committee for Control and Supervision of Experiments on Animals (CCSEA) and Institutional Animal Ethical Committee (IAEC)-NIPER-Hyderabad Telangana, India, and according to Animal Research Reporting of In Vivo Experiments (ARRIVE) guidelines [50] after getting approval of protocol no NIP/10/2019/PC/343 and NIP/05/2020/PC/385. Eight-week-aged C57BL/6 male mice were procured and housed in polycarbonate cages (three to four mice per cage) in a temperature-controlled room (22 ± 2 °C) with a 12-h light–dark cycle. They are allowed to feed on a normal pellet diet (NPD) and water ad libitum to get acclimatized in the institutional animal facility. Animals were weighed and randomized into different groups. Further, they were categorized into two groups, i.e., NPD (Norm pellet diet) and HFD (60% Kcal from fat), and continued the same for a further 4 months/16 weeks. After 3 months on the HFD, a single dose of 100 mg/kg STZ freshly prepared in chilled citrate buffer (pH 4.5) was administered intraperitoneally [51]. After 24 h of STZ administration, fasting blood glucose was examined by using a glucometer and commercially available Accurex kit. Once the hyperglycemia was confirmed, animals were continued on HFD for the further 4 weeks. Once glucose tolerance and insulin insensitivity were established, neurobehavioral studies were conducted for NPD and HFD fed animals to assess cognitive impairment after 16 weeks.
C-176 administration
After 13 weeks of diet manipulation, diabetic animals were further divided into two groups: T2DM + Veh (N = 20) and T2DM + C-176 (N = 20). The normal control animal (N = 20) was continued on NPD. The dose of C-176 was chosen based on previous studies. C-176 was administered for 6 weeks at 4 mg/kg for every alternate day. The drug stock was prepared in DMSO at 10 mg/ml and diluted in 5% Tween-80 and 5% PEG-400. The study was exploratory and hence the endpoint was not predefined. After the completion of dosing, neurobehavioral parameters were evaluated, and tissue was processed as mentioned in (Fig. 11) [52,53,54].
Biochemical estimation
To ensure that the animal exhibits T2DM characteristics, fasting blood glucose (FBG), oral glucose tolerance test (OGTT), intraperitoneal insulin tolerance test (IPTT), and lipid profile studies were carried out.
Plasma lipid profile
Blood was collected in the heparinized tube and centrifuged at 5000 rpm for 15 min to separate plasma. The plasma total cholesterol (TC) and total triglyceride (TG) levels were estimated using a commercially available Accurex kit.
Fasting blood glucose (FBG)
The FBG level was estimated using a commercially available Accurex kit at the end of the study.
Behavioral and cognitive analysis
Behavioral experiments were conducted using a blinding strategy. One of the co-authors who were not aware of the grouping was involved during the experiment but not during the data analysis.
Forced alteration test (novel arm entry test in Y-maze)
It is used to assess working memory and exploratory behavior which is based on the concept that mice explore unfamiliar environments. It comprised 10-min sample trials (T1) followed by 5 min of test trial/memory retrieval (T2) by keeping 1 h of inter-trial interval (ITI). In T1, the mouse was positioned at the end of the start arm facing away from the center. The entry to the third arm (the novel arm) of Y-maze was blocked while keeping the other two arms open for exploration. After the sample trial, the mouse was returned to its home cage for a 1-h inter-trial interval. In T2, the restriction in the third arm was removed, and the mouse was again placed into the start arm and then allowed to freely access all three arms of the maze. An arm entry was considered when 85% of a mouse’s body entered the arm. Time in novel arm (%) was defined as the time spent in the novel arm divided by the time spent in all arms during the first minute of the retrieval trial T2 [55, 56].
Assessment of intermediate-term recognition memory: novel object recognition test (NORT)
Novel object recognition trials were conducted in square test boxes (40 × 40 × 35 cm). Each test box consisted of an overhead camera. Two distinct objects were used: a triangle bottle painted white and a round, spherical bottle of a different color. The habituation phase of 10 min each for over 2 days preceded the testing phase. On testing day 3, mice were permitted to explore the empty test box for 20 min as a part of the habituation trial and 24 h after the habituation trial (on day 4, a sample trial was conducted). During the sample trial, mice were placed into the empty test box facing away from the objects. After 5 min of the accustomization phase, two similar sample objects were introduced into the box. The mouse was allowed to investigate the objects for 10 min before it was returned to its cage. After 60 min, NORT was conducted by placing the mouse into the empty test box again. After an initial 5-min accustomization phase, one old object from the sample trial and one newer object were introduced, and object interaction was recorded for 10 min. Object interaction was defined as an event where a mouse’s head was within 2 cm of the object and directed toward the object. The percent time spent exploring the novel versus a familiar object was calculated [57, 58].
Spatial learning and memory in the Morris water maze
The water maze, a circular pool (diameter, 150 cm), filled with opaque water (nonfat milk powder, 2 °C) was hypothetically divided into four quadrants. Mice were subjected to the training of four trials/day for 7 days, with 2 days of visible followed by 5 days of the hidden platform. During visible training, the flagged platform was positioned 1 cm above the water surface in the absence of extra MAZE cues. Each mouse underwent a 60-s trial to find the platform. The mouse was removed from the pool after locating the platform and remaining on it 5 s. Mice were introduced into the maze at varying locations during each trial to avoid procedural bias. During the visible platform trials, the platform location was changed for each trial between the four quadrants to avoid procedural bias. Mice were trained to seek the platform during the hidden platform trial in the presence of four extra MAZE signals positioned at four quadrants for spatial reference and orientation. The platform location was constant in the SW quadrant. After 24 h of the last trial, the probe trial was performed for 30 s where the platform was removed to assess spatial memory retention. The experiment was recorded using ANY-maze software (ANY-maze, version 6.1). The parameters like latency to enter into the target zone and number of platform crossings on the day of the probe trial were recorded [57, 59].
Microscopy study
Perfused brains were further stored in PFA for 24 h followed by preserving them in 30% sucrose. The brain was frozen as OCT (optimum cutting temperature) block, and then, coronal cryosection of various thicknesses ranging from 25 to 30 µ was carried out. Sections were stored at − 20 °C before processing for various staining like immunofluorescences.
Cell culture
Cell maintenance
BV2 and neuro2a cell lines were obtained from Elabscience, USA, and NCCS (Pune, India) respectively, and used between passage numbers 5 and 16. Cells were cultured in DMEM-high glucose media supplemented with FBS (10%) and streptomycin/penicillin (1%). They were cultured at 37 °C in a humidified atmosphere of 95% air and 5% CO2 [60, 61].
Palmitate conjugate preparation
One hundred-millimolar sodium palmitate stock was prepared in sterile water by heating at 70 °C. The 5% endotoxin-free BSA (sigma) stock in media was prepared. Five-millimolar PA-BSA conjugate was prepared freshly before use in a 1:20 ratio and filtered through a 0.2-µm syringe filter [46]. The palmitate dose was chosen based on the MTT test and protein expression study [45].
Stock preparation
CMA was solubilized in DMSO to prepare a stock of 1000 µg/ml. IFN-β stock was prepared by reconstituting in sterile PBS. The working IFN-β dose was chosen based on a published report and protein expression study.
Neuro2a conditioned media collection and treatment to BV2 cell
Neuro2a cells were plated in a six-well plate at a density of 1 × 105 cells/well. Cells were exposed to PA-BSA conjugate for 24 h. The conditioned media were collected for ELISA. In a separate experiment, the conditioned media were collected and transferred to the BV2 cell for western blotting and immunocytochemistry.
Mitochondrial membrane potential (Ψm) (JC1 assay)
The cells were seeded in six-well plates at a density of 5 × 104 cells/well. After 24 h, cells were exposed to PA-BSA conjugate for 12 h. Cells were incubated with 5 µM of JC-1 dye (T3168, Invitrogen) for 40 min, followed by PBS washing for 30 min. The images were captured under a fluorescence microscope (Nikon ECLIPSE Ti-U, Japan) [62].
Assessment of intracellular reactive oxygen species by DCFDA staining
The BV2 cells were plated in a six-well plate at a density of 5 × 10.4 cells/well. After 24 h, cells were exposed to PA-BSA conjugate and BSA for 18 h. Cells were incubated with 10 mM of DCFDA (cat. no. D399, Invitrogen by Thermo Fisher Scientific, Waltham, MA) dye for 30 min. Cells were washed thrice with PBS for 5 min each. The fluorescence intensity was observed under a fluorescence microscope at 20 × magnification (Nikon ECLIPSE Ti-U, Tokyo, Japan) [63]
MitoSOX staining for estimation of mitochondrial superoxide anion (O2.−)
The BV2 cells were seeded in a six-well plate, at a density of 5 × 104 cells/well, and kept for overnight incubation. After 24 h, cells were exposed to PA-BSA conjugate and BSA for 18 h followed by incubation with 5 mM MitoSOX (cat. no. M36008, Invitrogen by Thermo Fisher Scientific, Waltham, MA) for 15 min at 37 °C. After incubation, cells were washed with PBS, and images were captured under the fluorescence microscope (Nikon ECLIPSE Ti-U, Tokyo, Japan) [64].
Enzyme-linked immunosorbent assay (ELISA)
For quantifying the inflammatory marker, tissue was homogenized in T-PER (Tissue Protein extraction reagent) (cat. no. 78510) buffer followed by sonication (20 pulses for 10 s). The supernatants were collected by centrifuging the homogenate at 12,000 rpm for 5 min at 4 °C. Total protein concentration was estimated using Bradford reagent (BIORAD). Further, the inflammatory markers were quantified according to the procedure mentioned in the commercially available kit for IFN-β, TNF-α, IL-6, and IL-4 levels in tissue, and cell supernatants [57].
Protein expression study
Western blot
Total proteins from cells were extracted using RIPA (radioimmunoprecipitation assay buffer) (cat. no. R0278) supplemented with a protease (cat. no. P8340)-phosphatase (cat. no. P-0044) inhibitor cocktail in 1:200. The lysed samples were sonicated using a probe sonicator at 20 pulses for 10 s and allowed for incubation on ice followed by centrifugation at 12,000 rpm for 20 min. The supernatant was collected, and protein estimation was carried out by using Bradford reagent (BIORAD) keeping BSA as standard. The hippocampus and cortex tissue were also homogenized in T-PER buffer supplemented with protease and phosphate inhibitor cocktail in 1:200. The homogenized sample was further sonicated and allowed to centrifuge at 12,000 g for 20 min. The supernatant was collected, and protein was estimated using Bradford (BIORAD). Twenty- to forty-microgram protein samples were resolved using SDS-PAGE gel. Resolved proteins were transferred to the nitrocellulose membrane (BIORAD, cat. no. 1620112). The membrane corresponding to the desired protein was first blocked by incubating with 3% BSA followed by incubating them overnight at 4 °C with primary antibodies cGAS (31659 T), p-STING (72971S), p-TBK (5483T), p-IRF3 (29047T), TFAM (SC-166965), p-STAT1 (SC-8394), IFN-β (SC-57201), BAX (SC-7480), and β-actin (SC-47778). On the second day after washing with TBST, the membrane was incubated with corresponding HRP-tagged anti-rabbit (SC-2357) or anti-mouse (SC-516102) or anti-goat (SC-2354) secondary antibody for 2 h at room temperature. The chemiluminescence signal was captured using a Fusion-FX imager (Vilber Lourmat, Germany) to visualize the protein band, and their relative intensities were quantified by densitometry using ImageJ software (version 1.48, NIH, USA).
