Abstract
TET enzymes (TET1-3) are dioxygenases that oxidize 5-methylcytosine (5mC) into 5-hydroxymethylcytosine (5hmC) and are involved in the DNA demethylation process. In line with the observed 5hmC abundance in the brain, Tet genes are highly transcribed, with Tet3 being the predominant member. We have previously shown that Tet3 conditional deletion in the brain of male mice was associated with anxiety-like behavior and impairment in hippocampal-dependent spatial orientation. In the current study, we addressed the role of Tet3 in female mice and its impact on behavior, using in vivo conditional and inducible deletion from post-mitotic neurons. Our results indicate that conditional and inducible deletion of Tet3 in female mice increases anxiety-like behavior and impairs both spatial orientation and short-term memory. At the molecular level, we identified upregulation of immediate-early genes, particularly Npas4, in both the dorsal and ventral hippocampus and in the prefrontal cortex. This study shows that deletion of Tet3 in female mice differentially affects behavioral dimensions as opposed to Tet3 deletion in males, highlighting the importance of studying both sexes in behavioral studies. Moreover, it contributes to expand the knowledge on the role of epigenetic regulators in brain function and behavioral outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epigenetic regulation represents a crucial set of mechanisms impacting gene regulation, neural differentiation, and synaptic plasticity, which ultimately affects a panoply of cognitive functions such as learning and memory [1]. Histone tail modifications, noncoding RNAs, and DNA (de)methylation are the main types of epigenetic regulatory mechanisms [2]. The ten-eleven translocation (TET) family of enzymes consists of three dioxygenases involved in the DNA demethylation process, converting 5-methylcytosine (5mC) into 5-hydroxymethylcytosine (5hmC) and subsequently 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC) [3, 4]. These modified nucleotides can then be substituted by unmodified cytosines through the action of thymine DNA glycosylase (TDG) and base excision repair (BER) [5, 6]. In addition to taking part in the DNA demethylation process, 5hmC is thought to be a stable modification that is highly prevalent in the brain and dynamically regulated by neural activity [7, 8].
In adult mice, TET enzymes have been implicated in learning and memory processes [9,10,11,12,13,14] [reviewed in 15]. Interestingly, Tet3 is the most transcribed member in the brain, but its role is still largely unexplored [16, 17]. The main limitation has been the lethal phenotype observed in the constitutive Tet3 knockout (KO) mouse model [18]. Nonetheless, it has been described that fear extinction leads to Tet3-mediated accumulation of 5hmC within the infralimbic prefrontal cortex [14]. Transcriptional analysis in the hippocampus 30 min after contextual fear conditioning showed that genes related to synaptic plasticity and memory are sensitive to Tet3 upregulation [19]. Regarding neurophysiology, it was shown that synaptic activity bi-directionally regulates neuronal Tet3 expression since Tet3 levels increase when synaptic activity is elevated with bicuculline and decrease when global synaptic activity is reduced in the presence of tetrodotoxin, and that TET3 regulates synaptic transmission via DNA oxidation and repair pathways in hippocampal neurons [20]. Our recent work showed that TET3 is present in mature neurons and is not detected in astrocytes of the mouse cortex and hippocampal brain regions [21]. Conditional and inducible deletion of Tet3 in neurons increases anxiety-like behavior in male mice, with a concomitant increase in corticosterone levels and impaired spatial orientation. Moreover, it modifies the expression of genes related to the hippotalamic-pituitary-adrenal (HPA) axis and neuronal activity [21].
During the last decades, mouse studies on anxiety and cognition have been performed mostly in males, with concomitant sex-biased interpretations. There are intrinsic biological variances between males and females, which are important to consider in brain (dys)function. The most evident is hormonal regulation; however, genetic and epigenetic factors are also key variables to consider when unraveling the sex-specific differences in brain function [22].
Here, we evaluated the impact of Tet3 neuronal deletion on the behavior of female mice. In this work, we showed that Tet3 conditional knockout (cKO) females presented an increased anxiety-like behavior, assessed by the elevated plus maze (EPM) and open-field (OF) tests, and an impairment in spatial orientation, demonstrated by a decrease in the use of hippocampal-dependent strategies in the Morris water maze (MWM), as we previously observed in male mice. Interestingly, we found significant sex-specific impairment of short-term memory in female mice with Tet3 deletion, not observed in Tet3 cKO males. At the molecular level, we observed increased expression of Npas4 and c-Fos immediate-early genes in both the dorsal and ventral hippocampus of Tet3 cKO females. Therefore, with this study, we reinforce the involvement of Tet3 in anxiety-like behavior and spatial orientation and suggest a new role for Tet3, specifically in the acquisition of short-term memory in female mice. This study contributes to a deeper understanding of sex-specific epigenetic regulation of brain function.
Materials and Methods
Animals
Experiments were performed as previously described [21] using mice with inducible Tet3 deletion in forebrain post-mitotic neurons, Tet3fl/fl; Camk2a-CreERT2 (Tet3 cKO) in C57BL/6 N&B6;129S6 mixed background, and the respective littermate controls – mice homozygous for the Tet3 floxed allele, but not carrying Cre-recombinase, Tet3fl/fl (Ctrl). Tet3fl/fl were kindly provided by Wolf Reik lab [23, 24]. Animals were genotyped by PCR analysis using genomic DNA and primers specific to Cre-recombinase and the floxed Tet3 allele. Detection of flox transgene was performed using a primer specific to the fragment, which allowed detection of the deleted or floxed allele (Supplementary Table S1a; Supplementary Fig. S2a). Experiments started at 6 weeks of age, and the mice were euthanized at 16 weeks of age.
