Abstract
Sepsis is a life-threatening organ dysfunction that is caused by a dysregulated host response to infection. Surviving patients have cognitive and memory damage that started during sepsis. These neurologic damages have been associated with increased BBB permeability and microglial activation. However, a few discrete studies have seen over the years pointing to the potential role of astrocytes in the pathophysiology of neurological damage after sepsis. The purpose of this article is to review information on the potential role of astrocytes during sepsis, as well as to provoke further studies in this area. These published articles show astrocytic activation after sepsis; they also evidence the release of inflammatory mediators by these cells. In this sense, the role of astrocytes should be better elucidated during sepsis progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection [1]. This syndrome frequently affects individuals in intensive care units around the world and presents high rates of mortality and morbidity, with a prevalence of up to 60% in hospitalized patients [2]. In sepsis, the inflammatory response is amplified in such a way that it produces damage to organs other than those affected by the initial infection, and the brain is among those affected, which generates acute and long-term neurological changes [3]. For years, studies have associated the neurological damage in sepsis with an increased permeability of brain barriers caused by the exacerbated release of pro-inflammatory cytokines and peripheral oxidative stress [4], and this would activate immune cells in the brain, such as microglia [5].
Brain signaling can occur both as a response to invasion by microorganisms and in the absence of an infectious agent, as observed in sepsis [6]. Pro-inflammatory cytokines, reactive oxygen/nitrogen species, and patterns associated to tissue damage are among the molecules capable of upregulating brain cells by migrating from periphery through the meninges and brain barriers that protect the CNS; thus, the inflamed brain barriers lose their function of high selectivity, facilitating the influx of toxic molecules into the brain [3]. When passing through brain barriers, such molecules face the brain defense lines, which deposit in the microglia, an inflammatory and precursor cell of macrophages, a “saving” function for neurons. Consequently, microglia are activated and secrete inflammatory mediators, such as cytokines and reactive oxygen species, within the brain parenchyma to combat pathogens and restore homeostasis [7].
However, the mediators released by microglia may play a harmful role, especially in diseases where peripheral inflammation is chronic, sustained, or exacerbated [8]. In this sense, microglia-induced inflammation will activate the cells that compose the brain barriers, thus maintaining their high permeability to peripheral blood and restarting a cell activation cycle [3].
Nevertheless, this cycle does not only involve microglial cells [9]. Possibly, and especially during sepsis, astrocytes are being neglected in terms of their role in protecting from or enhancing neuroinflammation. In a simplistic form, microglia and astrocytes should restore homeostasis; however, here, they lose their role as good guys and become villains. However, these are only cells that are hyper stimulated by the flood of inflammatory mediators from the inflamed peripheral tissues and in situ production by themselves. In this review, we intend to create a new perspective regarding the role of astrocytes during sepsis, encouraging research on this topic.
Astrocytes in the Healthy CNS
Astrocytes represent the most abundant glial cells in the human brain (Verkhratsky & Parpura, 2015). Astrocytes were believed to be a homogeneous population; however, this hypothesis is increasingly being refuted [10]. Like microglia, astrocytes can exhibit different morphologies [11], and based on their morphology and spatial organization, astrocytes are classified into two basic subtypes: protoplasmic and fibrous astrocytes [12]. Protoplasmic astrocytes are found throughout gray matter and show several highly branched and bushy processes, which extend their end-feet to blood vessels and enwrap them to form the glial limiting membrane, which is the outermost wall of the BBB. Fibrous astrocytes, on the other hand, are mainly located within the white matter and have a stellate shape with smooth and long processes [13]. This type of astrocyte expresses high levels of glial fibrillary acidic protein (GFAP) as compared with protoplasmic astrocytes [14].
Astrocytic cells, together with neurons, microglia, pericytes, endothelial cells and the basement membrane, form the neurovascular unit, a structure that involves multicellular relationships to establish a functional coupling between the brain and blood vessels [15]. Astrocytes exhibit different activities to maintain the normal functioning of the CNS, for example, provide almost complete coverage of the cerebral vasculature [16]. Also, with the release of prostaglandins (PGE), nitric oxide (NO), and arachidonic acid (AA), they can control the local blood flow of the CNS [17]. Its role in the metabolism is related to its ability to respond to neuronal activity, being able to increase the rate of glucose uptake, glycolysis, and lactate release in the extracellular space, contributing to neuronal function [18].