Immunofluorescence (IF)
The frozen brain section was taken out from − 20 °C and kept for drying. Sections were given three washes of PBST followed by antigen retrieval in citrate buffer (pH 6) in the microwave oven at 80 °C and washing in PBS. Sections were blocked in 3% BSA in PBST for 1 h and then allowed to react with primary antibodies NeuN (MAB8377, 1:100), IBA (32725S, 1:50 and 17198S, 1:200), GFAP (3670S, 1:100), p-Tau (SC-32275, 1:100), p-STING (1:100), dsDNA (SC-58749, 1:50), IFN-β (1:100), p-STAT1 (1:100), CD16/32 (SC-20052, 1:50), CD206 (SC-376108, 1:100) synaptophysin (ab14692, 1:200), and PSD95 (36233S, 1:100) dilution for overnight. On the second day after retrieving the primary antibody, sections were washed three times with PBS followed by incubation with Alexa fluor-conjugated secondary antibodies at 4 µg/ml for 2–4 h (A11008, A11003, A21245, A11007). After incubation, tissue sections were washed thrice with PBS and mounted with Fluorosheild DAPI (Sigma Aldrich). Images were captured under 20 × , 40 × /1.30, and 63 × oil under the confocal microscope (LEICA, DMi8), and the mean fluorescent intensity was analyzed using ImageJ software.
Immunocytochemistry (ICC)
Cells were grown on the poly-L-lysine-coated coverslip. Cells were treated with the indicated concertation of PA-BSA conjugate for 24 h. Later cells were washed with PBS, fixed with 4% PFA, and permeabilized with 0.1% triton X-100 in PBS. Further cells were washed followed by blocking with 3% BSA containing 0.3 M glycine for 1 h. Later, cells were incubated with appropriate primary antibodies dsDNA (1:100), TFAM (1:100), p-STING (1:100), IBA (1:100), p-STAT1(1:100), and CD16/32 (1:200) for overnight. On the second day, cells were washed and incubated with secondary Alexa flour-conjugated secondary antibody for 2 h. Finally, cells were washed thrice with PBS, and coverslips were mounted with Fluorosheild DAPI on the slide (Sigma Aldrich). Images were captured under 40 × /1.30 oil under the confocal microscope (LEICA, DMi8), and the mean fluorescent intensity was analyzed using ImageJ software. For the Mitotracker colocalization study, after 24 h of treatment, cells were incubated with a 5 µM Mitotracker for 30 min and were fixed with 4% PFA after brief PBS washing. The rest of the protocol follows the same steps as discussed above.
Mitochondrial DNA-deficient cells generation
Neuro2a cells were grown in DMEM-HG supplemented with 10% FBS 100 μg/ml and streptomycin/penicillin (1%). They were cultured at 37 °C, in a humidified atmosphere of 95% air and 5% CO2 with the addition of pyruvate (100 μg/ml), uridine (50 μg/ml), and ethidium bromide (EtBr) at 1 μg/ml for 7 days [18, 65, 66].
Quantitative real-time polymerase chain reaction (RT-qPCR) assay
RNA was isolated from the tissue using the commercially available TRIzol reagent (Invitrogen). Total RNA was quantified, and their purity was checked in nanodrop (Thermo Fisher). The TAKARA cDNA synthesis kit (cat. no. 6110 A) was employed for synthesizing complementary DNA (cDNA). Further, quantitative PCR reaction was carried out using SYBR® Green Master Mix, ROX from Takara, and appropriate forward and reverse primers (Table S1) using Instrument, Quant Studio 7 Pro Design with Analysis 2.6.0 Real-Time PCR System Applied Biosystem (Thermo Fisher). Relative mRNA expression changes were calculated from the comparative threshold cycle (Cq) value relative to mouse GAPDH.
Cytosolic mitochondrial DNA isolation and quantification by RT-qPCR assay
To decipher the presence of mitochondrial DNA in the cytosol, the nuclear and mitochondrial contaminant-free cytosolic fraction was isolated from tissue. Tissue was homogenized in buffer with the composition of (5 mM HEPES-Tris (pH 7.4), 225 mM sucrose, 75 mM mannitol, and 1 mM EGTA). The homogenate was divided into two equal parts. One part is then centrifuged at 1300 g for 3 min, and the supernatant is again spun at 20,000 g for 10 min. The resultant supernatant was collected as the cytosolic fraction. Further, the cytosolic fraction (300 μl) was used to isolate cytosolic DNA, respectively, using the commercially available NucleoSpin Tissue kit (MACHERY-NAGEL, GmbH & Co, KG GERMANY, 740,952) according to the manufacturer’s instructions. The copy number of mtDNA-encoding NADH dehydrogenase (ND1) was measured by qPCR. The Cq value of mtDNA abundance from whole-cell extract served as a normalization control for the mtDNA values obtained from the cytosolic fractions [18, 67].
Statistical significance
GraphPad Prism 8.2.2 software (GraphPad, San Diego, CA, USA) was used to assess the significant differences. All measurements were carried out in triplicates, with a minimum of three independent experiments. Student’s t-tests were used to compare two distinct groups. To compare more than two groups one-way or two-way Analysis of Variance (ANOVA) followed by the Bonferroni post-hoc test was used. Quantitative data are presented as mean ± SEM. Statistical significance was considered at *p < 0.05, **p < 0.01, and ***p < 0.001.
Result
Activation of cGAS/STING-mediated Type-1 interferon response is involved in T2DM-associated central changes
The HFD with a single 100 mg/kg STZ administration led to significant behavioral, neuroinflammatory, and neurodegenerative changes in the mice’s brains (Fig. S-1) [45, 68, 69]. The cortex and hippocampus of the diabetic brain have shown increased p-Tau deposition (p < 0.01, p < 0.01) compared to normal control (Fig. 1a, b, e). Furthermore, decreased synaptophysin (p < 0.01, p < 0.01) and PSD95 (p < 0.01, p < 0.05) synaptic protein expression was also observed in the hippocampus and cortex of diabetic brain compared to the normal control (Fig. 1c, d, f, g). There are possibilities of various underlying pathomechanisms, contributing to brain changes in diabetic subjects. One such mechanism, cGAS/STING-mediated Type-1 IFN response, is well reported in obesity-based, aging-related, and other neurodegenerative diseases like MS, and the HTT model. The protein expression study has revealed a significant increase in cGAS (p < 0.001, 286%) (p < 0.001, 190%) and p-STING (p < 0.001, 395%) (p < 0.001, 369%) expression in the hippocampus and cortex of diabetic mice compared to normal control. The downstream of cGAS/STING activation is the phosphorylation of TBK and IRF3. The level of both p-TBK and p-IRF3 was also found to be significantly elevated (p < 0.001, 222%) (p < 0.01, 247%) in the hippocampus and (p < 0.01, 227%) (p < 0.01, 376%) cortex tissue of diabetic compared to the NPD-fed animal (Fig. 2a, b, c, d). The qPCR study also revealed the increased mRNA fold in cGAS (p < 0.01, 2.15-fold), STING (p < 0.01, 8.08-fold), and TBK (p < 0.01, 5.84-fold) (Fig. 2e) in the hippocampus tissue. The central nervous system (CNS) is enriched with neurons, microglia, and astrocytes.
Diabetes-associated metabolic syndrome led to phosphorylated-Tau deposition and synapse loss in the brain. a, b Immunofluorescence image showing p-Tau deposit in the DG area of the hippocampus and layers I–II of the cortex (40 × magnification with oil). c, d Immunofluorescence image showing the synaptophysin and PSD-95 expression in the DG area of the hippocampus and layers I–II of the cortex of the normal and diabetic brain (20x magnification). e Scatter plot with a bar graph showing the mean fluorescence intensity of p-Tau as a percentage of control (n = 3). f, g Scatter plot with the bar graph showing the mean fluorescent intensity of synaptophysin and PSD-95 as a percentage of control in the hippocampus and cortex. Data are represented as mean ± SEM. Comparisons between groups were done by using a two-tailed unpaired t-test. p-value on the figure indicates significance, *p < 0.05, **p < 0.01, vs. NC group. HC (hippocampus), CORT (cortex)
cGAS/p-STING-mediated Type-1 interferon response was upregulated in the diabetic brain. a, b Western blot image of cGAS, p-STING, p-IRF3, and p-TBK of the hippocampus and cortex tissue lysate and c, d scatter plot with a bar graph showing densitometric analysis of the blot of NC and T2DM group as a percentage of normal control (n = 4). e Scatter plot with a bar graph showing mRNA fold change in cGAS, STING, and TBK of NC and T2DM group. Data are represented as mean ± SEM. Comparisons between groups were done by using a two-tailed unpaired t-test. p-value on the figure indicates significance, ***p < 0.001, **p < 0.01, vs. NC group
Therefore, to observe the relative predominance of cell type for p-STING expression, a colocalization study of p-STING with NeuN (neuronal marker), IBA (microglia marker), and GFAP (astrocyte marker) was carried out. The colocalization study has shown the relative abundance of p-STING to neurons is significantly higher compared to microglia (p < 0.05, 92%) and astrocytes (p < 0.001, 245%) (Fig. 3a, b, c, d). Further, the relative mean fluorescent intensity of p-STING was also found to be significantly (p < 0.001, 384%) elevated in the diabetic brain compared to the NPD-fed animal (Fig. 3e).