Mice were housed (five per cage) under standard laboratory conditions (12 h light/12 h night cycles (8–20 h) at a temperature of 22–24 ℃, relative humidity of 55%, and with ad libitum access to water and food). Housing conditions were enriched with paper rolls and soft paper. All experiments were conducted in accordance with EU Directive 2010/63/EU and NIH guidelines on animal care and experimentation and approved by the Portuguese Government/Direção Geral de Alimentação e Veterinária (DGAV) with the project reference 0421/000/000/2017.
Tamoxifen Administration
Mice were injected intraperitoneally twice daily with 50 mg/kg of tamoxifen (Sigma, St. Louis, MO; T-5648), dissolved in corn oil (Sigma; C-8267) at 20 mg/ml, for 5 consecutive days, with 7 days break, followed by 5 additional consecutive days of tamoxifen administration.
Behavioral Analysis
The behavior testing was conducted 1 month after the last tamoxifen injection, during the light phase with habituation to testing rooms for 30 min before each test. The behavioral assessment was performed following this order: Elevated plus maze (EPM), open-field (OF), forced swimming test (FST), novel object recognition (NOR), and Morris water maze (MWM). All behavioral data analysis was performed with the researcher blinded to the genotype. A detailed explanation of each behavioral test is provided in the supplementary methods.
Determination of Estrous Cycle Stage
Vaginal smears were collected immediately after each behavioral test to determine the stage of the estrous cycle. Vaginal smears were performed by inserting a drop of sterile 0.9% saline solution in the vagina with the help of a 1 ml syringe, collecting the cell suspension by inserting a small plastic inoculation loop, and performing a smear into a glass slide. Smears were air-dried, fixed in 96% ethanol for 5 min, and stained using the Papanicolaou protocol. Briefly, smears were hydrated in tap water, stained with Harris hematoxylin for 1 min, rinsed in running tap water for 2 min, regressively stained by a single dip in alcohol–acid solution, rinsed in tap water for 2 min, dehydrated in 96% ethanol for 1 min, stained with orange G for 1 min, washed in 96% ethanol for 1 min, stained with Eosin Azure 50 for 1 min, dehydrated in a decreasing series of alcohol concentration, and cleared with xylene. Slides were analyzed under a light microscope, and the proportion of cornified epithelial cells, nucleated epithelial cells, and leukocytes was used for the determination of the estrous cycle phases [25].
Serum Corticosterone Levels
Blood samples for basal measurements of corticosterone were collected one week before the behavior assessment. Two independent collections were made at two different time points, 8 a.m. and 8 p.m., on two consecutive days. The blood was quickly collected after a small incision in the tail of the animals and then centrifuged at 13,000 rpm for 10 min, and the supernatant was removed and stored at − 80 ℃ until use. Total corticosterone levels (corticosterone free in circulation and bound to corticosteroid-binding globulin) in serum were measured by radioimmunoassay using the Corticosterone ELISA kit (Enzo Life Sciences, New York, USA), according to the manufacturer’s instructions.
DNA/RNA Extraction
Brains were obtained after deep anesthesia with a mixture of ketamine (75 mg/kg, i.p.; Imalgene 1000, Merial, EUA) and medetomidine (1 mg/kg, i.p.; Dorbene Vet, Pfizer, EUA), and transcardially perfused with 0.9% saline. Brain regions (PFC, dorsal/ventral hippocampus, and amygdala) were macrodissected by the same experienced researcher, and tissue samples were stored at − 80 ℃. Brain tissue was homogenized using Trizol® reagent (Invitrogen). Both nucleic acids were extracted according to the manufacturer’s instructions. RNA was treated with DNase I (Thermo Scientific), and a total of 500 ng of RNA was used for cDNA synthesis using the qScript cDNA SuperMix (Quanta Biosciences, USA).
qRT-PCR
cDNA was diluted 1:10 and used as a template for quantitative real-time PCR reactions using the 5 × HOT FIREPol EvaGreen qPCR supermix (Solis Biodyne) and primers designed to specifically amplify each mRNA of interest (Supplementary Table S1b). Amplification reactions were performed in duplicate and cycle threshold (Ct) data obtained in a 7500 Fast Real-time PCR System (Applied Biosystems). The relative abundance of each gene of interest was calculated on the basis of the ΔΔCt method [26]
3D Reconstruction of Neurons
Neuronal reconstruction was performed as previously described by Antunes et al. [21]. CA1 neurons were identified by their typical triangular soma-shape and apical dendrites extending toward the striatum radiatum. For each experimental group, three animals were assessed, and for each one, a minimum of three neurons per area were reconstructed and evaluated (a minimum of 9 neurons per area). Neurons were selected for reconstruction following these criteria: (i) identification of soma within the pyramidal layer of CA1; (ii) full impregnation along the entire length of the dendritic tree; (iii) no morphological changes attributable to incomplete dendritic impregnation of Golgi-Cox staining or truncated branches. The dendritic reconstruction was performed at 100 × (oil) magnification using a motorized microscope (BX51, Olympus) and Neurolucida software (Microbrightfield). The analyzed dendritic features were total length, number of endings and nodes, and Sholl analysis (number of dendrite intersections at radial intervals of 20 mm). Dendritic spine density (calculated as the number of spines/dendritic length) was evaluated in proximal and distal segments of dendrites. To identify changes in spine morphology, spines in the selected segments were classified into thin, mushroom, thick, or ramified [27] and the proportion of spines in each category was calculated for each neuron.