Despite the high metabolic activity, astrocytes use at most 15% of the total brain energy in the form of glucose. In fact, the rates of glucose uptake and glycolysis are high; however, as the oxidative phosphorylation rates are low compared to the astrocytic rates, astrocytes serve as a source of lactate production. The lactate is the main substrate for neuronal functioning during cerebral activation, and through the astrocyte-neuron lactate shuttle [19, 20].
In addition to lactate release, astrocytes are part of the tripartite synapses, which are synapses composed of two neurons and one astrocyte. These cells act synergistically with a functional unit for the functioning of plasticity and adequate for the release of neurotransmitters [12, 21].
Astrocytes in Pre-clinical Models of Sepsis
One of the most used animal models of human disease for the study of sepsis is the cecal ligation and perforation model (CLP) [22]. Briefly, a 3-cm midline laparotomy was performed to expose the cecum and adjoining intestine. The cecum was tightly ligated with a 3–0 silk suture in the middle of its length (below the ileocecal valve), perforated once with a 14-gauge needle, squeezed gently to extrude a small amount of feces through the perforation site, and returned to the peritoneal cavity, and the laparotomy was closed with 4–0 silk sutures [23]. This model is well accepted in the international literature for reproducing polymicrobial sepsis and allowing the induction of different degrees of severity, similarly to what is found in humans. Also, this model is effective to study neurological dysfunction during and after sepsis [24].
Astrocytes from CLP animals display an increased gene expression of pro-inflammatory cytokines, such as tumor necrosis factor- α (TNF-α), interleukin (IL)-1β, IL-6, IL-18, monocyte chemoattractant protein-1 (MCP-1), and cyclooxygenase-2 (COX-2), with a reduction in IL-10, accompanied by augmented levels of Toll-like receptor (TLR)-2 mRNA expression but no changes either in TLR4 or in vascular endothelial growth factor (VEGF) gene expression [25]. These results are like those found in rat brain tissue homogenate after CLP [26]. This cytokine profile has been closely related to the action of microglial cells [27], but interestingly, a study provided evidence that mediators released by peripheral blood mononuclear cells (PBMC) directly promote astrocyte reactivity during sepsis, independently of microglia [28], corroborating our previous finding that sepsis causes astrocytic activation [26].
The Table 1 summarizes the main findings concerning CLP-induced sepsis effects on astrocytes. The studies point the occurrence of astrocytic activation as early as 4 h after polymicrobial sepsis [29], which may persist for up to 15 days after sepsis [30]. The hippocampus was the most prevalent brain structure evaluated in the studies.
The animal model of sepsis by CLP generates endotoxemia in animals, especially due to the presence of gram-negative bacteria. Therefore, the administration of lipopolysaccharide (LPS) alone can mimic the inflammatory response that occurs in sepsis in animal models of human diseases. The effects of LPS on astrocytic activation in animal models are shown in Table 2. The systemic effects of LPS induce important changes in the brain of animals submitted to sepsis, and these studies demonstrate a more mechanistic evaluation involving the astrocytes, although they also show astrocytic activation and release of pro-inflammatory cytokines provoked by LPS injection.
However, most information about the role of astrocytes comes from studies in cell culture. However, in sepsis, these profiles are still little explored; Table 3 presents the studies carried out with astrocyte culture stimulated essentially with LPS. In addition to the astrocytic activation being evident, there is evidence of alteration in inflammatory mediators, which supports the hypothesis of a greater influence of these cells on the pathophysiology of sepsis. Recent, study has shown that neuroinflammation or ischemia induced two different types of reactive astrocytes, referred to as “A1” and “A2” [43]. It was shown that the A1 phenotype positively regulates different classical complement cascade genes and complement 3 (C3), which is harmful to neurons and oligodendrocytes. On the other hand, the A2 phenotype induced by ischemia positively regulates many neurotrophic factors that promote the survival and growth of neurons [44]. In fact, melatonin was able to reduce the number of A1 astrocytes and increase the number of A2 astrocytes in the periventricular white matter of neonatal septic rats [45].
Although there is a lot of evidence about the activation of astrocytic cells after sepsis or LPS stimulus, however, there is no direct evidence of the interaction between these cells’ with the BBB. The role of BBB dysfunction after sepsis has been extensively studied, and its impairment is known to play an important role in acute neuroinflammation and long-term damage after sepsis [3]. However, in some other publications, the authors strongly suggest that astrocytes release several substances that regulate the function of the BBB since it is formed [56, 57]. In this sense, probably, the changes in astrocytic profiles could influence mostly the BBB function after sepsis, leading to increase the neuroinflammation and the neuronal damage e after this the cognitive decline.