Relative colocalization of p-STING to neuron, microglia, and astrocyte. a, b, c Immunofluorescence image showing p-STING colocalization to astrocyte (GFAP), microglia (IBA), and neuron (NeuN), between NC and T2DM brain in CA1 area of the hippocampus; d scatter plot with a bar graph showing relative colocalization of p-STING to neuron, microglia, and astrocyte in the hippocampus (n = 3); e scatter plot with a bar graph showing mean fluorescence intensity of p-STING in terms of percentage of control between NC and T2DM brain (n = 3). Data are represented as mean ± SEM. Comparisons between groups were done by using one-way ANOVA (Fig. d) and two-tailed unpaired t-test (Fig. e). p-value on the figure indicates significance, *p < 0.05, **p < 0.01, ***p < 0.001 vs. NC and p-STING/NeuN group
T2DM and palmitate-mediated lipotoxicity cause dsDNA leakage in brain and neuro2a cells
As we have mentioned while introducing the topic, cGAS/STING-mediated Type-1 IFN response may happen due to endogenous factors leading to chronic sterile inflammation. One such endogenous factor is dsDNA, mainly the leaked mtDNA to cytosol, further activating the cGAS. The immunofluorescence study showed the presence of significantly elevated dsDNA in both the hippocampus (p < 0.001, 353%) (dentate gyrus area) and cortex (p < 0.01, 589%) compared to the normal control brain (Fig. 4a, b, c, d).
Type-1 IFN response in the diabetic brain caused by cytosolic mtDNA. a, b Immunofluorescence image showing dsDNA in the DG area of hippocampus and layers I–II of cortex tissue (under 20 × magnification). c, d Scatter plot with a bar graph showing the mean fluorescent intensity of dsDNA as a percentage of control in the hippocampus and cortex. e Scatter plot with a bar graph showing cytosolic mtDNA (ND1) fold change in the brain by qPCR (n = 3). Data are represented as mean ± SEM. Comparisons between groups were done by using a two-tailed unpaired t-test. p-value on the figure indicates significance, **p < 0.01
Elevated free fatty acid (FFA) uptake in the brain of the obesity-based experimental model and increased palmitate levels in overweight humans with underlying diseases of dyslipidemia and diabetes were also linked with central inflammation and cognitive impairment. Based on the above evidence, PA-BSA conjugate was used at the cellular level to elucidate if the dsDNA leakage occurs. Based on our standardization data in the lab and available published data, the two doses of palmitate, i.e., P-200 and P-300, were used for the Mitotracker-dsDNA colocalization ICC study. The leakage of dsDNA was very evident at the P-300 dose compared to vehicle control (p < 0.001, 573%) (Fig. 5a, b). To investigate whether cytosolic DNA is of mitochondrial origin or not, qPCR study of the cytosolic content of tissue using the mtDNA-specific primer ND1 was performed. The relative abundance of cytosolic mtDNA in the diabetic brain was significantly elevated (p < 0.01, 5.25-fold change) compared to age-matched normal control (Fig. 4e). To establish the role of palmitate in the cGAS/STING pathway, we used the CMA, a selective STING activator, and carried out western blot study. CMA at 300 µg/ml (p < 0.001, 140%) and 300 µM PA-BSA conjugate (p < 0.01, 57%) led to a significant increase in p-STING expression compared to only BSA-treated cells (Fig. 5c, d). Western blot study was carried out for cGAS and p-STING exploring the effect of different incubation hours (12, 18, and 24 h) of palmitate on cells. The 300 µM of PA-BSA has shown significantly elevated p-STING expression (p < 0.001) between 18 and 24 h compared to only BSA-treated cells. Moreover, the 24-h incubation effect on p-STING expression was equivalent to CMA-300 (Fig. S2).
Palmitate-mediated lipotoxicity effect on the dsDNA leakage, mitochondrial membrane potential change, and p-STING expression in neuro2a cells. a Immunofluorescence image showing colocalization of dsDNA to Mitotracker of BSA as vehicle control (upper panel) and 300 µm PA-BSA (lower panel) exposed neuro2a cells. b Scatter plot with a bar graph showing fragments of cytosolic mtDNA in PA-BSA-treated cell compared to BSA control as the percentage of control (n = 3). c, d Western blot of p-STING and its densitometric analysis for BSA, CMA at the dose of 200 and 300 µg/ml and palmitate at 300 µm. e, f Fluorescent image showing JC1 stain of neuro2a cell, left panel (JC-1 aggregate, red color), middle panel (JC monomer, green color), right panel (merge image), scatter plot with the bar graph showing the ratio of JC aggregate to JC monomer fluorescent mean intensity as percentage of BSA exposed cell control. Data are represented as mean ± SEM. Comparisons between groups were done by using one-way ANOVA (Fig. B.1) and two-tailed unpaired t-test (Figs. A.1,C.1). The p-value in the figure indicates significance, **p < 0.01, ***p < 0.001 vs. BSA exposed group
cGAS/STING-mediated Type 1 interferon signaling is due to mitochondrial dysfunction in the brain and neuro2a cells
We showed the involvement of the cGAS/p-STING-mediated Type-1 IFN response due to leaked mtDNA to the cytoplasm. Now, the leakage of the mtDNA could be due to mitochondrial dysfunction. The mitochondrial being the powerhouse of the cell shows an overwhelming response under stress. Later was linked with BAX pore formation, and improper packaging of mtDNA due to deficient TFAM function. Western blot study revealed a significant increase in the BAX (p < 0.01, 144%) (p < 0.01, 207%) and decrease in TFAM (p < 0.01, 35%) (p < 0.05, 49%) expression in the hippocampus and cortex of the diabetic brain respectively compared to normal control (Fig. 6a, b, c, d). Moreover, the 300 µM of PA-BSA led to a significant decrease in TFAM mean fluorescent intensity (p < 0.01, 72%) in cells compared to only BSA-treated cells (Fig. 6e, f). The altered mitochondrial membrane potential (MMP/Ψm) gives insight into the mitochondrial stress implicating the altered permeability across the membrane. Palmitate significantly altered the MMP/Ψm of neuro2a cells indicating a significant loss in red/green ratio at 300 µM dose compared to normal control (p < 0.001, 90%) (Fig. 5e, f). To assess the interrelation of p-STING expression to mitochondria, we have carried out the colocalization study of Mitotracker to p-STING. The 300 µM of PA-BSA dose led to a significant increase in the p-STING expression (p < 0.01, 393%) compared to only BSA-treated cells. With the increase in the p-STING expression, a concomitant decrease in the Mitotracker fluorescent intensity was also observed as the ratio of p-STING/Mitotracker was also found to be significantly elevated (p < 0.01, 248%) (Fig. 6g, h, i). Further, to decipher the mtDNA-mediated Type-1 IFN response, cells were transformed to mitochondrial deficient by incubating them with ethidium bromide (EtBr) for 7 days and further exposing them to PA-BSA. According to the protein expression study, 300 µM of PA-BSA exposure exhibited significantly elevated expression of p-STING (p < 0.001, 127%), p-IRF3 (p < 0.01, 71%), p-TBK (p < 0.001, 190%), and p-NF-Κβ (p < 0.001, 131%) compared to cells which undergone EtBr treatment (EtBr + P300) before palmitate (Fig. 6j, k).
Mitochondrial dysfunction involved in mtDNA-mediated Type-1 interferon response in the diabetic brain and palmitate-mediated lipotoxic stress in cells. a, b TFAM and BAX western blot image of the hippocampus and cortex tissue lysate; c, d scatter plot with the bar graph showing densitometric analysis of normal control (n = 3) and diabetic group (n = 3); e, f effect of BSA as vehicle control (upper panel) and 300 µm palmitate (lower panel) on TFAM expression in neuro2a cells. Scatter plot with the bar graph showing mean fluorescence intensity of TFAM as the percentage of control (n = 3); g immunofluorescence image showing the effect of BSA and P-300 on colocalization of p-STING with Mitotracker under 40 × magnification. Left panel (p-STING), middle panel (Mitotracker), right panel (merge image). h Scatter plot with the bar graph showing the mean fluorescent intensity of p-STING, i scatter plot with the bar graph showing the ratio of mean fluorescent intensity of p-STING to Mitotracker (n = 3), j, k western blot image of p-STING, p-IRF, p-TBK of only BSA, EtBr + P300, P300, only EtBr-treated neuro2a cells and the scatter plot with the bar graph showing the densitometric analysis of p-STING, p-IRF3, p-TBK, and NF-κB. Data are represented as mean ± SEM. Comparisons between groups were done by using one-way ANOVA (Fig. k) and two-tailed unpaired t-tests (Fig. c, d, f, h, i). The p-value on the figure indicates significance, *p < 0.05, **p < 0.01, ***p < 0.001 vs. NC (in brain tissue) and BSA-treated group (in neuro2a cells). $$p < 0.01, $$$ p < 0.001 vs. EtBr-1 + P300-treated cells (in Fig. k)
Activation of cGAS/STING leads to an increase in the IFN-β level
The p-STING upon its activation phosphorylates TBK, IRF3, and IκB kinase (IKK)-related kinase and increases IFN-\(\beta\) release. We performed the IF study for IFN-β expression in tissue. The mean fluorescent intensity was found to be significantly higher in both the hippocampus (p < 0.001, 490%) and cortex (p < 0.01, 231%) compared to normal control (Fig. 7a, b, c, d). We have also performed ELISA to quantify IFN-β in tissue and palmitate-treated neuro2a cell supernatant. The IFN-β level was found to be significantly higher in the hippocampus (p < 0.001, 117%) and cortex (p < 0.001, 116%) of the diabetic group compared to the normal control. Similarly, the IFN-β level in the supernatant of palmitate-treated neuro2a cells was also significantly elevated (p < 0.01, 117%) compared to vehicle control (Fig. 7f, g, h). Further, the qPCR result has unveiled a relative increase in the mRNA level of IFNAR in the hippocampus (p < 0.01, 17-fold mRNA change) compared to normal control (Fig. 7e).