Statistical Analysis
A confidence interval of 95% was assumed for hypothesis testing. Normality was assumed for all continuous variables after testing with the Shapiro–Wilk test. Homoscedasticity and sphericity were assumed for all respective variables after testing with Levene’s and Mauchly’s tests, respectively. For the comparison of two means, the two-tailed unpaired Student’s t-test was carried out with the two-stage step-up method of Benjamini, Krieger, and Yekuteli used for multiple comparisons correction. For the comparison of means with two independent variables, a factorial analysis of variance (ANOVA) was performed; for one independent and one repeated measures variable, a mixed-design ANOVA was used and post-hoc analysis was performed using the Sidak correction. For the comparison of proportions, the two-sided chi-square test was carried out. Appropriate effect size measures were reported for all statistical tests. All statistical analyses were carried out using SPSS 22.0® or GraphPad Prism 8.0® and are detailed in Supplementary Table S2.
Results
Tet3 Ablation in Adult Mouse Neurons Does not Affect Tet1 nor Tet2 Expression
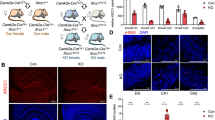
To characterize the function of Tet3 in mature neurons of adult female mice, we used a previously described Tet3 cKO mouse model [23, 24], in which exon 5 of the coding sequence of the Tet3 gene is flanked by LoxP sequences for Cre-induced site-specific recombination. We crossed this mouse line with a Camk2a-CreERT2 transgenic line that expresses a tamoxifen-inducible Cre-recombinase under the control of the mouse Camk2a (calcium/calmodulin-dependent protein kinase II alpha) promoter region. Tamoxifen administration at 6 weeks of age led to Tet3 downregulation in Camk2a-expressing neuronal populations in the cortex, hippocampus, amygdala, and other structures (Supplementary Fig. S1) [21, 28]. Tet3 deletion was assessed by genotyping PCR and RT-PCR, after behavioral analysis, at 16 weeks of age (Fig. 1a; Supplementary Fig. S2a,b).
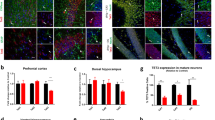
Tet3 cKO female mice showed increased anxiety-like behavior and no alterations in depressive-like behavior. (a) Scheme illustrating the protocol used to induce Tet3 deletion and the behavior paradigm timeline. Six-week-old mice were injected intraperitoneally with 50 mg/kg of tamoxifen twice a day for 5 consecutive days, with 7 days break, followed by injections for 5 additional consecutive days. Animals were submitted to behavioral testing 1 month after the last tamoxifen injection and euthanized after this assessment. (b, c) Anxiety-like behavior was tested both in the elevated plus maze (EPM) (b) and in the open-field test (OF) (c), showing increased anxiety-like behavior in Tet3 cKO female mice. (d) The presence of depressive-like behavior was assessed in the forced swimming test (FST) (n = 13–17 per group), showing no deficits in Tet3 cKO female mice. (e) Basal serum concentration of corticosteroids in control and Tet3 cKo mice, both in the morning and at night, revealed a significant increased production by Tet3 cKO mice (n = 9-10 per group). Quantifications are presented as the mean ± SEM. (b–d) Two-tailed Student’s t-test; *p < 0.05; (e) adjusted two-tailed Student’s t-test. *p < 0.05
Tet3 conditional knockout (Tet3 cKO) mice presented a significant reduction of Tet3 mRNA levels in all forebrain regions analyzed – prefrontal cortex, amygdala, dorsal, and ventral hippocampus (Supplementary Fig. S1a–d) (t-test, p < 0.05). Also, downregulation of Tet3 did not result in significant changes in Tet1 or Tet2 transcription levels in any of the brain regions analyzed (Supplementary Fig. S1a–d).
Tet3 Deletion in Female Mice Leads to Increased Anxiety-like Behavior, Impaired Spatial Orientation, and Impaired Short-term Memory
We tested the performance of Tet3 cKO female mice in different behavioral paradigms to assess its impact on emotional and cognitive domains (Fig. 1a). Two behavioral tests were performed to assess anxiety-like behavior – the elevated-plus maze (EPM) and the open-field (OF) – and to assess depressive-like behavior, the forced swimming test (FST). To evaluate cognitive performance, we used the Morris water maze (MWM) test and the novel object recognition (NOR) test. Importantly, females’ estrous cycle was assessed at the end of each behavioral paradigm, and all females (controls and cKOs) were in the luteal phase (Supplementary Fig. S3), suggesting an arrested estrous cycle as a consequence of tamoxifen administration.