Astrocytes in the Human Septic Brain
Data about the astrocytes activation in humans are scarce; however, evidence suggests that, as occurs with microglia, astrocytes also change from a resting to an activated state when stimulated [58]. Human brain astrocytes are more diverse, more complex, and in greater numbers when compared to the rodent brain [59, 60].
In the post-mortem analysis of tissue from the right frontal lobe of 3 sepsis cases, an elevation of the GFAP marker was identified, thus indicating high astrocytic activity in the region when compared to controls [61]. Other studies with pediatric, adult, and elderly septic patients show in common an elevation of the s-100β marker at the expense not only of microglial activation, but also of astrocytic activation. This activation was associated with longer delirium duration, higher delirium severity, and in-hospital mortality [12, 62, 63].
Conclusion
In summary, there is robust preclinical evidence of astrocytic activation after sepsis and few clinical studies. The inflammatory stimulus generated by polymicrobial sepsis (CLP model) or by the LPS stimulus can generate systemic responses that influence the functioning of astrocytes in the brain (Fig. 1). However, little is known about the effects of this activation on the pathophysiology of sepsis. In view of the data presented here, the emerging need to establish the role of astrocytes in the pathophysiology of neurological dysfunction during sepsis becomes evident. Some questions still need scientific evidence, for example, how homeostatic functions of astrocytes are affected in sepsis, how occurs the astrocyte activation after BBB activation, and how astrocytes interact with the BBB after this. These and other questions may explain how astroglial activation can contribute to the long-term consequences of sepsis.
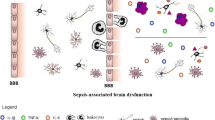
Role of astrocytes in sepsis development. During sepsis, pathogens, inflammatory and oxidative mediators are released in the blood. These mediators interact with the cells of blood brain barrier, increasing your selective permeability (1). Lipopolysaccharide (LPS) and other molecules entry in the brain parenchyma (2), lead to activation of glial cells, such as astrocytes and microglia. Activated astrocytes can activate microglia cells (3), besides release reactive oxygen species (4) and cytokines (5). These events culminate in neuronal damage (6). On the other hand, stressed neurons can also activate microglia (7) and increase de neuroinflammatory response in septic brain. There is also evidence of astrogliosis during sepsis (8). Thus, astrocytes may play a much more important role in sepsis than has been attributed to them
Data Availability
Not applicable.
References
Van Der Poll T, Van De Veerdonk FL, Scicluna BP, Netea MG (2017) The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol 17(7):407–420. https://doi.org/10.1038/nri.2017.36
Rudd KE, Johnson SC, Agesa KM et al (2020) Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. The Lancet 395:200–211. https://doi.org/10.1016/S0140-6736(19)32989-7
Danielski LG, Giustinadella A, Badawy M et al (2018) Brain barrier breakdown as a cause and consequence of neuroinflammation in sepsis. Mol Neurobiol 55:1045–1053. https://doi.org/10.1007/s12035-016-0356-7
Barichello T, Generoso JS, Collodel A et al (2021) The blood-brain barrier dysfunction in sepsis. Tissue Barriers 9. https://doi.org/10.1080/21688370.2020.1840912
Mazeraud A, Righy C, Bouchereau E et al (2020) Septic-associated encephalopathy: a comprehensive review. Neurotherapeutics 17:392–403. https://doi.org/10.1007/S13311-020-00862-1
Akrout N, Sharshar T, Annane D (2009) Mechanisms of brain signaling during sepsis. Curr Neuropharmacol 7:296–301. https://doi.org/10.2174/157015909790031175
Deng YY, Fang M, Zhu GF et al (2013) Role of microglia in the pathogenesis of sepsis-associated encephalopathy. CNS Neurol Disord - Drug Targets 12:720–725. https://doi.org/10.2174/18715273113126660178