Diabetes and palmitate-mediated lipotoxic stress elevated interferon-β expression. a, b Immunofluorescence image showing IFN-β expression for DG area of the hippocampus and II–III layer of cortex tissue (under 63 × magnification with oil) for NC and T2DM brain. c, d Scatter plot with the bar graph showing the mean fluorescent intensity of IFN-β as a percentage of control for the hippocampus and cortex tissue (n = 3). e Scatter plot with the bar graph showing quantification of IFNAR by qPCR in the hippocampus (n = 3). f, g, h Scatter plot with the bar graph showing IFN-β quantification by ELISA in the hippocampus, cortex tissue, and cell supernatant of neuro2a cells as the percentage of control (n = 3). Data are represented as mean ± SEM. Comparisons between groups were done by using a two-tailed unpaired t-test. p-value on the figure indicates significance, *p < 0.05, **p < 0.01, ***p < 0.001 vs. NC (in brain tissue) and BSA-treated group (in neuro2a cells)
Increased IFN-β led to p-STAT1-mediated microglial activation
The proinflammatory microglia phenotype promotes neuroinflammation by releasing proinflammatory mediators and contributes to synapse loss. IFN-β acts via IFNAR and further transduces the signaling via the JAK-STAT pathway. Western blot study showed a significant increase in the p-STAT1 expression in both the hippocampus (p < 0.01, 315%) and cortex (p < 0.01, 260%) compared to the diabetic brain (Fig. 8e, f, g, h). The colocalization study by IF showed the percentage overlap of IBA with p-STAT1 was significantly higher in the hippocampus (p < 0.01, 190%) and cortex (p < 0.01, 353%) compared to the normal control brain (Fig. 8a, b, c, d). To decipher the neuroglia crosstalk, we had to carry out a comparative study between conditioned media from PA-BSA-treated neuronal cells, direct exposure of IFN-β, and PA-BSA. As we have seen in Fig. 3, p-STING also colocalizes with IBA; therefore, we explored BV2 cell’s response under lipotoxic stress. The palmitate exposure has increased the cGAS, p-STING, p-IRF3, and p-TBK expression in a dose-dependent manner (Fig. S3a). The 300 µM of PA-BSA has also significantly increased the cellular ROS (p < 0.001, 90%) and mitochondrial superoxide (p < 0.01, 134%) in the BV2 cells (Fig. 9a, b, c, d). Further, to decipher that IFN-β transduces its function via p-STAT1, western blot, and ICC study for p-STAT1, relative expression on microglia was carried out. The BV2 cells were exposed to 100 IU-IFN-β, conditioned media from 300 µM PA-BSA-treated neuro2a cell, and direct exposure to 300 µM PA-BSA. The dose IFN-100 IU was chosen based on the previous publication and standardization study (Fig. S3b, c). IFN-100 IU (p < 0.001, 463%), P-300 (p < 0.01, 133%), and conditioned media (p < 0.001, 272%) have significantly elevated the p-STAT1 expression compared to BSA-treated cell (Fig. 9e, f).
The implication of p-STAT1-mediated microglia activation in diabetic brain. a, b Immunofluorescence image showing colocalization of p-STAT1 with microglia (IBA) in the CA area of hippocampus and layers I–II of the cortex tissue (under 63 × magnification with oil) for NC and T2DM brain. c, d Scatter plot with the bar graph showing relative colocalization of p-STAT1 to IBA as the percentage of control in hippocampus and cortex tissue (n = 3). e, f, g, h Western blot image of p-STAT1 in the hippocampus and cortex and scatter plot with the bar graph showing densitometric analysis of p-STAT1 between normal control (n = 3) and diabetic group (n = 4). Data are represented as mean ± SEM. Comparisons between groups were done by using the two-tailed unpaired t-test. p-value on the figure indicates significance, *p < 0.05, **p < 0.01 vs. NC
Effect of palmitate-mediated lipotoxic stress on neuroglia crosstalk. a, b Fluorescence image showing MitoSOX and DCFDA stain of BV2 cell representing mitochondrial superoxide generation and cellular ROS generation (20 × magnification). c, d Scatter plot with the bar graph showing the relative mean fluorescent intensity of MitoSOX and DCFDA for BSA- and P300-treated BV2 cells. e, f Western blot image of p-STAT1 for BSA, 300 µm PA-BSA, conditioned media from palmitate-treated neuro2a cell, 100 IU interferon-β, and scatter plot with the bar graph showing the p-STAT1 densitometric analysis. Data are represented as mean ± SEM. Comparisons between groups were done by using one-way ANOVA (Fig. f) and a two-tailed unpaired t-test (Fig. c, d). p-value on the figure indicates significance, **p < 0.01, ***p < 0.001 vs. BSA-treated group (in BV2 cells)
IFN-100 IU (p < 0.01, 843%), P-300 (p < 0.05, 486%), and neuro2a cell conditioned media (p < 0.05, 713%) have significantly elevated the p-STAT1 mean fluorescent intensity compared to BSA-treated cell (Fig. 10a, c). Further, we used CD16/32 surface markers with IBA to illustrate their expression pattern by using a double immunofluorescence labeling study. An increase in CD16 is associated with the inflammatory state of microglia. To do so, BV2 cells were exposed to 100 IU-IFN-β, conditioned media from 300 µM PA-BSA-treated neuro2a cells, and direct exposure to 300 µM PA-BSA as well. A significant increase in the CD-16 fluorescent intensity in IFN-100 IU (p < 0.01, 613%), P-300 (p < 0.05, 380%), and conditioned media (p < 0.05, 403%)-treated cells was observed compared to the BSA-treated cell (Fig. 10b, d). The present section depicts the IFN-β-mediated neuron-microglia crosstalk in the transformation of the proinflammatory phenotype of microglia through p-STAT1 activation.
Effect of palmitate-mediated lipotoxic stress and IFN-β-mediated neuroglia crosstalk to microglia. a, c Immunofluorescence image of BV2 cell for p-STAT1 for BSA, 300 µm PA-BSA, conditioned media from palmitate-treated neuro2a cell, 100 IU interferon-β, and scatter plot with the bar graph showing the p-STAT1 mean fluorescent intensity as the percentage of control. b, d Immunofluorescence image of BV2 cell for CD-16 for BSA, 300 µm PA-BSA, conditioned media from palmitate-treated neuro2a cell, 100 IU interferon-β, and scatter plot with the bar graph showing the p-STAT1 mean fluorescent intensity as the percentage of control. Data are represented as mean ± SEM. Comparisons between groups were done by using a two-tailed unpaired t-test. p-value on the figure indicates significance, *p < 0.05, **p < 0.01, ***p < 0.001 vs. BSA-treated group (in BV2 cells)
The above results convey the unprecedented role of the cGAS/STING pathway in the metabolic stress associated with central dysfunction. Further, we selected C-176 for animal study to evaluate its neuroprotective potential.
STING inhibition improved cognitive performance in diabetic mice
In the present study, animals under HFD + STZ were further divided into groups, one continued on HFD with vehicle administration and another received C-176 at 4 mg/kg dose. The timeline of the study is illustrated in (Fig. 11a). HFD + STZ administration animals have shown a significant increase in body weight (p < 0.001, 42%), whereas C-176 treatment did not bring any significant decrease (p > 0.05) in body weight (Fig. 11b). The C-176 brought a significant decrease in IPTT (p < 0.05) and OGTT (p < 0.01) profiles, which demonstrates the improvement in insulin sensitivity and glucose tolerance (Fig. 11c, d). The diabetic group of animals has shown a significant increase in plasma FBG (p < 0.001, 239%), TC (p < 0.001, 234%), and TG (p < 0.001, 77%) compared to NPD-fed animals. A significant change in FBG (p < 0.05, 26%), TC (p < 0.05, 28%), and TG (p < 0.01, 29%) was observed in the C-176-treated group compared to diabetics (Fig. 11e, f, g). Previously, cGAS/STING signaling active involvement in NAFLD, liver cirrhosis, and other related metabolic syndromes was explored. Hence, it could be inferred that STING inhibition in peripheral organs might be responsible for improved lipid profile in C-176-treated animals.
C-176 treatment effect on the metabolic parameter in T2DM mice. a Study design and the timeline for C-176 treatment study; b body weight change; c, d IPTT and OGTT between NC, T2DM, and T2DM + C-176 (n = 6) between 0 and 120 min. e, f, g Effect of C-176 treatment on plasma glucose, total cholesterol, and triglycerides level. h, i, j Effect of C-176 treatment on plasma TNF- α, IL-6, and IL-4 level. Data are represented as mean ± SEM. Comparisons between groups were done by using two-way ANOVA and one-way ANOVA followed by “Bonferroni’s multiple comparisons post hoc tests.” p-value on the figure indicates significance, ***p < 0.001 vs. NC, and #p < 0.05, ##p < 0.01 vs. T2DM group
Diabetic animals also exhibited a significant increase in plasma proinflammatory cytokines such as TNF-a (p < 0.001, 231%), IL-6 (p < 0.001, 383%), and a decrease in anti-inflammatory cytokines IL-4 (p < 0.001, 61%) compared to non-diabetics. C-176 treatment significantly improved the plasma inflammatory profile by exhibiting a decrease in TNF-a (p < 0.01, 58%) and IL-6 (p < 0.01, 57%) and an increase in IL-4 (p < 0.05, 84%) levels compared to diabetics (Fig. 11h, i, j).
The neurobehavioral assessment demonstrated that C-176-treated animals showed a significant improvement in spatial working memory in NAET in Y-Maze (p < 0.01) and exploratory behavior or episodic memory (p < 0.05) in the NORT test as well (Fig. 12a, b). C-176-treated group of animals showed improved spatial learning (p < 0.01) and memory consolidation in terms of a decrease in escape latency (p < 0.01) and an increase in the number of platform crossing (p < 0.01) in the MWM test (Fig. 12c, d, e, f, and g).
C-176 treatment effect on behavioral performance. a Scatter plot with a bar graph showing the novel arm entry test in Y-Maze (n = 9), b bar graph showing the percentage time spent in novel arm relative to familiar arm in the NORT test (n = 7). Graphical representation of cognitive performance in MWM as c escape latency for days 1–2 representing task learning (visible platform). d Escape latency for days 3–7 representing spatial learning (invisible platform) and e, f escape latency and platform crossing on probe trial day representing consolidated memory (n = 9). g Representative track plot displaying swim patterns during memory probe trial 24 h after the last training session. Comparisons between groups were done by using one-way ANOVA followed by “Bonferroni’s multiple comparisons post hoc tests.” p-value on the figure indicates significance, *p < 0.05, **p < 0.01 vs. familiar object in NORT; **p < 0.01, **p < 0.001 vs. NC; and ##p < 0.01 vs. T2DM
STING inhibition alleviated the expression of the Type-1 interferon response pathway
We have carried out the western blot study to observe relative changes in protein expression involved in Type-1 IFN response. C-176 has brought a significant decrease in the p-STING (p < 0.01, 51%) (p < 0.01, 61%) in both hippocampus and cortex tissue. The immunofluorescent study has also shown a significant decrease in the p-STING fluorescence intensity in the hippocampus (p < 0.001, 49%) and cortex (p < 0.01, 55%) in the C-176-treated brain compared to the diabetic brain. The C-176-treated group has also shown a decrease in the protein expression of p-IRF3 (p < 0.001, 59%) (p < 0.01, 42%), and p-TBK (p < 0.01, 39%) (p < 0.05, 51%) in the hippocampus and cortex respectively compared to the diabetic brain. The C-176-treated brain also showed a significant decrease in p-NF-Kβ expression (p < 0.01, 46%) (p < 0.05, 37%) in the hippocampus and cortex which was found to be elevated (p < 0.001, 239%) (p < 0.001, 381%) in the diabetic brain compared to normal compared to diabetic animals (Fig. 13).