Tet3 cKO mice spent less time in the open arms of the EPM when compared to the control group (t-test, p = 0.02; Fig. 1b). Also, in the OF, Tet3 cKO female mice displayed a decreased percentage of time spent in the center of the arena (t-test, p = 0.05; Fig. 1c), indicating an anxiety-like behavior in both tests. In the FST, Tet3 cKO and control mice displayed similar immobility levels (p = 0.824; Fig. 1d), indicating no alterations in learned helplessness. Taking into account the involvement of the HPA axis in the modulation of behavior, we determined the basal corticosterone levels in the serum of control and Tet3 cKO mice. We did not detect any differences at nadir, but in the zenith phase, Tet3 cKO female mice presented reduced corticosterone levels when compared to control mice (adjusted t-test, p < 0.01; Fig. 1e).
We also evaluated the impact of Tet3 conditional deletion on cognitive function. Experimental groups were subjected to the reference memory task of the MWM test, a task dependent on hippocampal function. Both Tet3 cKO and control mice were able to successfully learn the task, as confirmed by the decreasing latencies during the trials (Fig. 2a). In the probe trial, both control and Tet3 cKO mice presented similar performances, shown by the same preference (percentage of time swum) for the goal quadrant where the platform was located during the acquisition phase (t-test, p = 0.109) (Fig. 2b). However, analysis of the strategies adopted by mice to find the escape platform, divided into random searching/scanning (non-hippocampal strategies) or directed strategies (hippocampal strategies) [29], revealed that Tet3 cKO female mice used significantly less hippocampal-dependent strategies than control mice (chi-square test, p = 0.022; Fig. 2c), indicating deficits in spatial orientation.
Tet3 cKO female mice show impairment of spatial learning and short-term recognition memory. (a–c) Morris water maze test. Spatial acquisition performances were recorded during the 4-day training. (a) Escape latency. (b) Time in the target quadrant. (c) Cognitive strategies to reach the hidden platform during water maze learning. A schematic representation and color code for each group of strategy and the average prevalence by trial number are shown. Representation of the percentage of mice using directed strategies (hippocampal-dependent strategies) (n = 8–11 per group). Results indicate a decrease in hippocampal-dependent strategies in Tet3 cKO female mice. (d–f) Novel object recognition test. Animals were allowed to explore two identical objects for 10 min. (d) After an interval of 1 h in their home cages, the novel object was displaced to the opposite side of the box and mice were allowed to explore this new configuration, evaluating spatial recognition memory (displacement). (e) After 24 h, mice returned to the arena, where one of the familiar objects was replaced by a novel one, evaluating long-term memory. (f) After 24 h, two new objects were placed in the box and mice were allowed to explore them; 1 h after, one object was replaced by a novel one and the animals were placed in the arena to evaluate short-term memory (n = 14–18 per group). Results show impairment in short-term memory in Tet3 cKO female mice. Quantifications are presented as mean ± SEM. (a) Mixed ANOVA, genotype; (b) two-tailed Student’s t-test; (c) chi-square test; *p < 0.05; (d–f) two-tailed Student’s t-test, ***p < 0.001
To further clarify the impact of Tet3 deletion on hippocampal function, we performed the NOR test, which evaluates recognition memory. In this task, Tet3 cKO and control mice showed similar time exploring the object displaced after a short period of time (1 h) (t-test, p = 0.872; Fig. 2d), indicating normal object location memory. Moreover, Tet3 cKO and control mice dedicated similar percentages of time exploring the novel object displayed 24 h after being exposed to two equal objects (familiar), indicating no deficits in long-term object recognition memory (t-test, p = 0.708; Fig. 2e). After 24 h, two new objects were used and, 1 h after, replaced with a novel one to evaluate short-term memory. Here, Tet3 cKO females displayed a decreased discrimination percentage, indicating an impairment in short-term memory (t-test, p < 0.001; Fig. 2f).
Thus, our results indicate that conditional and inducible deletion of Tet3 in female mice increases anxiety-like behavior and impairs both spatial orientation and short-term memory. On the other hand, no alterations in learned helpless, object location, and recognition memory were observed.
Tet3 cKO Mice Displayed Increased Expression of Neuronal Activity-regulated Genes in Forebrain Regions
Considering our previous work which showed that Tet3 cKO male mice presented an increase in immediate-early genes (IEGs) expression [21], we evaluated whether Tet3 deletion in females resulted in the same deregulation. We analyzed the prefrontal cortex (PFC), dorsal and ventral hippocampus (dHip and vHip), and amygdala (Fig. 3a–d), observing the increased expression of several neuronal activity-regulated genes in Tet3 cKO female mice, particularly of Npas4 which was significantly increased in the prefrontal cortex and in the dorsal and ventral hippocampus (adjusted t-test, Npas4: PFC, p = 0.047; dHip, p = 0.047; vHip, p = 0.010; Fig. 3a–c), which is in line with our previous observations in the male hippocampus. In the PFC, Egr1 (a.k.a. Zif268) expression was also significantly increased in Tet3 cKO mice (adjusted t-test, Egr1: p = 0.045; Fig. 3a). In the ventral hippocampus, c-Fos was also significantly increased (adjusted t-test, c-fos: p = 0.007; Fig. 3c). In the amygdala, only Creb1 was significantly increased in Tet3 cKO females when compared to control littermates (adjusted t-test, p = 0.006).