Michels M, Steckert A V., Quevedo J et al (2015) Mechanisms of long-term cognitive dysfunction of sepsis: from blood-borne leukocytes to glial cells. Intensive Care Medicine Experimental 3. https://doi.org/10.1186/s40635-015-0066-x
Verkhratsky A, Ho MS, Vardjan N et al (2019) General pathophysiology of astroglia. Advances in Experimental Medicine and Biology. Springer, New York LLC, pp 149–179
Chaboub LS, Deneen B (2013) Astrocyte form and function in the developing central nervous system. Semin Pediatr Neurol 20:230–235. https://doi.org/10.1016/J.SPEN.2013.10.003
Reid JK, Kuipers HF (2021) She doesn’t even go here: the role of inflammatory astrocytes in CNS disorders. Front Cell Neurosci 15. https://doi.org/10.3389/FNCEL.2021.704884
Villarreal A, Vogel T (2021) Different flavors of astrocytes: revising the origins of astrocyte diversity and epigenetic signatures to understand heterogeneity after injury. Int J Mol Sci 22. https://doi.org/10.3390/IJMS22136867
Oberheim NA, Takano T, Han X et al (2009) Uniquely hominid features of adult human astrocytes. J Neurosci 29:3276–3287. https://doi.org/10.1523/JNEUROSCI.4707-08.2009
Escartin C, Galea E, Lakatos A et al (2021) Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci 24:312–325. https://doi.org/10.1038/S41593-020-00783-4
Schaeffer S, Iadecola C (2021) Revisiting the neurovascular unit. Nat Neurosci 24:1198–1209. https://doi.org/10.1038/S41593-021-00904-7
Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP (2010) The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58:1094–1103. https://doi.org/10.1002/GLIA.20990
Gordon GRJ, Mulligan SJ, MacVicar BA (2007) Astrocyte control of the cerebrovasculature. Glia 55:1214–1221. https://doi.org/10.1002/GLIA.20543
Bélanger M, Allaman I, Magistretti PJ (2011) Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14:724–738. https://doi.org/10.1016/J.CMET.2011.08.016
Bordone MP, Salman MM, Titus HE et al (2019) The energetic brain – a review from students to students. J Neurochem 151:139–165. https://doi.org/10.1111/JNC.14829
Itoh Y, Esaki T, Shimoji K et al (2003) Dichloroacetate effects on glucose and lactate oxidation by neurons and astroglia in vitro and on glucose utilization by brain in vivo. Proc Natl Acad Sci U S A 100:4879–4884. https://doi.org/10.1073/PNAS.0831078100
Araque A, Parpura V, Sanzgiri RP, Haydon PG (1999) Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22:208–215. https://doi.org/10.1016/S0166-2236(98)01349-6
Poli-de-Figueiredo LF, Garrido AG, Nakagawa N, Sannomiya P (2008) Experimental models of sepsis and their clinical relevance. Shock 30(Suppl 1):53–59. https://doi.org/10.1097/SHK.0B013E318181A343
Fink MP, Heard SO (1990) Current research review laboratory models of sepsis and septic shock.
Savi FF, de Oliveira A, de Medeiros GF et al (2021) What animal models can tell us about long-term cognitive dysfunction following sepsis: a systematic review. Neurosci Biobehav Rev 124:386–404. https://doi.org/10.1016/J.NEUBIOREV.2020.12.005
Bellaver B, dos Santos JP, Leffa DT et al (2018) Systemic inflammation as a driver of brain injury: the astrocyte as an emerging player. Mol Neurobiol 55:2685–2695. https://doi.org/10.1007/s12035-017-0526-2
Danielski LG, Della GA, Bonfante S et al (2020) NLRP3 Activation contributes to acute brain damage leading to memory impairment in sepsis-surviving rats. Mol Neurobiol 57:5247–5262. https://doi.org/10.1007/s12035-020-02089-9
Moraes CA, Zaverucha-Do-valle C, Fleurance R et al (2021) Neuroinflammation in sepsis: molecular pathways of microglia activation. Pharmaceuticals 14. https://doi.org/10.3390/PH14050416
Bellaver B, Rocha AS, Souza DG et al (2019) Activated peripheral blood mononuclear cell mediators trigger astrocyte reactivity. Brain Behav Immun 80:879–888. https://doi.org/10.1016/j.bbi.2019.05.041