C-176 effect on p-STING, p-IRF3, p-TBK, and p-NF-Κβ protein expression. a, b Western blot image of p-STING, p-IRF3, p-TBK, and p-NF-Κβ in the hippocampus and cortex. c, d Scatter plot with the bar graph showing the densitometric analysis of blot. e, f Immunofluorescence image of p-STING in the CA1 area of the hippocampus and layer II of cortex tissue. g Scatter plot with bar graph representing the mean fluorescent intensity of p-STING in hippocampus and cortex as the percentage of control. Data are represented as mean ± SEM. Comparisons between groups were done by using one-way ANOVA followed by “Bonferroni’s multiple comparison post hoc test” (Fig. c, d) and two-tailed unpaired t-test (Fig. g). p-value on the figure indicates significance, **p < 0.01, ***p < 0.001 vs. NC and #p < 0.05, ##p < 0.01, ###p < 0.001 vs. T2DM
STING inhibition decreased the microglia activation and neuroinflammatory changes
We discussed, in the previous section, how cGAS/STING signaling eventually causes the release of IFN-β which leads to activation of neighbor microglial cells through p-STAT1. C-176 treatment has brought a significant decrease in the p-STAT1 expression both in the hippocampus (p < 0.05, 53%) and cortex (p < 0.05, 54%) tissue compared to the diabetic brain. The treated group also showed a decrease in IFN-β expression in the hippocampus (p < 0.05, 29%) and cortex (p < 0.05, 51%) (Fig. 14a, b, c, d). ELISA was also carried out for the quantification of IFN-β and TNF-α. The C-176-treated group showed a significant decrease in the IFN-β level (p < 0.01, 50%) (p < 0.05, 46%) and TNF-α (p < 0.01, 43%) (p < 0.01, 57%) in the hippocampus and cortex tissue compared to diabetic control (Fig. 14e, f).
C-176 alleviated the expression of IFN-β-mediated Type-1 IFN response. a, b Western blot image of p-STAT1 and IFN-β and their densitometric analysis in the hippocampus and cortex. c, d Densitometric analysis of p-STAT1 and IFN-β in the hippocampus and cortex. e, f IFN-β and TNF-α quantification by ELISA in the hippocampus and cortex tissue. g The mRNA fold changes in IFN-β, IL-6, IL-1β, CXCL10, and CCR7 by qPCR analysis in the hippocampus. Data are represented as mean ± SEM. Comparisons between groups were done by using one-way ANOVA followed by “Bonferroni’s multiple comparison post hoc tests.” The p-value on the figure indicates significance, **p < 0.01, ***p < 0.001 vs. NC and #p < 0.05, ##p < 0.01 vs. T2DM
Further, the qPCR study revealed a decrease in the relative mRNA level of IFN-β in the hippocampus (p < 0.010, 0.55-fold) and TNF-α (p < 0.001, 0.51-fold) in C-176-treated brain compared to diabetics. IL-6, IL-1β, and CXCL10 represent proinflammatory, whereas CCR7 represents the anti-inflammatory microglial state. The qPCR study revealed a significant increase in the relative mRNA level of IL-6, IL-1β, and CXCL10 in the hippocampus (p < 0.01, 3.78-fold; p < 0.01, 6.1-fold; p < 0.01, 3.78-fold) tissue compared to the normal control. C-176 treatment has brought a significant decrease in mRNA (p < 0.01, 0.57-fold; p < 0.05, 0.70-fold; p < 0.01, 0.61-fold) compared to diabetic brain.
The CCR7 mRNA level was found to be significantly decreased in the diabetic brain (p < 0.01, 0.83-fold) compared to the normal control depicting the loss of the neuroprotective phenotype of microglia. The C-176 administration did not bring any significant increase in the mRNA level of CCR7 (p > 0.05, 0.24-fold) compared to the diabetic brain (Fig. 14g).
We have also carried out the IF study of the brain section using CD16/IBA and CD206/IBA double labeling for microglia. The diabetic brain has shown a significant increase in CD-16 (p < 0.001, p < 0.001) and a decrease in CD206 (p < 0.001, p < 0.001) positive microglia expression in the diabetic brain compared to normal control. C-176 treatment has led to a significant decrease in CD-16 (p < 0.001, p < 0.01) and an increase in CD206 (p < 0.001, p < 0.001) positive microglia both in the hippocampus and cortex tissue compared to the diabetic brain (Fig. 15).
Effect of C-176 treatment on expression of CD16 and CD206 positive microglia. a, b Immunofluorescence image showing colocalization of CD16 with microglia (IBA) in CA1 area of the hippocampus and layers II–III of cortex under (40 × magnification with oil). c, d Immunofluorescence image showing colocalization of CD206 with microglia (IBA) in the hippocampus and cortex under (40 × magnification with oil). e, f Scatter plot with bar graph representing the CD16 and CD206 + ve cell in hippocampus and cortex as the percentage of control. Data are represented as mean ± SEM. Comparisons between groups were done by using one-way ANOVA followed by “Bonferroni’s multiple comparison post hoc tests.” The p-value on the figure indicates significance, **p < 0.01, ***p < 0.001 vs. NC and ###p < 0.001 vs. T2DM
Discussion
Diabetes-associated metabolic syndrome imparts decreased cognitive performance, increased incidence of glial activation, and neuroinflammation [47, 70,71,72,73]. HFD + STZ-induced animal models have exhibited cognitive decline and AD-like pathological hallmarks [45, 74, 75]. The metabolic disease-associated cognitive impairment has been linked with neuroinflammatory changes and synaptic weakening with underlying neuroimmune dyshomeostasis involving neurons and glia [24, 45, 68, 69]. Likewise, the present study explores the possibilities of the neuroimmune mechanism underpinning the diabetes-induced cognitive impairment and the outcome suggests that (i) cGAS/p-STING-mediated Type-1 IFN response has a causal relation to diabetes-associated central neuroinflammatory changes and cognitive impairment as increased cGAS, p-STING, p-TBK, p-IRF3, and IFN-β expression were observed; (ii) cytosolic presence of mtDNA led to the cGAS/p-STING-mediated sterile Type-1 IFN response activation; (iii) changes in mitochondrial dynamics under sustained metabolic stress might lead to the mtDNA leakage to the cytoplasm as elevated BAX and decreased TFAM expression were noted; (iv) the IFN-β/IFNAR/p-STAT1-mediated neuroglia interaction causing the microglia activation; (iv) C-176, a small molecule, restored both hippocampus- and cortex-dependent cognitive performance and decreased the microglia activation by mitigating the p-STING, p-TBK, p-IRF3, p-STAT1, and IFN-β level. Further C-176 treatment attenuated the proinflammatory microglia marker such as IL-1β, CXCL-10, IL-6, TNF-α, and CD-16. Therefore, the above results emphasize the significance of Type-1 IFN response in causing metabolic stress-associated brain changes. As diabetes progresses, sustained peripheral insulin resistance, poor glycemic control, BBB damage, decreased BDNF, brain insulin resistance, reduced capillary blood flow, neuroinflammation, and oxidative stress jeopardize cognitive function with characteristics of underlying plaque deposition, tau pathology, and synapse loss [76,77,78]. Previously, HFD-fed mice have shown an increase in amyloid plaque and phosphorylated-Tau deposit by increasing amyloid converting enzyme, BACE, and APP (amyloid precursor proteins) expression [46]. Consistent with the above, immunostaining revealed the p-tau presence in the HFD-fed brain compared to NPD-fed control (Fig. 1), impaired cognitive function (Fig. S1) (Fig. 12) with underlying decreased synaptophysin, a presynaptic, and PSD-95; a postsynaptic protein expression was observed in the hippocampus and cortex of diabetic animal compared to the normal control (Fig. 1). Later could be associated with glial activation causing elaborate surge of proinflammatory cytokines such as TNF-α (Fig. 14) and excitatory neurotransmitters damaging the synapse [79, 80]. Immunoblot and qPCR study revealed elevated cGAS/STING/TBK/IRF3 expression in the diabetic brain. The p-STING expression was more pronounced in the neuron compared to microglia and astrocyte of the diabetic brain (Fig. 3). It might signify the neuron being the ultimate resort of undergoing central dysfunction. However, the study does not claim or negate the involvement of glial cells.
Prior studies have emphasized that cytosolic DNA acts as the sensor for cGAS/p-STING activation under various stressors like TNF-α, cisplatin, palmitic acid, high glucose, lipopolysaccharide, ischemic reperfusion injury, and hypoxic insult besides aging [13, 15, 18, 50, 66, 81]. In the present study, we emphasized the fact that T2DM-associated metabolic stress also led to the mtDNA release which activates cGAS/STING-mediated Type-1 IFN response in the brain and the neuronal cell. The IF study revealed the cytosolic presence of dsDNA in both the cortex and hippocampus. Further, the qPCR result confirmed the nature of the DNA was of mitochondrial origin (Fig. 4). Moreover, the palmitate-mediated lipotoxic stress also led to the leakage of mtDNA to the cytosol from the mitochondrial-rich site in the neuro2a cells in Mitotracker-dsDNA colocalization study (Fig. 5). The comparative study of palmitate with CMA further emphasizes the fact that p-STING activation is via cGAS activation under lipotoxicity whereas the CMA-mediated p-STING activation was independent of cGAS activation.
The mtDNA leakage can be associated with various underlying reasons such as BAX-mediated pore formation [66], mtDNA packaging defect due to deficient TFAM (transcription factors A, mitochondria) function, mitochondrial oxidative stress [18, 34, 36, 38], decreased mitochondrial membrane potential, architectural damage of mitochondrial cristae, necroptotic executioner, i.e., MLKL-anchored [82, 83], amyloid plaque, and due to excessive TNF response too [15]. Diabetes being a chronic disease could propagate various mitochondrial stressors, and therefore, scrutinizing to single factor causing mtDNA release would be a predicament. Meanwhile, we observed an elevated Bax expression with decreased TFAM in both the hippocampus and cortex of the diabetic brain compared to normal control (Fig. 6). Besides, other concomitant factors might work coherently in causing mtDNA release. Additionally, the PA-BSA conjugate triggered increased cellular ROS, mitochondrial ROS, and alteration in MMPs (Ψm) in cells leading to mitochondrial stress (Figs. 5 and 9). The sustained mitochondrial stress might result in deficient TFAM expression in the cell as we have also seen decreased TFAM expression in the cell under lipotoxic stress (Fig. 6) [84, 85]. Further, the colocalization study of p-STING with Mitotracker has shown that the increase in p-STING expression corresponds with a concomitant decrease in Mitotracker expression (Fig. 6). STING upon activation translocates to the perinuclear space for executing its downstream function [66], which can be observed in our study, where p-STING concentrates toward perinuclear space under lipotoxic stress. Mitochondrial-deficient neuro2a cells upon EtBr exposure failed to show p-STING, p-TBK, and p-IRF expression even under lipotoxic stress, further championing the significance of mtDNA in Type-1 IFN response (Fig. 6). Mitoprotective agents could be beneficial in regulating excessive CGAS/STING activation. In the line, melatonin, a mitoprotective molecule, exhibited neuroprotection by decreasing the mtDNA release [18]. One of the limitations of the present study is that we have not been able to specify one prime reason behind mtDNA leakage to the cytoplasm. However, later would have been possible by employing knocking down or using the specific inhibitor. Therefore, it also opens the horizon for further investigation at the subcellular level. The cGAS/STING-mediated Type-1 IFN response is related to IFN-β release and its autocrine and paracrine effects. Later has been explored in various disease scenarios such as TBI-related neuroinflammation, cisplatin-induced tubular inflammation, rheumatoid arthritis, and other NDDs associated with increased IFN-β release. T2DM is a chronic metabolic disease that leads to neuroinflammation due to an imbalance in proinflammatory and anti-inflammatory cytokines. Changes in the metabolite profile and regression of peripheral inflammatory mediators due to BBB damage also led to brain inflammation. Earlier intracerebroventricular (ICV) administration of PA-BSA conjugate to the brain has brought the cognitive decline associated with insulin insensitivity and increased microglial and astrocytic reactivity mediated by increased TNF-α [47].