Tet3 cKO female mice showed an increase in the expression of neuronal activity-regulated genes. (a–d) mRNA expression of immediate-early genes (IEGs) in forebrain regions was measured by qPCR in controls and Tet3 cKO animals in the (a) prefrontal cortex, (b) dorsal hippocampus, (c) ventral hippocampus, and (d) amygdala (n = 4–6 per group). Quantifications are presented as the mean ± SEM. (a–d) Two-tailed Student’s t-test; *p < 0.05, **p < 0.01
Taking into account the implication of dendritic and spine remodeling in the neuroplastic process, known to modulate cognitive performance, we analyzed neuronal and spines morphology in the dorsal and ventral CA1 hippocampus sub-regions. No differences were found between Tet3 cKO and control mice in the parameters evaluated, namely dendritic length, spine density, and type (Fig. 4a–h).
Three-dimensional morphometric analysis of Golgi-impregnated neurons of the dorsal and ventral hippocampus sub-regions reveals no major alterations by Tet3 conditional deletion in neuronal morphology and dendritic spine density. Overall, Tet3 cKO animals display no differences for all the parameters evaluated. (a,e) Total length; (b,f) sholl analysis; (c,g) global spine density; (d,h) spines type analysis. n = 9–15 neurons; 3 mice per group. Quantifications are presented as the mean ± SEM. (a,b,e,f) Factorial ANOVA; (c,g) two-tailed Student’s t-test; (d,h) factorial ANOVA, genotype
Discussion
This study addresses the effects of Tet3 ablation in mature neurons of female mice and adds novel evidence that supports Tet3 role as a modulator of complex behavior in the adult mouse brain, as we previously described in the male mice [21]. Specifically, we observed that ablation of Tet3 in female mature neurons leads to an increase in anxiety-like behavior and impairs spatial orientation, showing that, for these behavioral dimensions, Tet3 function is most likely gender-independent, as similar results were observed for male mice.
Previous studies have addressed the role of another member of the TET family, TET1, in emotional behavior. Although Tet1 KO mice showed normal anxiety and depression-related behaviors in one study [11], another study showed that selective deletion of Tet1 in nucleus accumbens (NAc) neurons produced antidepressant-like effects in several behavioral tests [30]. In contrast, in our model, in which we deleted Tet3 specifically in mature forebrain neurons, we observed an anxiety-like phenotype, which was independent of gender. A recent study on human TET3 deficiency, with genetic variants detected in eight families, showed common phenotypic features such as intellectual disability and/or global developmental delay, hypotonia, autistic traits, movement disorders, growth abnormalities, and facial dysmorphism [31]. Moreover, consistent with our results, anxiety and short-term memory deficits were reported in two mothers that were heterozygous for the TET3 mutated allele. Indeed, Tet3 cKO females showed a significant impairment in short-term recognition memory, contrary to what was observed in males. It is essential to note that while patients bearing homozygous TET3 mutations have much more severe phenotypes than Tet3 cKO mice (male and female), the mutation in patients was present throughout development, while loss of Tet3 in mice was only induced in adulthood. This indicates that additional roles of TET3 in development are also key for normal behavior in adult life.
Regarding object location and long-term recognition memories, Tet3 cKO females do not display any impairment, in agreement with our previous observations in males. Importantly and contrarily to long-term memory which requires gene expression and new protein synthesis, short-term memory only involves the alteration of preexistent proteins [32]. Thus, the loss of Tet3 as a DNA demethylation agent and the putative consequent alteration of gene expression cannot explain this impairment by itself. We speculate that Tet3 deletion, which starts several weeks before the behavioral assessment, can introduce permanent structural and/or functional neuronal alterations and consequently affect the short-term memory function. Since short-term memory is dependent on the PFC and hippocampal regions [33], we hypothesize that Tet3 deletion impairs neuronal structure and/or function in these regions, resulting in short-term memory deficits. Nevertheless, it remains elusive how males and females are differentially affected by Tet3 deletion, specifically in this type of memory. It is known that males and females present diverse sex-specific differences induced by environmental, hormonal, and (epi)genetic factors [22]; studies unraveling epigenetic mechanisms to explain differences in brain function are only now emerging. Particularly relevant for the short-term memory differences could be the variation of the DNA (de)methylation pattern according to gender. Indeed, it is already known that the female brain has higher levels of DNA methylation, with more methylated CpG sites than males [34]. Moreover, the opposite pattern for 5-hydroxylmethylcytosine is observed in the males’ prefrontal cortex, presenting higher 5hmC levels compared to females [22]. Therefore, the targets of the activity of TET enzymes, namely TET3, can be different in males and females, explaining the variance in short-term memory performance between sexes.