Rotaru-Zavaleanu AD, Neacşu AI, Cojocaru A et al (2021) Heterogeneity in the number of astrocytes in the central nervous system after peritonitis. Curr Health Sci J 47:164–169. https://doi.org/10.12865/CHSJ.47.02.03
Tian J, Tai Y, Shi M et al (2020) Atorvastatin relieves cognitive disorder after sepsis through reverting inflammatory cytokines, oxidative stress, and neuronal apoptosis in hippocampus. Cell Mol Neurobiol 40:521–530. https://doi.org/10.1007/s10571-019-00750-z
Moraes CA, Santos G, Spohr TCLS et al (2015) Activated microglia-induced deficits in excitatory synapses through IL-1β: implications for cognitive impairment in sepsis. Mol Neurobiol 52:653–663. https://doi.org/10.1007/s12035-014-8868-5
Catalão CHR, Santos-Júnior NN, da Costa LHA et al (2017) Brain oxidative stress during experimental sepsis is attenuated by simvastatin administration. Mol Neurobiol 54:7008–7018. https://doi.org/10.1007/s12035-016-0218-3
Huang CT, Lue JH, Cheng TH, Tsai YJ (2020) Glycemic control with insulin attenuates sepsis-associated encephalopathy by inhibiting glial activation via the suppression of the nuclear factor kappa B and mitogen-activated protein kinase signaling pathways in septic rats. Brain Res 1738. https://doi.org/10.1016/j.brainres.2020.146822
Xiong CQ, Zhou HC, Wu J, Guo NZ (2019) The protective effects and the involved mechanisms of tanshinone IIA on sepsis-induced brain damage in mice. Inflammation 42:354–364. https://doi.org/10.1007/s10753-018-0899-z
Catalão CHR, Santos-Junior NN, da Costa LHA et al (2020) Simvastatin prevents long-term cognitive deficits in sepsis survivor rats by reducing neuroinflammation and neurodegeneration. Neurotox Res 38:871–886. https://doi.org/10.1007/s12640-020-00222-z
Montoya A, Elgueta D, Campos J et al (2019) Dopamine receptor D3 signalling in astrocytes promotes neuroinflammation. Journal of Neuroinflammation 16. https://doi.org/10.1186/S12974-019-1652-8
Alexander JJ, Jacob A, Cunningham P et al (2008) TNF is a key mediator of septic encephalopathy acting through its receptor, TNF receptor-1. Neurochem Int 52:447–456. https://doi.org/10.1016/j.neuint.2007.08.006
Beck-Schimmer B, Baumann L, Restin T et al (2017) Sevoflurane attenuates systemic inflammation compared with propofol, but does not modulate neuro-inflammation: a laboratory rat study. Eur J Anaesthesiol 34:764–775. https://doi.org/10.1097/EJA.0000000000000668
Semmler A, Okulla T, Sastre M et al (2005) Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat 30:144–157. https://doi.org/10.1016/J.JCHEMNEU.2005.07.003
Hasegawa-Ishii S, Inaba M, Umegaki H et al (2016) Endotoxemia-induced cytokine-mediated responses of hippocampal astrocytes transmitted by cells of the brain-immune interface. Sci Rep 6. https://doi.org/10.1038/srep25457
Lu Y, Yang Y, Peng Z et al (2020) Silencing IFNγ inhibits A1 astrocytes and attenuates neurogenesis decline and cognitive impairment in endotoxemia. Biochem Biophys Res Commun 533:1519–1526. https://doi.org/10.1016/j.bbrc.2020.10.084
Fu HQ, Yang T, Xiao W et al (2014) Prolonged neuroinflammation after lipopolysaccharide exposure in aged rats. PLoS One 9:106331. https://doi.org/10.1371/JOURNAL.PONE.0106331
Liddelow SA, Guttenplan KA, Clarke LE et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. https://doi.org/10.1038/NATURE21029
Fujita A, Yamaguchi H, Yamasaki R et al (2018) Connexin 30 deficiency attenuates A2 astrocyte responses and induces severe neurodegeneration in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride Parkinson’s disease animal model. J Neuroinflammation 15:1–20. https://doi.org/10.1186/S12974-018-1251-0/FIGURES/11
Jiang S, Wang H, Zhou Q et al (2021) Melatonin ameliorates axonal hypomyelination of periventricular white matter by transforming A1 to A2 astrocyte via JAK2/STAT3 pathway in septic neonatal rats. J Inflamm Res 14:5919–5937. https://doi.org/10.2147/JIR.S337499