In the present study, we have seen a marked increase in the IFN-β level in both diabetic brain and supernatant of PA-BSA-exposed neuro2a cells (Fig. 7). Type-1 IFN response occurs via IFNAR as IFNAR knockout cells failed to show Type-1 IFN response [24]. Although we did not explore much on IFNAR except the qPCR study which shows a relative increase in IFNAR mRNA level in the diseased brain (Fig. 7), Nazmi et al. have discussed how chronic degenerating neurons in the prion disease model transforms microglia phenotype via STING-mediated Type-1 IFN response [24]. IFN-β conducts its response via STAT1 on microglia, the increased p-STAT1 expression, in the diseased brains. Moreover, colocalization study has shown a higher percentage of IBA colocalizing with p-STAT1 in the diseased brains, further leading to the release of proinflammatory cytokines (Fig. 8). Meanwhile, we should not overrule the scenario of p-STING colocalizing with microglia in the diabetic brain. PA-BSA has also shown a dose-dependent increase in cGAS/STING/TBK/IRF3/NF-κβ expression in BV2 cells (Fig. S3). BV2 cells have also exhibited increased cellular ROS, mitochondrial superoxide, and a decrease in MMP after exposure to PA-BSA-mediated lipotoxic stress. To delineate neuroglia crosstalk, conditioned media from palmitate-insulted neuro2a cells have also led to increase in the p-STAT1 expression in the BV2 cell marking the ongoing IFN-\(\beta\)-mediated JAK-STAT signaling (Figs. 9, 10). Additionally, CD16/32 and CD206, the two surface markers, are being attributed to different microglia/macrophage populations [86]. An increase in CD16 and a decrease in CD206 are associated with the inflammatory state of DAM or M1 microglia, whereas the increase in CD206 and decrease in CD16 are attributed to the protective state of DAM or M2 microglia perse [87, 88]. It can be noted that BV2 cell’s exposure to conditioned media from PA-BSA-insulted neuro2a cell has also increased the CD16/32 mean intensity characterizing the inflammatory microglia (Fig. 10). To investigate the STING inhibition on neuroprotective potential, C-176, a nitrofuran derivative, is one of the potential small molecules which have inhibited the STING-mediated IFN-β reporter activity. They have synthesized various analogs and assessed their STING inhibitory activity in both in vivo and in vitro setups [49]. C-176 has shown neuroprotection in the case of subarachnoid hemorrhage, MACAO, and traumatic brain injury. Besides, it has also mitigated cisplatin-induced tubular inflammation, obesity-associated liver disease, NAFLD, and liver steatosis by inhibiting p-STING-mediated downstream signaling. C-176 treatment has shown improvement in metabolic profile in terms of improved glucose tolerance and insulin sensitivity without lending any change in body weight and blood glucose. However, later also decreased the inflammatory cytokines (Fig. 11). It was noteworthy to see the improved learning and memory performance among C-176-treated animals signifying the importance of Type-1 IFN response in maintaining brain homeostasis.
The protein expression study has shown that C-176 treatment has decreased the p-STING, p-IRF, p-TBK, and p-NF- \(\kappa \beta\) expression in both the cortex and hippocampus (Figs. 11, 12).
The dichotomy around terming the disease-associated microglia has always been an inquisitive topic among researchers. New roadmaps are being proposed in recent publications to nominate microglia in different disease scenarios. Meanwhile, microglial polarization in terms of the M1/M2 paradigm where M1 promotes neuroinflammation and M2 is neuroprotection is also being extensively used and published. Our results have shown that C-176 treatment has decreased the expression of p-STAT1, IFN- \(\beta ,\) and TNF-α in both the cortex and hippocampus with a decrease in the mRNA fold of IFN-β as well (Fig. 14). Abolishing ER stress-mediated Type-1 IFN response has promoted M2 microglia and has inhibited T-cell infiltration, further providing neuroprotection in the case of TBI [89]. In ischemic stroke conditions, C-176 abolished the STING activation further decreasing the M1 and increasing the M2 microglia population providing neuroprotection [14]. In other instances, IFN-β/IFNAR response has been reported to be neuroprotective [90]. The IFN-β-mediated microglia activation promotes inflammatory signals via CXCL10. The diabetic brain has shown a marked increase in the relative mRNA level of CXCL10 and a decrease in CCR7 which contributes to neuroprotection. C-176 treatment prevented the restraining of the microglia population toward inflammatory phenotype as the relative decrease in mRNA level of CXCL10, IL-6, and IL-1β was also noted. On the other side, C-176-treated brains were found to be enriched with more neuroprotective microglia as the increase in relative mRNA level of CCR7 was reported. In addition, the C-176 treatment showed decreased CD16 + ve and increased CD206 + ve microglial population in both the cortex and hippocampus (Fig. 15). C-176 treatment by decreasing neuroinflammation caused the restoration of the cortex- and hippocampus-dependent cognitive performance.
To the best of our knowledge, we have been able to underline the significance of cGAS/p-STING-mediated Type-1 IFN response in the case of diabetic brain and neuroglia crosstalk under lipotoxic stress. Figure 16 briefly explains the mechanism and conclusion for the present research work. Meanwhile, the study also has its share of limitations: such as it discusses only the downstream effect of p-STING inhibition and does not cater to the upstream p-STING activation in detail. What if we can strengthen the mitochondria, will that be enough to restrain the elaboration of Type-1 IFN response? Type-1 IFN response is also responsible for the antiviral effect, and therefore, to what extent mitigation of IFN response is justified? In addition, TBK itself has been reported to be involved in autophagic loss and Tau deposit which opens a different perspective to see the entire neuronal health concerning tauopathy.
Mechanism of type 2 diabetes and lipotoxicity-induced neuroinflammation involving Type-1 IFN response. T2DM-like chronic disease-associated lipotoxic stress, insulin resistance, and free fatty acid (FFA) uptake led to the mitochondrial stress causing the mtDNA translocation to cytoplasm where it activates the cGAS/p-STING signaling. p-STNIG further activates p-TBK and p-IRF3. TBK may also contribute to the p-TAU deposit. The p-IRF3, after translocating to the nucleus, activates and promotes the transcription of ISGs causing the increased production of IFN-β. The released IFN-β may act via both autocrine and paracrine manner via IFNAR. The neuroglia crosstalk happens via IFN-β/IFNAR interaction, which transduces the incoming signaling via STAT-1, further increasing the transcription of ISGs via acting through interferon signaling responsive element (ISRE) and promoting the release of proinflammatory cytokines. The interaction also causes microglia activation termed disease-associated microglia (DAM). Metabolic stress also disturbs neuroimmune homeostasis in the brain. The activated microglia may cause synaptic damage, further weakening the signal transmission across the synapse. C-176 by inhibiting the p-STING alleviated the Type-1 IFN response and restored the brain function
Data availability
The data which supports this study is available on request from the corresponding author upon appropriate request.
