Abstract
This study aimed to gain insights into the pathophysiology underlying PLA2G6-associated neurodegeneration that is implicated in three different neurological disorders, suggesting that other, unknown genetic or environmental factors might contribute to its wide phenotypic expression. To accomplish this, we downregulated the function of pla2g6 in the zebrafish nervous system, performed parkinsonism-related phenotypic characterization, and determined the effects of gene regulation upon the loss of pla2g6 function by using RNA sequencing and downstream analyses. Pla2g6 deficiency resulted in axonal degeneration, dopaminergic and motor neuron cell loss, and increased β-synuclein expression. We also observed that many of the identified, differentially expressed genes were implicated in other brain disorders, which might explain the variable phenotypic expression of pla2g6-associated disease, and found that top enriched canonical pathways included those already known or suggested to play a major role in the pathogenesis of Parkinson’s disease. Our data support that pla2g6 is relevant for cranial motor development with significant implications in the pathophysiology underlying Parkinson’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has become increasingly apparent that modifier genes play a considerable role in the phenotypic heterogeneity of Mendelian disorders, as reflected by the incomplete penetrance, intrafamilial variation, and varying clinical severity, as reported in many of them [1, 2]. Indeed, genes involved in neurological diseases are often associated with more than one phenotype, meaning that mutations in the same gene are associated with the development of two or more neurological phenotypes. This suggests that other, unknown genetic or environmental factors might contribute to the phenotypic expression of these disorders [3]. This is the case of PLA2G6, mutations of which cause three different neurological phenotypes in humans, including neurodegenerative diseases with increased basal ganglia iron, such as infantile neuroaxonal dystrophy (INAD), and neurodegeneration with brain iron accumulation (NBIA-type 2), as well as atypical (or complex) juvenile parkinsonism [4,5,6]. PLA2G6 encodes for a Ca2+-independent phospholipase A2 group 6, a multifaceted enzyme essential for membrane remodeling in neurons that plays a role in phospholipid remodeling, signal transduction, cell proliferation, and endoplasmic reticulum stress-mediated apoptosis [7, 8]. Both INAD and NBIA are characterized by the high iron deposition in the globus pallidus and the presence of axonal swellings throughout the central nervous system (CNS). INAD is an infantile disorder that manifests with intellectual disability and progressive neuroregression, leading to severe disability and death in the first decade of one’s life, while NBIA onsets between infancy and the third decade of life and manifests with progressive dystonia, spasticity, ataxic gait, neuropsychiatric abnormalities, and optic atrophy or retinal degeneration [9]. The PLA2G6-associated parkinsonism is usually characterized by severe akinetic-rigid syndrome, dystonia, and cognitive impairment, with not always evidence of iron deposition in the basal ganglia [6, 10]. Taken together, there is an increased need to develop experimental models to further understand these complex and phenotypically heterogeneous neurodegenerative conditions.
The zebrafish has become a widely used model system to study developmental biology and gene function, as a functional nervous system that allows zebrafish larvae to perform complex behaviors such as swimming, and prey capture is established by 4–5 days of larval development [11, 12]. The small size and transparency of the early larvae allows the visualization of the complete CNS and makes the zebrafish an excellent system in which one can express fluorescent proteins to label neurons for morphological analysis and to assess the subcellular localization of tagged proteins. The zebrafish catecholaminergic (CA) system, including dopamine-releasing neurons and a set of dopamine receptors, has been described and genetically analyzed [13]. Most of the CA system components described in the adult brain are already apparent by 3 days postfertilization (dpf) [14, 15]. Further, extensive functional conservation between human genes implicated in parkinsonism and their corresponding zebrafish orthologues has been reported [16,17,18]. Loss of pink1 function causes impaired dopaminergic (DA) neuron development and locomotor function [19], mitochondrial complex I deficiency and DA neuronal cell loss have been described in parkin-deficient zebrafish models [20], and deletion of the WD40 domain of lrrk2 results in DA neuronal loss and axonal disorganization in the zebrafish developing brain [21].
Therefore, in order to gain insights into the pathophysiology underlying PLA2G6-associated neurodegeneration, we downregulated the expression of pla2g6 by using morpholino antisense oligonucleotides (MOs) and examined its associated phenotypic manifestations, including brain motor and DA neuronal organization. To explore whether the effects of gene regulation might contribute to pla2g6-associated phenotypic variability, we determined the zebrafish transcriptome-wide changes and established a set of functional biological networks by integrating different types of biological data, including but not limited to genetics, transcriptomics, metabolomics, and proteomics.