Chen X-L, Wang Y, Peng W-W et al (2018) Effects of interleukin-6 and IL-6/AMPK signaling pathway on mitochondrial biogenesis and astrocytes viability under experimental septic condition. https://doi.org/10.1016/j.intimp.2018.04.020
Hua X, Wang Y, Yang C et al (2019) Effect of incubation with lipopolysaccharide and interferon-γ on reactive astrogliosis. J Integr Neurosci 18:415–421. https://doi.org/10.31083/J.JIN.2019.04.1138
Wang Q, Zhao Y, Sun M et al (2014) 2-Deoxy-d-glucose attenuates sevoflurane-induced neuroinflammation through nuclear factor-kappa B pathway in vitro. Toxicol In Vitro 28:1183–1189. https://doi.org/10.1016/J.TIV.2014.05.006
Fernandes A, Silva RFM, Falcão AS et al (2004) Cytokine production, glutamate release and cell death in rat cultured astrocytes treated with unconjugated bilirubin and LPS. J Neuroimmunol 153:64–75. https://doi.org/10.1016/j.jneuroim.2004.04.007
Rama Rao KV, Jayakumar AR, Tong X et al (2010) Marked potentiation of cell swelling by cytokines in ammonia-sensitized cultured astrocytes. J Neuroinflammation 7:66. https://doi.org/10.1186/1742-2094-7-66
Korcok J, Wu F, Tyml K et al (2002) Sepsis inhibits reduction of dehydroascorbic acid and accumulation of ascorbate in astroglial cultures: intracellular ascorbate depletion increases nitric oxide synthase induction and glutamate uptake inhibition. J Neurochem 81:185–193. https://doi.org/10.1046/j.1471-4159.2002.00814.x
Peng W, Huang J, Zheng Y et al (2019) UCP2 silencing aggravates mitochondrial dysfunction in astrocytes under septic conditions. Mol Med Rep 20:4459–4466. https://doi.org/10.3892/mmr.2019.10721
Sun YB, Zhao H, Mu DL et al (2019) Dexmedetomidine inhibits astrocyte pyroptosis and subsequently protects the brain in in vitro and in vivo models of sepsis. Cell Death Dis 10. https://doi.org/10.1038/s41419-019-1416-5
Falcão AS, Fernandes A, Brito MA et al (2005) Bilirubin-induced inflammatory response, glutamate release, and cell death in rat cortical astrocytes are enhanced in younger cells. Neurobiol Dis 20:199–206. https://doi.org/10.1016/j.nbd.2005.03.001
Bian Y, Zhao X, Li M et al (2013) Various roles of astrocytes during recovery from repeated exposure to different doses of lipopolysaccharide. Behav Brain Res 253:253–261. https://doi.org/10.1016/j.bbr.2013.07.028
Sweeney MD, Sagare AP, Zlokovic BV (2018) Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14:133–150
Daneman R, Prat A (2015) The blood–brain barrier. Cold Spring Harbor Perspectives in Biology 7. https://doi.org/10.1101/cshperspect.a020412
Shulyatnikova T, Verkhratsky A (2020) Astroglia in sepsis associated encephalopathy. Neurochem Res 45:83–99. https://doi.org/10.1007/S11064-019-02743-2
Oberheim NA, Goldman SA, Nedergaard M (2012) Heterogeneity of astrocytic form and function nancy. Methods Mol Biol 814:23–45. https://doi.org/10.1007/978-1-61779-452-0
Vasile F, Dossi E, Rouach N (2017) Human astrocytes : structure and functions in the healthy brain. Brain Struct Funct 222:2017–2029. https://doi.org/10.1007/s00429-017-1383-5
Warford J, Lamport A-C, Kennedy B, Easton AS (2017) Human brain chemokine and cytokine expression in sepsis: a report of three cases. Can J Neurol Sci/JCanadien des Sciences Neurologiques 44:96–104. https://doi.org/10.1017/cjn.2016.310
Khan BA, Perkins AJ, Prasad NK et al (2020) Biomarkers of delirium duration and delirium severity in the ICU. Crit Care Med 48:353–361. https://doi.org/10.1097/CCM.0000000000004139
Jorge-Ripper C, Alemán MR, Ros R et al (2017) Prognostic value of acute delirium recovery in older adults. Geriatr Gerontol Int 17:1161–1167. https://doi.org/10.1111/GGI.12842
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by Lucinéia Gainski Danielski and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Danielski, L.G., Giustina, A.D., Gava, F.F. et al. The Many Faces of Astrocytes in the Septic Brain. Mol Neurobiol 59, 7229–7235 (2022). https://doi.org/10.1007/s12035-022-03027-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03027-7