References
Gustafson DR, McFarlane SI (2018) Epidemiology of type 2 diabetes and dementia. Type 2 Diabetes Dement 5–27. https://doi.org/10.1016/B978-0-12-809454-9.00002-0
Moran C, Beare R, Phan TG et al (2015) Type 2 diabetes mellitus and biomarkers of neurodegeneration. Neurology 85:1123–1130. https://doi.org/10.1212/WNL.0000000000001982
André C, Dinel A, Ferreira G, et al (2014) Brain, behavior, and immunity diet-induced obesity progressively alters cognition, anxiety-like behavior and lipopolysaccharide-induced depressive-like behavior: focus on brain indoleamine 2,3-dioxygenase activation 41:10–21. https://doi.org/10.1016/j.bbi.2014.03.012
Chakraborty A, Hegde S, Praharaj SK et al (2021) Age related prevalence of mild cognitive impairment in type 2 diabetes mellitus patients in the indian population and association of serum lipids with cognitive dysfunction. Front Endocrinol (Lausanne) 12. https://doi.org/10.3389/FENDO.2021.798652
Panyawattanakit C, Wongpradit W, Kanhasing R, Kulalert P (2022) Cognitive impairment and associated factors among older adults with diabetes in a Suburban Primary Health Center in Thailand. Dement Geriatr Cogn Disord 51:175–181. https://doi.org/10.1159/000524132
Lin CF, Liu HC, Lin SY (2022) Kidney function and risk of physical and cognitive impairment in older persons with type 2 diabetes at an outpatient clinic with geriatric assessment implementation. Diabetes, Metab Syndr Obes Targets Ther 15:79. https://doi.org/10.2147/DMSO.S341935
Junyi P, Siou L, Simeng W et al (2022) Pathogenesis and research progress of diabetes and cognitive impairment. J Clin Rev Case Reports 7:82. https://doi.org/10.33140/jcrc.07.05.02
Moran C, Phan TG, Chen J et al (2013) Brain atrophy in type 2 diabetesregional distribution and influence on cognition. Diabetes Care 36:4036–4042. https://doi.org/10.2337/DC13-0143
Biessels GJ (2013) Brain MRI correlates of cognitive dysfunction in type 2 diabetes: the needle recovered from the haystack? Diabetes Care 36:3855–3856. https://doi.org/10.2337/dc13-1501
Frison E, Proust-Lima C, Mangin JF et al (2021) Diabetes mellitus and cognition: pathway analysis in the MEMENTO Cohort. Neurology 97:E836–E848. https://doi.org/10.1212/WNL.0000000000012440
Rollins CPE, Gallino D, Kong V et al (2019) Contributions of a high-fat diet to Alzheimer’s disease-related decline: a longitudinal behavioural and structural neuroimaging study in mouse models. NeuroImage Clin 21:101606. https://doi.org/10.1016/j.nicl.2018.11.016
Jana A, Wang X, Leasure JW et al (2022) Increased Type I interferon signaling and brain endothelial barrier dysfunction in an experimental model of Alzheimer’s disease. Sci Reports 121(12):1–16. https://doi.org/10.1038/s41598-022-20889-y
Yuan L, Mao Y, Luo W et al (2017) cro Palmitic acid dysregulates the Hippo – YAP pathway and inhibits angiogenesis by inducing mitochondrial damage and activating the cytosolic DNA sensor cGAS – STING – IRF3 signaling mechanism. J Biol Chem 292:15002–15015. https://doi.org/10.1074/jbc.M117.804005
Kong L, Li W, Chang E et al (2022) mtDNA-STING axis mediates microglial polarization via IRF3/NF-κB signaling after ischemic stroke. Front Immunol 13. https://doi.org/10.3389/FIMMU.2022.860977
Willemsen J, Neuhoff MT, Hoyler T et al (2021) TNF leads to mtDNA release and cGAS/STING-dependent interferon responses that support inflammatory arthritis. Cell Rep 37:109977. https://doi.org/10.1016/j.celrep.2021.109977
Gamdzyk M, Doycheva DM, Araujo C et al (2020) cGAS/STING pathway activation contributes to delayed neurodegeneration in neonatal hypoxia-ischemia rat model: possible involvement of LINE-1. Mol Neurobiol 57:2600–2619. https://doi.org/10.1007/S12035-020-01904-7
Liao Y, Cheng J, Kong X et al (2020) HDAC3 inhibition ameliorates ischemia/reperfusion-induced brain injury by regulating the microglial cGAS-STING pathway. Theranostics 10:9644–9662. https://doi.org/10.7150/thno.47651
Jauhari A, Baranov SV, Suofu Y et al (2020) Melatonin inhibits cytosolic mitochondrial DNA-induced neuroinflammatory signaling in accelerated aging and neurodegeneration. J Clin Invest 130:3124–3136. https://doi.org/10.1172/JCI135026
Yu Y, Zhang Y, Zhao X et al (2019) STING-mediated inflammation in Kupffer cells contributes to progression of nonalcoholic steatohepatitis Find the latest version: STING-mediated inflammation in Kupffer cells contributes to progression of nonalcoholic steatohepatitis. 129:546–555. https://doi.org/10.1172/JCI121842
Taylor JM, Moore Z, Minter MR, Crack PJ (2018) Type-I interferon pathway in neuroinflammation and neurodegeneration: focus on Alzheimer’s disease.125:797–807. https://doi.org/10.1007/s00702-017-1745-4
Geng K, Ma X, Jiang Z et al (2023) High glucose-induced STING activation inhibits diabetic wound healing through promoting M1 polarization of macrophages. Cell Death Discov 91(9):1–16. https://doi.org/10.1038/s41420-023-01425-x
Ablasser A, Chen ZJ (2019) CGAS in action: Expanding roles in immunity and inflammation. Science 363:eaat8657. https://doi.org/10.1126/science.aat8657
Chung KW, Dhillon P, Huang S et al (2019) Mitochondrial damage and activation of the STING pathway lead to renal inflammation and fibrosis article mitochondrial damage and activation of the STING pathway lead to renal inflammation and fibrosis. Cell Metab 30:784-799.e5. https://doi.org/10.1016/j.cmet.2019.08.003
Nazmi A, Field RH, Griffin EW et al (2019) Chronic neurodegeneration induces type I interferon synthesis via STING, shaping microglial phenotype and accelerating disease progression. Glia 67:1254–1276. https://doi.org/10.1002/glia.23592
Piantadosi CA (2020) Mitochondrial DNA, oxidants, and innate immunity. Free Radic Biol Med.152:455–461. https://doi.org/10.1016/j.freeradbiomed.2020.01.013
Motwani M, Pesiridis S, Fitzgerald KA (2019) DNA sensing by the cGAS–STING pathway in health and disease. Nat Rev Genet 20:657–674. https://doi.org/10.1038/S41576-019-0151-1
Abreha MH, Ojelade S, Dammer EB et al (2021) TBK1 interacts with tau and enhances neurodegeneration in tauopathy. J Biol Chem 296:100760. https://doi.org/10.1016/j.jbc.2021.100760
Balka KR, Louis C, Saunders TL et al (2020) TBK1 and IKKε act redundantly to mediate STING-induced NF-κB responses in myeloid cells. Cell Rep 31:107492. https://doi.org/10.1016/j.celrep.2020.03.056
Pokatayev V, Hasin N, Chon H et al (2016) RNase H2 catalytic core Aicardi-Goutières syndrome–related mutant invokes cGAS–STING innate immune-sensing pathway in mice. J Exp Med 213:329–336. https://doi.org/10.1084/JEM.20151464
Rice GI, Forte GMA, Szynkiewicz M et al (2013) Assessment of interferon-related biomarkers in Aicardi-Goutières syndrome associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and ADAR: A case-control study. Lancet Neurol 12:1159–1169. https://doi.org/10.1016/S1474-4422(13)70258-8
Crow YJ (2015) Manel N (2015) Aicardi-Goutières syndrome and the type I interferonopathies. Nat Rev Immunol 157(15):429–440. https://doi.org/10.1038/nri3850
Preeti K, Sood A, Fernandes V (2021) Metabolic regulation of glia and their neuroinflammatory role in Alzheimer’s disease. Cell Mol Neurobiol.42. https://doi.org/10.1007/s10571-021-01147-7
Ho GT, Boyapati RK, Tamborska A, Dorward DA (2017) Advances in the understanding of mitochondrial DNA as a pathogenic factor in inflammatory diseases. F1000Res 6. https://doi.org/10.12688/F1000RESEARCH.10397.1
Pérez-Treviño P, Velásquez M, García N (2020) Mechanisms of mitochondrial DNA escape and its relationship with different metabolic diseases. Biochim Biophys Acta Mol Basis Dis 1866. https://doi.org/10.1016/J.BBADIS.2020.165761
Yuzefovych LV, Pastukh VM, Ruchko MV et al (2019) Plasma mitochondrial DNA is elevated in obese type 2 diabetes mellitus patients and correlates positively with insulin resistance. PLoS One 14. https://doi.org/10.1371/JOURNAL.PONE.0222278
Yuzefovych LV, Musiyenko SI, Wilson GL, Rachek LI (2013) Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS One 8. https://doi.org/10.1371/JOURNAL.PONE.0054059
Ruchko M, Gorodnya O, LeDoux SP et al (2005) Mitochondrial DNA damage triggers mitochondrial dysfunction and apoptosis in oxidant-challenged lung endothelial cells. Am J Physiol - Lung Cell Mol Physiol 288. https://doi.org/10.1152/AJPLUNG.00255.2004
West AP, Shadel GS (2017) Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat Rev Immunol 17:363–375. https://doi.org/10.1038/NRI.2017.21
Clausen BH, Lambertsen KL, Babcock AA et al (2008) Interleukin-1beta and tumor necrosis factor-alpha are expressed by different subsets of microglia and macrophages after ischemic stroke in mice. 18:1–18. https://doi.org/10.1186/1742-2094-5-46
Konno H, Yamauchi S, Berglund A et al (2018) Suppression of STING signaling through epigenetic silencing and missense mutation impedes DNA damage mediated cytokine production. Oncogene 37:2037–2051. https://doi.org/10.1038/s41388-017-0120-0
Peng Y, Zhuang J, Ying G et al (2020) Stimulator of IFN genes mediates neuroinflammatory injury by suppressing AMPK signal in experimental subarachnoid hemorrhage. J Neuroinflammation 17:1–20. https://doi.org/10.1186/s12974-020-01830-4
Kim DY, Kim SR, Jung UJ (2020) Myricitrin ameliorates hyperglycemia, glucose intolerance, hepatic steatosis, and inflammation in high-fat diet/streptozotocin-induced diabetic mice. Int J Mol Sci 21. https://doi.org/10.3390/ijms21051870
Li X yan, Lu S shuang, Wang H lun et al (2018) Effects of the fenugreek extracts on high-fat diet-fed and streptozotocin-induced type 2 diabetic mice. Anim Model Exp Med 1:68–73. https://doi.org/10.1002/ame2.12004
Barrière DA, Noll C, Roussy G et al (2018) Combination of high-fat/high-fructose diet and low-dose streptozotocin to model long-term type-2 diabetes complications. Sci Rep 8. https://doi.org/10.1038/s41598-017-18896-5
Preeti K, Fernandes V, Sood A et al (2023) Necrostatin-1S mitigates type-2 diabetes-associated cognitive decrement and lipotoxicity-induced neuro-microglia changes through p-RIPK-RIPK3-p-MLKL axis. Metab Brain Dis 38:1581–1612. https://doi.org/10.1007/S11011-023-01185-8
Kim JY, Lee HJ, Lee SJ et al (2017) Palmitic acid-BSA enhances amyloid-β production through GPR40-mediated dual pathways in neuronal cells: involvement of the Akt/mTOR/HIF-1α and Akt/NF-κB pathways. Sci Rep 7:1–16. https://doi.org/10.1038/s41598-017-04175-w
Melo HM, Seixas da Silva GDS, Sant’Ana MR et al (2020) Palmitate is increased in the cerebrospinal fluid of humans with obesity and induces memory impairment in mice via pro-inflammatory TNF-α. Cell Rep 30:2180–2194.e8. https://doi.org/10.1016/j.celrep.2020.01.072
Zang N, Cui C, Guo X et al (2022) cGAS-STING activation contributes to podocyte injury in diabetic kidney disease. iScience 25:105145. https://doi.org/10.1016/j.isci.2022.105145