Materials and Methods
Zebrafish Housing and Maintenance
Zebrafish (TL wt strains and Tg(olig2:EGFP) transgenic lines) were maintained and used following the approved National Human Genome Research Institute Animal Care and Use Committee protocols at the Columbia University zebrafish facility and the Icahn School of Medicine at Mount Sinai (ISMMS). Microinjections were performed in one-cell-stage embryos by using an IM-300 microinjector (Narishige International USA, Inc., Amityville, NY, USA). Injected (morphant) and noninjected (wt) embryos were raised in petri dishes with fish water at 28.5 °C (model: SYM 1.1CF120V; VWR, Radnor, PA, USA) for normal development up to 5 dpf. Euthanasia was carried out by immobilization by submersion in ice water (5 parts ice/1 part water, 0–4 °C) for at least 20 min to ensure death by hypoxia.
Fixation
1-Phenyl 2-thiourea (PTU) at 0.3 mg/ml was used to inhibit pigmentation. When the desired developmental stage was reached, dechorionated embryos were either fixed in 4% of paraformaldehyde (PFA) in 1× PBS overnight at 4 °C or in fresh sweet fix medium (4% PFA with 4% sucrose in 1×PBS) at 25 °C, 1 h of fixation per day of development. After fixation, the sweet fix medium was removed and embryos were washed in PBS overnight at 4 °C, followed by 3 washes of 10 min in PBTr (phosphate-buffered saline, 0.5% Triton-X100) on a shaker and by dehydratation in 50% methanol/50% PBTr solution for 5 min. Embryos were stored at − 20 °C in 100% methanol.
Pla2g6 Knockdown and Rescue Experiments
MOs targeting the splice site of exon 14 of pla2g6 (pla2g6-x14 MO) were designed through GeneTools website (GeneTools, LLC, Philomath, OR, USA; Supplementary Table 1). MO working solutions (0.35 mM) were prepared with nuclease-free water and 0.05% of phenol red (Sigma-Aldrich, Co., St. Louis, MO, USA). Cell death was suppressed by co-injecting with a p53 MO. A mean of 214 ± 46 embryos was injected and 241 ± 26 embryos were kept uninjected (wt) per experiment. Eight independent experiments were performed. Efficacy of MOs was evaluated by WISH, qPCR, and Sanger sequencing (Fig. 1). For phenotypic rescue, cDNA from human PLA2G6 (Clone ID: HsCD00304171) was obtained from the DNASU Plasmid Repository (www.dnasu.org). hPLA2G6 plasmid was linearized with KpnI for 1 h at 37 °C followed by precipitation using 3 M Na acetate and 2 volumes of 100% ethanol and incubated for at least 1 h at − 20 °C. DNA was then pelleted and its quality was checked in a 0.8% agarose gel. DNA was transcribed into RNA using the MegaScript T7 kit according to the manufacturer’s instructions (Ambion, Thermo Fisher Scientific Inc., Waltham, MA, USA), followed by a lithium chloride (LiCl) precipitation with 30 μl of LiCl and 30 μl of nuclease-free water and an overnight incubation at − 20 °C. Intact RNAs were then stored at − 80 °C in 5 μl aliquots. Images were obtained by using a Nikon SMZ1500 dissection microscope and a Nikon DS-Ri1 Camera (TV Lens 0.55× DS) (Nikon, Tokyo, Japan).