Regarding transcriptional regulation in Tet3 cKO mice, we investigated the expression of a variety of known neuronal activity regulatory genes. These genes are known to play important roles in diverse cellular processes such as neurotransmission, neuronal plasticity, learning, and memory [35, 36]. We have previously shown that Npas4 and c-Fos are upregulated in the hippocampus of Tet3 cKO male mice [21]. Notably, and contrary to Tet3 function in our model, Tet1 was shown to be a positive regulator of IEGs expression, with Tet1 KO animals showing a reduction in mRNA levels of neural-activity-regulated genes, namely Npas4, c-Fos, Arc, and Egr2 [11, 12]. Surprisingly, we observed that Tet3 deletion in post-mitotic forebrain neurons increases the expression of IEGs in the hippocampus (c-Fos and Npas4, as we previously observed in Tet3 cKO male mice), in the PFC (Npas4 and Egr1), and in the amygdala (Creb1). Given that anxiety in rodents involves strong interaction between brain regions such as PFC, amygdala, and hippocampus [37], we suggest that the anxiety-like behavior can be attributed to the hyperactivation of excitatory neurons in these regions, which is in accordance with the results showing that endogenous Tet3 negatively regulates excitatory synaptic transmission in young mice [38]. Thus, on the one hand, the aberrant increase in Npas4 and c-Fos transcript levels in the dorsal hippocampus of Tet3 cKO mice might lead to a dysregulation of neuronal activity and possibly explain the spatial orientation impairment; on the other hand, the impairment in the use of directed strategies reflects alteration of the goal-directed behavior and can be associated with the anxiety-like behavior, since the capacity to demonstrate goal-directed behavior involves suppression of emotional states, namely anxiety [39, 40]. Curiously, we observed decreased levels of corticosterone at the zenith time point in Tet3 cKO females, differently from our observations in males [21].
Throughout the behavioral assessment, we observed an arrest of mice in the estrous cycle, which is a probable consequence of tamoxifen administration. Indeed, CreERT2 recombinases are insensitive to endogenous estrogen, but activated by the synthetic estrogens receptor (ER) antagonist 4-hydroxytamoxifen (OHT), which is metabolized from 3,4-dihydroxy tamoxifen [33]; the OHT has a high affinity to the ERα and ERβ estrogen receptors, interfering with the normal estrogens binding to these receptors and with the estrous cycle regulation [41]. Thus, as all females were in the luteal phase of the estrous cycle, it was possible to perform the behavior characterization in a more homogeneous sample.
In conclusion, this work complements the previous observations in male mice with a conditional deletion of Tet3 in mature neurons and contributes to the current knowledge on the mechanisms modulating adult mouse behavior, specifically introducing new insights into females’ brain function regulation.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Feng J, Fouse S, Fan G (2007) Epigenetic regulation of neural gene expression and neuronal function. Pediatr Res 61(5 Pt 2):58r–63r. https://doi.org/10.1203/pdr.0b013e3180457635
Snijders C, Bassil KC, de Nijs L (2018) Methodologies of neuroepigenetic research: background, challenges and future perspectives. Prog Mol Biol Transl Sci 158:15–27. https://doi.org/10.1016/bs.pmbts.2018.04.009
Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y (2010) Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 466(7310):1129–1133. https://doi.org/10.1038/nature09303
Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, He C, Zhang Y (2011) Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 333(6047):1300–1303. https://doi.org/10.1126/science.1210597
Liu S, Wang J, Su Y, Guerrero C, Zeng Y, Mitra D, Brooks PJ, Fisher DE, Song H, Wang Y (2013) Quantitative assessment of Tet-induced oxidation products of 5-methylcytosine in cellular and tissue DNA. Nucleic Acids Res 41(13):6421–6429. https://doi.org/10.1093/nar/gkt360
Branco MR, Ficz G, Reik W (2011) Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nat Rev Genet 13(1):7–13. https://doi.org/10.1038/nrg3080
Hahn MA, Szabó PE, Pfeifer GP (2014) 5-Hydroxymethylcytosine: a stable or transient DNA modification? Genomics 104(5):314–323. https://doi.org/10.1016/j.ygeno.2014.08.015
Guo JU, Su Y, Zhong C, Ming GL, Song H (2011) Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell 145(3):423–434. https://doi.org/10.1016/j.cell.2011.03.022
Zhang RR, Cui QY, Murai K, Lim YC, Smith ZD, Jin S, Ye P, Rosa L, Lee YK, Wu HP, Liu W, Xu ZM, Yang L, Ding YQ, Tang F, Meissner A, Ding C, Shi Y, Xu GL (2013) Tet1 regulates adult hippocampal neurogenesis and cognition. Cell Stem Cell 13(2):237–245. https://doi.org/10.1016/j.stem.2013.05.006