Haag SM, Gulen MF, Reymond L et al (2018) Targeting STING with covalent small-molecule inhibitors. Nature 5597713(559):269–273. https://doi.org/10.1038/s41586-018-0287-8
du Sert NP, Hurst V, Ahluwalia A et al (2020) The arrive guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol 18:1–12. https://doi.org/10.1371/journal.pbio.3000410
Park KA, Jin Z, Lee JY et al (2020) Long-lasting exendin-4 fusion protein improves memory deficits in high-fat diet/streptozotocin-induced diabetic mice. Pharmaceutics 12:1–18. https://doi.org/10.3390/pharmaceutics12020159
Ding C, Song Z, Shen A et al (2020) Small molecules targeting the innate immune cGAS-STING-TBK1 signaling pathway. Acta Pharm Sin B 10:2272–2298. https://doi.org/10.1016/j.apsb.2020.03.001
Cao L, Xu E, Zheng R et al (2022) Traditional Chinese medicine Lingguizhugan decoction ameliorate HFD - induced hepatic - lipid deposition in mice by inhibiting STING - mediated inflammation in macrophages. Chin Med 1–16. https://doi.org/10.1186/s13020-021-00559-3
Bi X, Du C, Wang X et al (2021) Mitochondrial damage-induced innate immune activation in vascular smooth muscle cells promotes chronic kidney disease-associated plaque vulnerability. 2002738:1–18. https://doi.org/10.1002/advs.202002738
Rom S, Zuluaga-Ramirez V, Gajghate S et al (2019) Hyperglycemia-driven neuroinflammation compromises BBB leading to memory loss in both diabetes mellitus (DM) type 1 and type 2 mouse models. Mol Neurobiol 56:1883–1896. https://doi.org/10.1007/s12035-018-1195-5
Yang S, Lee DK, Shin J et al (2017) Nec- 1 alleviates cognitive impairment with reduction of A b and tau abnormalities in. 9:61–77. https://doi.org/10.15252/emmm.201606566
Johnson LA, Zuloaga KL, Kugelman TL et al (2016) Amelioration of metabolic syndrome-associated cognitive impairments in mice via a reduction in dietary fat content or infusion of non-diabetic plasma. EBioMedicine 3:26–42. https://doi.org/10.1016/j.ebiom.2015.12.008
Kodali M, Attaluri S, Madhu LN et al (2021) Metformin treatment in late middle age improves cognitive function with alleviation of microglial activation and enhancement of autophagy in the hippocampus. Aging Cell 20:e13277. https://doi.org/10.1111/acel.13277
Nunez J (2008) Morris water maze experiment. J Vis Exp. https://doi.org/10.3791/897
Marwarha G, Claycomb K, Schommer J et al (2016) Palmitate-induced endoplasmic reticulum stress and subsequent C/EBPα homologous protein activation attenuates leptin and insulin-like growth factor 1 expression in the brain. Cell Signal 28:1789. https://doi.org/10.1016/J.CELLSIG.2016.08.012
Urso CJ, Zhou H (2021) Palmitic acid lipotoxicity in microglia cells is ameliorated by unsaturated fatty acids. Int J Mol Sci 22:1–18. https://doi.org/10.3390/ijms22169093
Hu L, Zhang S, Wen H et al (2019) Melatonin decreases M1 polarization via attenuating mitochondrial oxidative damage depending on UCP2 pathway in prorenin-treated microglia. PLoS ONE 14:1–18. https://doi.org/10.1371/journal.pone.0212138
Khan I, Preeti K, Kumar R et al (2023) Piceatannol promotes neuroprotection by inducing mitophagy and mitobiogenesis in the experimental diabetic peripheral neuropathy and hyperglycemia-induced neurotoxicity. Int Immunopharmacol 116:109793. https://doi.org/10.1016/j.intimp.2023.109793
Khan I, Preeti K, Kumar R et al (2022) Activation of SIRT1 by silibinin improved mitochondrial health and alleviated the oxidative damage in experimental diabetic neuropathy and high glucose-mediated neurotoxicity. 0:1–17. https://doi.org/10.1080/13813455.2022.2108454
Warren EB, Aicher AE, Fessel JP, Konradi C (2017) Mitochondrial DNA depletion by ethidium bromide decreases neuronal mitochondrial creatine kinase: implications for striatal energy metabolism. PLoS One 12. https://doi.org/10.1371/journal.pone.0190456
Ouchi H, Hirokawa N, Maekawa H et al (2019) Mitochondrial damage causes inflammation via cGAS-STING signaling in acute kidney injury article mitochondrial damage causes inflammation via cGAS-STING signaling in acute kidney injury. Cell Rep 29:1261-1273.e6. https://doi.org/10.1016/j.celrep.2019.09.050
Bai J, Cervantes C, Liu J et al (2017) DsbA-L prevents obesity-induced inflammation and insulin resistance by suppressing the mtDNA release-activated cGAS-cGAMP-STING pathway. Proc Natl Acad Sci U S A 114:12196–12201. https://doi.org/10.1073/pnas.1708744114
Fernandes V, Preeti K, Sood A et al (2023) Neuroepigenetic changes in DNA methylation affecting diabetes-induced cognitive impairment. Cell Mol Neurobiol 43:2005–2020. https://doi.org/10.1007/s10571-022-01278-5
Sood A, Fernandes V, Preeti K et al (2023) Fingolimod alleviates cognitive deficit in type 2 diabetes by promoting microglial M2 polarization via the pSTAT3-jmjd3 axis. Mol Neurobiol 60:901–922. https://doi.org/10.1007/s12035-022-03120-x
De CP, Matarese G (2018) Neuropharmacology leptin and ghrelin: sewing metabolism onto neurodegeneration. Neuropharmacology 136:307–316. https://doi.org/10.1016/j.neuropharm.2017.12.025
Wijesekara N, Araujo R, De FFG, Fraser PE (2018) Neuropharmacology Impaired peripheral glucose homeostasis and Alzheimer ’ s disease. Neuropharmacology 136:172–181. https://doi.org/10.1016/j.neuropharm.2017.11.027
Duarte AI, Santos MS, Oliveira CR, Moreira PI (2018) Neuropharmacology brain insulin signalling, glucose metabolism and females ’ reproductive aging: a dangerous triad in Alzheimer’s disease. Neuropharmacology 136:223–242. https://doi.org/10.1016/j.neuropharm.2018.01.044
Vieira MNN, Lima-filho RAS, De FFG (2018) Neuropharmacology connecting Alzheimer’s disease to diabetes: underlying mechanisms and potential therapeutic targets. Neuropharmacology 136:160–171. https://doi.org/10.1016/j.neuropharm.2017.11.014
Takeda S, Sato N, Uchio-Yamada K et al (2010) Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Aβ deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A 107:7036–7041. https://doi.org/10.1073/pnas.1000645107
Jeon BT, Jeong EA, Shin HJ et al (2012) Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes 61:1444–1454. https://doi.org/10.2337/DB11-1498
Neumann K, Rojo L, Navarrete L et al (2008) Insulin resistance and Alzheimer’s disease: molecular links & clinical implications. Curr Alzheimer Res 5:438–447. https://doi.org/10.2174/156720508785908919
Keller JN, Schmitt FA, Scheff SW et al (2005) Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology 64:1152–1156. https://doi.org/10.1212/01.wnl.0000156156.13641.ba
Riederer P, Korczyn AD, Ali SS et al (2017) The diabetic brain and cognition. J Neural Transm 124:1431–1454. https://doi.org/10.1007/s00702-017-1763-2
Santello M, Toni N, Volterra A (2019) Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci 22:154–166. https://doi.org/10.1038/s41593-018-0325-8
Greenhalgh AD, David S, Bennett FC (2020) Immune cell regulation of glia during CNS injury and disease. Nat Rev Neurosci 21:139–152. https://doi.org/10.1038/s41583-020-0263-9
Guo Y, Gu R, Gan D et al (2020) Mitochondrial DNA drives noncanonical inflammation activation via cGAS–STING signaling pathway in retinal microvascular endothelial cells. Cell Commun Signal 18:1–12. https://doi.org/10.1186/s12964-020-00637-3
Sarhan J, Liu BC, Muendlein HI et al (2019) Constitutive interferon signaling maintains critical threshold of MLKL expression to license necroptosis. Cell Death Differ 332–347. https://doi.org/10.1038/s41418-018-0122-7
Yang Y, Wu M, Cao D et al (2021) ZBP1-MLKL necroptotic signaling potentiates radiation-induced antitumor immunity via intratumoral STING pathway activation. Sci Adv 7:1–16. https://doi.org/10.1126/sciadv.abf6290
Yuzefovych L, Wilson G, Rachek L (2010) Different effects of oleate vs palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: Role of oxidative stress. Am J Physiol-Endocrinol Metab 299. https://doi.org/10.1152/AJPENDO.00238.2010
Viader A, Sasaki Y, Kim S et al (2013) Aberrant schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy. Neuron 77:886–898. https://doi.org/10.1016/j.neuron.2013.01.012
Jurga AM, Paleczna M, Kuter KZ (2020) Overview of general and discriminating markers of differential microglia phenotypes. Front Cell Neurosci 14:1–18. https://doi.org/10.3389/fncel.2020.00198
Chang CF, Wan J, Li Q et al (2017) Alternative activation-skewed microglia/macrophages promote hematoma resolution in experimental intracerebral hemorrhage. Neurobiol Dis 103:54–69. https://doi.org/10.1016/J.NBD.2017.03.016
Yang J, Zhao Y, Zhang L et al (2018) RIPK3/MLKL-mediated neuronal necroptosis modulates the M1/M2 polarization of microglia/macrophages in the ischemic cortex. 2622–2635. https://doi.org/10.1093/cercor/bhy089
Sen T, Saha P, Gupta R et al (2020) Aberrant ER stress induced neuronal-IFNβ elicits white matter injury due to microglial activation and T-cell infiltration after TBI. J Neurosci 40:424–446. https://doi.org/10.1523/JNEUROSCI.0718-19.2019
Gilli F, Valentino P, Caldano M et al (2008) Expression and regulation of IFNalpha/beta receptor in IFNbeta-treated patients with multiple sclerosis. Neurology 71:1940–1947. https://doi.org/10.1212/01.WNL.0000327340.50284.8D
Acknowledgements
The authors would like to acknowledge the financial support provided by the Department of Pharmacology and Toxicology, NIPER Hyderabad, and the Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India, for carrying out this work.
Funding
This work is supported by the National Institute of Pharmaceutical Education and Research, Hyderabad, and the Department of Pharmaceuticals, Ministry of Chemical and Fertilizers, Govt. of India.
Author information
Authors and Affiliations
Contributions
All authors take responsibility for the integrity and accuracy of this manuscript. KP, DKK, and SBS contributed to the conceptualization, designing, and seamless execution of the research; KP performed studies, evaluated data, and wrote the manuscript; AS, VF, and IK performed studies, evaluated data, and reviewed the manuscript; DKK and SBS reviewed, edited the manuscript, and supervised the research work.
Corresponding authors
Ethics declarations
Ethics approval
This present study was conducted under the regulation of the Committee for Control and Supervision of Experiments on Animals (CCSEA) and Institutional Animal Ethical Committee (IAEC)-NIPER-Hyderabad Telangana, India, and according to Animal Research Reporting of In Vivo Experiments (ARRIVE) guidelines [50] after getting approval of protocol no NIP/10/2019/PC/343 and NIP/05/2020/PC/385.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Preeti, K., Sood, A., Fernandes, V. et al. Experimental Type 2 diabetes and lipotoxicity-associated neuroinflammation involve mitochondrial DNA-mediated cGAS/STING axis: implication of Type-1 interferon response in cognitive impairment. Mol Neurobiol 61, 6217–6244 (2024). https://doi.org/10.1007/s12035-024-03933-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-024-03933-y