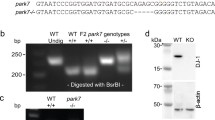
Downregulation of pla2g6 expression. a Microscopy imaging of wt and morphant embryos. Severe morphological defects, such as small body, brain abnormalities, and curvy tail, were observed in morphant embryos. b WISH with sense (left side) and antisense (central and right sides) pla2g6 probes indicates the loss of pla2g6 expression in 24 hpf pla2g6-deficient embryos. c Downregulation of pla2g6 mRNA expression was noted at different developmental stages. d Sanger chromatogram sequences of wt (top) and morphant (bottom) embryos showing the loss of pla2g6 exon 14. An additional pla2g6 exon 13 was described in the NCBI zebrafish database (NM_213097, transcript variant XM_005161794.3); however, this is missing in the transcript variant XM_005161795.2. Exon 13 was not identified during sequencing, suggesting that this TL zebrafish line does not express the largest transcript
RNA Isolation and Gene Expression Analyses
RNA isolation was done at different developmental stages (24, 48, 72, 96, and 120 hpf). Pooled zebrafish embryos (20–50) were homogenized in 1 ml TRIzol reagent (Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA, USA) using a Pestle Grinder System (Fisher Scientific, Hampton, NH, USA). Precipitated RNA was resuspended in 50 μl of RNAse-free water and purified by using the RNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. RNA concentrations were determined by a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE, USA) and by a Qubit Fluorometric Quantitation using the Qubit RNA BR Assay Kit (Qubit, Thermo Fisher Scientific Inc., Waltham, MA, USA). Total RNA was reverse transcribed using SuperScript IV reverse transcriptase kit (Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA, USA) following the manufacturer’s instructions. Gene expression was carried out by qPCR using an Eco Real-Time PCR System (Illumina, San Diego, CA, USA), SYBRGreen PCR master mix (Life Technologies, Grand Island, NY, USA), and corresponding primers (Supplementary Table 1). Primer efficiency and gene quantification were determined by using the standard curve method. Samples were run in triplicates with a non-template control, consisting of a mixture of cDNAs at different developmental stages (18, 24, 48, 72, 96, and 120 hpf), included in each assay. Relative expression levels for our target genes were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and Beta-2 microglobulin (B2M) housekeeping genes by using the 2−ΔΔCT method [22].
Whole-Mount In Situ Hybridization
WISH was carried out as described by Thisse and Thisse [23]. Pla2g6, th1, and sncb sense and antisense mRNA probes were synthesized from previously purified PCR templates (E-Gel SizeSelect 2%; Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA, USA; Supplementary Material, Table 1). mRNA probes were then synthesized by using the DIG-RNA labeling T3/T7 kit (Roche, Basel, Switzerland) followed by a 2-h incubation step at 37 °C, purified using a SigmaSpin Post Reaction Purification column, and resuspended in RNAlater with 0.5 M EDTA (Sigma-Aldrich, Co., St. Louis, MO, USA). Fixed embryos were rehydrated through a series of washes with methanol/PBST for 5 min each step and permeabilized with 10 μg/ml Proteinase K at RT. Embryos were then postfixated with 4% paraformaldehyde (PFA) for 20 min to stop the Proteinase K digestion and washed in PBST 5 times for 5 min each. Prehybridization of embryos was performed by 4-h incubation at 68 °C in hybridization mix (50% formamide, 5× SSC, 1 M citric acid, 50 μg/ml heparin, 500 μg/ml tRNA, and 0.1% Tween 20) followed by overnight hybridization at 68 °C in 200 μl of hybridization mix containing 150 ng of DIG-labeled RNA probes. Embryos were washed in series of hybridization mix (without heparin and tRNA)/2× SSC at 70 °C and 0.2% SSC/PBST at RT, preincubated with 5% BSA-2% sheep serum in PBST for 3 h under agitation at RT, and incubated overnight with alkaline phosphatase anti-DIG antibody at 4 °C with agitation (Roche, Basel, Switzerland; 1:5000). The antibody was removed with PBST washes at RT, embryos were labeled with labeling mix (NBT/BCIP in alkaline Tris buffer) in the dark until the desired staining intensity was reached, and the reaction was stopped with a stop solution (1 mM EDTA and 0.1% Tween 20 in PBS), followed by 20 min fixation in 4% PFA. Embryos were kept in 100% glycerol at 4 °C in the dark until imaging. Images were obtained as previously described.