Gontier G, Iyer M, Shea JM, Bieri G, Wheatley EG, Ramalho-Santos M, Villeda SA (2018) Tet2 rescues age-related regenerative decline and enhances cognitive function in the adult mouse brain. Cell Rep 22(8):1974–1981. https://doi.org/10.1016/j.celrep.2018.02.001
Rudenko A, Dawlaty MM, Seo J, Cheng AW, Meng J, Le T, Faull KF, Jaenisch R, Tsai LH (2013) Tet1 is critical for neuronal activity-regulated gene expression and memory extinction. Neuron 79(6):1109–1122. https://doi.org/10.1016/j.neuron.2013.08.003
Kumar D, Aggarwal M, Kaas GA, Lewis J, Wang J, Ross DL, Zhong C, Kennedy A, Song H, Sweatt JD (2015) Tet1 oxidase regulates neuronal gene transcription, active DNA Hydroxy-methylation, object location memory, and threat recognition memory. Neuroepigenetics 4:12–27. https://doi.org/10.1016/j.nepig.2015.10.002
Kaas GA, Zhong C, Eason DE, Ross DL, Vachhani RV, Ming GL, King JR, Song H, Sweatt JD (2013) TET1 controls CNS 5-methylcytosine hydroxylation, active DNA demethylation, gene transcription, and memory formation. Neuron 79(6):1086–1093. https://doi.org/10.1016/j.neuron.2013.08.032
Li X, Wei W, Zhao QY, Widagdo J, Baker-Andresen D, Flavell CR, D’Alessio A, Zhang Y, Bredy TW (2014) Neocortical Tet3-mediated accumulation of 5-hydroxymethylcytosine promotes rapid behavioral adaptation. Proc Natl Acad Sci U S A 111(19):7120–7125. https://doi.org/10.1073/pnas.1318906111
Antunes C, Sousa N, Pinto L, Marques CJ (2019) TET enzymes in neurophysiology and brain function. Neurosci Biobehav Rev 102:337–344. https://doi.org/10.1016/j.neubiorev.2019.05.006
Szwagierczak A, Bultmann S, Schmidt CS, Spada F, Leonhardt H (2010) Sensitive enzymatic quantification of 5-hydroxymethylcytosine in genomic DNA. Nucleic Acids Res 38(19):e181. https://doi.org/10.1093/nar/gkq684
MacArthur IC, Dawlaty MM (2021) TET Enzymes and 5-hydroxymethylcytosine in neural progenitor cell biology and neurodevelopment. Front Cell Dev Biol 9:645335. https://doi.org/10.3389/fcell.2021.645335
Gu TP, Guo F, Yang H, Wu HP, Xu GF, Liu W, Xie ZG, Shi L, He X, Jin SG, Iqbal K, Shi YG, Deng Z, Szabo PE, Pfeifer GP, Li J, Xu GL (2011) The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature 477(7366):606–610. https://doi.org/10.1038/nature10443
Kremer EA, Gaur N, Lee MA, Engmann O, Bohacek J, Mansuy IM (2018) Interplay between TETs and microRNAs in the adult brain for memory formation. Sci Rep 8(1):1678. https://doi.org/10.1038/s41598-018-19806-z
Yu H, Su Y, Shin J, Zhong C, Guo JU, Weng YL, Gao F, Geschwind DH, Coppola G, Ming GL, Song H (2015) Tet3 regulates synaptic transmission and homeostatic plasticity via DNA oxidation and repair. Nat Neurosci 18(6):836–843. https://doi.org/10.1038/nn.4008
Antunes C, Da Silva JD, Guerra-Gomes S, Alves ND, Ferreira F, Loureiro-Campos E, Branco MR, Sousa N, Reik W, Pinto L, Marques CJ (2021) Tet3 ablation in adult brain neurons increases anxiety-like behavior and regulates cognitive function in mice. Mol Psychiatry 26(5):1445–1457. https://doi.org/10.1038/s41380-020-0695-7
Ratnu VS, Emami MR, Bredy TW (2017) Genetic and epigenetic factors underlying sex differences in the regulation of gene expression in the brain. J Neurosci Res 95(1–2):301–310. https://doi.org/10.1002/jnr.23886
Peat JR, Dean W, Clark SJ, Krueger F, Smallwood SA, Ficz G, Kim JK, Marioni JC, Hore TA, Reik W (2014) Genome-wide bisulfite sequencing in zygotes identifies demethylation targets and maps the contribution of TET3 oxidation. Cell Rep 9(6):1990–2000. https://doi.org/10.1016/j.celrep.2014.11.034
Santos F, Peat J, Burgess H, Rada C, Reik W, Dean W (2013) Active demethylation in mouse zygotes involves cytosine deamination and base excision repair. Epigenetics Chromatin 6(1):39. https://doi.org/10.1186/1756-8935-6-39
Byers SL, Wiles MV, Dunn SL, Taft RA (2012) Mouse estrous cycle identification tool and images. PLoS ONE 7(4):e35538. https://doi.org/10.1371/journal.pone.0035538
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif) 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Harris KM, Jensen FE, Tsao B (1992) Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci 12(7):2685–2705
Liu XB, Murray KD (2012) Neuronal excitability and calcium/calmodulin-dependent protein kinase type II: location, location, location. Epilepsia 53(Suppl 1):45–52. https://doi.org/10.1111/j.1528-1167.2012.03474.x
Graziano A, Petrosini L, Bartoletti A (2003) Automatic recognition of explorative strategies in the Morris water maze. J Neurosci Methods 130(1):33–44
Feng J, Pena CJ, Purushothaman I, Engmann O, Walker D, Brown AN, Issler O, Doyle M, Harrigan E, Mouzon E, Vialou V, Shen L, Dawlaty MM, Jaenisch R, Nestler EJ (2017) Tet1 in nucleus accumbens opposes depression- and anxiety-like behaviors. Neuropsychopharmacology 42(8):1657–1669. https://doi.org/10.1038/npp.2017.6
Beck DB, Petracovici A, He C, Moore HW, Louie RJ, Ansar M, Douzgou S, Sithambaram S, Cottrell T, Santos-Cortez RLP, Prijoles EJ, Bend R, Keren B, Mignot C, Nougues MC, Ounap K, Reimand T, Pajusalu S, Zahid M, Saqib MAN, Buratti J, Seaby EG, McWalter K, Telegrafi A, Baldridge D, Shinawi M, Leal SM, Schaefer GB, Stevenson RE, Banka S, Bonasio R, Fahrner JA (2020) Delineation of a human mendelian disorder of the DNA demethylation machinery: TET3 deficiency. Am J Hum Genet 106(2):234–245. https://doi.org/10.1016/j.ajhg.2019.12.007
Kandel ER, Dudai Y, Mayford MR (2014) The molecular and systems biology of memory. Cell 157(1):163–186. https://doi.org/10.1016/j.cell.2014.03.001
Preston AR, Eichenbaum H (2013) Interplay of hippocampus and prefrontal cortex in memory. Current Biol CB 23(17):R764–R773. https://doi.org/10.1016/j.cub.2013.05.041
Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, Russo SJ, Devine SE, McCarthy MM (2015) Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci 18(5):690–697. https://doi.org/10.1038/nn.3988
Loebrich S, Nedivi E (2009) The function of activity-regulated genes in the nervous system. Physiol Rev 89(4):1079–1103. https://doi.org/10.1152/physrev.00013.2009
Coutellier L, Beraki S, Ardestani PM, Saw NL, Shamloo M (2012) Npas4: a neuronal transcription factor with a key role in social and cognitive functions relevant to developmental disorders. PLoS ONE 7(9):e46604. https://doi.org/10.1371/journal.pone.0046604
Tovote P, Fadok JP, Luthi A (2015) Neuronal circuits for fear and anxiety. Nat Rev Neurosci 16(6):317–331. https://doi.org/10.1038/nrn3945
Wang L, Li MY, Qu C, Miao WY, Yin Q, Liao J, Cao HT, Huang M, Wang K, Zuo E, Peng G, Zhang SX, Chen G, Li Q, Tang K, Yu Q, Li Z, Wong CC, Xu G, Jing N, Yu X, Li J (2017) CRISPR-Cas9-mediated genome editing in one blastomere of two-cell embryos reveals a novel Tet3 function in regulating neocortical development. Cell Res 27(6):815–829. https://doi.org/10.1038/cr.2017.58
Jimenez JC, Su K, Goldberg AR, Luna VM, Biane JS, Ordek G, Zhou P, Ong SK, Wright MA, Zweifel L, Paninski L, Hen R, Kheirbek MA (2018) Anxiety cells in a hippocampal-hypothalamic circuit. Neuron 97(3):670-683.e676. https://doi.org/10.1016/j.neuron.2018.01.016
Yoshida K, Drew MR, Mimura M, Tanaka KF (2019) Serotonin-mediated inhibition of ventral hippocampus is required for sustained goal-directed behavior. Nat Neurosci 22(5):770–777. https://doi.org/10.1038/s41593-019-0376-5
Andersson KB, Winer LH, Mork HK, Molkentin JD, Jaisser F (2010) Tamoxifen administration routes and dosage for inducible Cre-mediated gene disruption in mouse hearts. Transgenic Res 19(4):715–725. https://doi.org/10.1007/s11248-009-9342-4
Acknowledgements
The authors would like to thank Wolf Reik and Julian Peat (Babraham Institute, Cambridge, UK) for providing the Tet3 floxed mice and Nuno Sousa (School of Medicine, University of Minho, Portugal) for critical reading of the manuscript.
Funding
This work was supported by National Funds through Foundation for Science and Technology (FCT) fellowships (PD/BD/106049/2015 to CA, PD/BD/128074/2016 to JDS, SFRH/BD/131278/2017 to ELC, SFRH/BD/101298/2014 to SGM, IF/01079/2014 to LP, IF/00047/2012 and CEECIND/00371/2017 to CJM); FCT project grant (PTDC/BIA-BCM/121276/2010) to C.J.M; EpiGeneSys Small Collaborative project to LP; BIAL Foundation Grant 427/14 to LP; and Nature Research Award for Driving Global Impact – 2019 Brain Sciences to LP. It was also co-funded by the Life and Health Sciences Research Institute (ICVS) and by the FEDER (NORTE-01–0145-FEDER-000013), through the Competitiveness Internationalization Operational Program (POCI), and by National funds, through the Foundation for Science and Technology (FCT) – project UIDB/50026/2020 and UIDP/50026/2020. Moreover, this work has been funded by ICVS Scientific Microscopy Platform, member of the national infrastructure PPBI – Portuguese Platform of Bioimaging (PPBI-POCI-01–0145-FEDER-022122; by National funds, through the Foundation or Science and Technology (FCT) – project UIDB/50026/2020 and UIDP/50026/2020).
Author information
Authors and Affiliations
Contributions
C.A designed the study, performed the experiments, analyzed the data, and wrote the manuscript; J.D.S. performed the statistical analysis; S.G.G. and N.D.A. helped with the behavioral tests and respective analysis; E.L.C. helped with the behavioral tests; L.P. and C.J.M. designed the study and wrote the final manuscript. All authors revised and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All experiments were conducted in accordance with EU Directive 2010/63/EU and NIH guidelines on animal care and experimentation and were approved by the Portuguese Authorities (DGAV) with the project reference 0421/000/000/2017.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Antunes, C., Da Silva, J.D., Guerra-Gomes, S. et al. Tet3 Deletion in Adult Brain Neurons of Female Mice Results in Anxiety-like Behavior and Cognitive Impairments. Mol Neurobiol 59, 4892–4901 (2022). https://doi.org/10.1007/s12035-022-02883-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02883-7