Immunohistochemistry
Zebrafish larvae were processed for immunohistochemistry using the protocol of Turner et al. [24]; 1–5 dpf dechorionated embryos were fixed using the sweet fixation method, as previously described. Eyes from embryos older than 24 hpf were dissected using a dissection needle, as described elsewhere [24]. Embryos were rehydrated with 50% methanol-50% PBTr, washed in PBTr 3 times for 5 min, permeabilized with proteinase K at RT for 5 min (24 hpf stage) or 1 min (dissected embryos any stage), and postfixed in 4% PFA at RT for 20 min. Embryos were blocked during at least 1 h at RT in immunohistochemistry blocking solution (IB) (10% normal goat serum/0.1% DMSO/PBTr) on a shaker, incubated with primary antibodies at 4 °C overnight in IB on a shaker, and washed 4 times for 30 min at RT in PBTr. Embryos were incubated with secondary antibodies at 4 °C overnight in IB on a shaker, washed 4 times for 30 min in PBTr on a shaker, and mounted for imaging in low melt agarose (0.5%) in glass bottom culture dishes (MatTek Corporation, Ashland, MA, USA). Images were acquired by using water immersion lens (×10, ×20, and ×40) in a Leica SP5 DM confocal microscope (Leica, Wetzlar, Germany) at the Microscopy Shared Resource Facility at the ISMMS and analyzed by using the ImageJ software (imagej.nih.gov). The primary antibody and concentrations used are described as follows: 6-11B-1 anti-acetylated α-tubulin antibody (GeneTex, Inc., Irvine, CA, USA), 1:200. Secondary antibody used and concentrations are described as follows: anti-mouse rhodamine Red X (Jackson Immunoresearch Labs, West Grove, PA, USA) 1:200. Measurements of the trigeminal ganglia were also performed through the ImageJ software.
Live Imaging
Alive embryos were anesthetized with 0.02% of tricaine solution (ethyl-3-aminobenzoate methanesulfonic acid, Sigma-Aldrich, Co., St. Louis, MO, USA). Embryos were imaged as previously described.
Statistical Analyses
Statistical analyses were performed using the GraphPad Prism software version 6.0 (GraphPad, La Jolla, CA, USA). Data on graphs are presented as mean ± SEM. Statistical differences between wild-type and morphant embryos were calculated by using the Mann-Whitney nonparametric test. *P < 0.01, **P < 0.001, and ***P < 0.0001.
RNA Sequencing (RNA-Seq)
Approximately 20 (morphant) to 40 (wt) embryos were pooled and used for RNA isolation, and total RNA was extracted as previously described. RNA libraries were prepared by using the TruSeq total RNA kits and sequenced by using 100 bp paired-end reads on a HiSeq2000 instrument (Illumina, San Diego, CA, USA). RNA-Seq data analysis was performed at ContigExpress (http://www.contigexpress.com/). Briefly, samples were mapped to the zebrafish genome sequence (Ensembl release 69) by using the splicing-aware mapper TopHat [25]. The resulting BAM files were processed for gene quantification in FPKM (fragments per kilobase per million) and differential expression analysis by using Cufflinks [26]. The identified differentially expressed genes (DEGs) (FDR ≤ 0.05) in each of the pairwise comparisons (wt versus morphant at 18, 48, and 72 hpf) were subject to GO and pathway enrichment analyses by using Gorilla [27] and KOBAS [28], respectively. Heatmaps of DEGs were generated by using the GENE-E software (http://www.broadinstitute.org/cancer/software/GENE-E/). Selected RNA-Seq data were validated through qPCR assays.
Ingenuity Pathway Analysis
The Ingenuity Pathway Analysis (IPA) software (Qiagen, Hilden, Germany) was used to perform downstream analyses (P value cutoff at 0.05). Relevant functional pathways, gene networks, as well as functional and disease clusters were identified through the Core analysis of IPA. The pathways most significant to the input data were determined by the Canonical Pathways Analysis tool based on two parameters: a ratio of the number of genes from the data set that map to the pathway divided by the total number of genes that map to the canonical pathway and a P value from the input data set (Fisher’s exact test right-tailed). Networks were algorithmically generated based on their connectivity and scored according to how relevant they are to the genes in the input dataset. The interactive Disease and Biological Functions tool was also used to predict the directional biological effects of the gene/protein sets and to visualize the diseases and functions as interactive nodes on networks and pathways.
Results
The Zebrafish pla2g6 and Human PLA2G6 Are Highly Conserved
BLAST search of the zebrafish genome identified a single gene homologous to human PLA2G6 (hPLA2G6), ENSDARG00000060921, on chromosome 22 (http://dgd.genouest.org/browse). The pla2g6 gene encoded an 818 amino acid protein, with an overall 52.6% identity and up to 70.2% similarity with hPLA2G6. Within the Ankyrin repeat region (ANK), the human and zebrafish amino acid sequences displayed 50.6% identity and 70.5% similarity. In the functionally relevant patatin domain region, the amino acid identity rose to 63.3% identity and 95% similarity.
Downregulation of pla2g6 Expression in Early Larval Development
To downregulate the pla2g6 function, we eliminated the pla2g6 exon 14 by using a MO targeting the intron 14 splice acceptor junction (pla2g6-x14 MO). The exon 14 was chosen because it contains the NBIA- and parkinsonism-associated p.R632W mutation [5, 29] and is a part of the Pat_PNPLA9 domain containing phospholipase enzymatic activity that is thought to be involved in multiple cellular processes, including phospholipid remodeling, signal transduction, cell proliferation, and apoptotic cell death [8].
After MO microinjections, several morphological defects, such as smaller body, brain abnormalities, and rigidity (i.e., curvy tail), were observed in pla2g6-deficient morphants (Fig. 1a). Mortality was observed in about 49.7% of the injected embryos and 42.8% of the noninjected embryos after 120 hpf. Pla2g6 expression, which was previously shown to be expressed in the zebrafish developing brain (Fig. 1b), was successfully downregulated in the early stages of development, including 18 (P = 1.63E-06), 24 (P = 2.43E-05), 48 (P = 0.003), and 72 hpf (P = 2.79E-05) (Fig. 1c). The pla2g6 exon 14 was effectively removed as shown by WISH, qPCR, and direct Sanger sequencing (Fig. 1b–d).
Pla2g6 Deficiency Causes DA Cell Loss
Genetic dissection of the DA and noradrenergic (NA) contributions of the CA tracts in 3 dpf larvae revealed that DA neurons predominantly constitute the hypothalamic and spinal CA tracts, whereas NA neurons make only a minor contribution [12]. DA projections are mainly and exclusively observed in the olfactory bulbs (OB), pretectum (Pr), preoptic region (PO), diencephalon (DC), endohypothalamic tract (eht), and caudal hypothalamus (CH), while a major contribution of NA neurons is mostly appreciable in the locus coeruleus (LC), medulla oblongata (MO), and area postrema (AP) [12]. Therefore, to explore the parkinsonism-associated phenotype, the expression patterns of DA cell markers, including tyrosine hydroxylase genes (th1 and th2) and dopamine transporter gene (dat), were examined for both injected and wt zebrafish embryos. Loss of th1, th2, and dat was observed at 18, 24, 48, 72, and 120 hpf in mutant larvae, versus wt, with th1 being the most significantly downregulated gene in all examined developmental stages (Fig. 2a). Because th1 is expressed higher in embryonic and early larval stages than th2 [14, 30], to localize DA cell loss, WISH was performed by using th1 as a marker. We observed pronounced DA cell loss across the forebrain, midbrain, and hindbrain in morphant embryos versus wt, being highly pronounced in the OB, DC, CH, and MO and moderate in the Pr, LC, and eht (Fig. 2b).
Downregulation of pla2g6 causes dopaminergic cell loss and increased scnb levels. a Decreased expression of three different dopaminergic neuron markers (th1, th2, and dat) was observed in morphant embryos versus wt. Only 18 hpf embryos are shown [P < 0.0001 (th1), P = 0.001 (th2), P = 0.01 (dat)]. RE: relative expression. b WISH for th1 expression (dark purple labeling, black arrows) in wt (left) and morphant (right) embryos is shown. Decrease of dopaminergic neurons is observed across the forebrain (telencephalom, diencephalon), midbrain (mesencephalon), and hindbrain (rhombencephalon) of morphant embryos, reducing highly in the olfactory bulbs (OB), diencephanlon (DC), caudal hypothalamus (CH), and medulla oblongata (MO), and moderately in the locus coeruleus (LC), pretectum (Pr), and endohypothalamic tract (eht). AAN: arch-associated, Sym: sympathetic. c qPCR relative expression shows a higher expression of sncb in pla2g6-deficient embryos when compared to wt counterparts [P = 0.02 (48 hpf)]. The graph shows the mean relative expression of 12 replicates for each sample. d WISH for sncb mRNA expression (48 hpf, dark purple labeling, black arrows) in wt (left) and morphant (right). High levels of scnb expression were observed across the habenula (Ha), forebrain (fb), midbrain (mb), and hindbrain (hb) in morphant embryos versus wt. gVIII: statoacoustic ganglion; gP: posterior lateral line ganglion; LHa: left-side habenula; RHa: right-side habenula. Values represent the means ± SEM. ***P < 0.0001, **P < 0.001, *P < 0.05
Pla2g6 Morphants Showed Elevated β-Synuclein (sncb) Expression
Synucleins, including α-, β-, and γ-synucleins, are a family of small neuronal proteins that are exclusively found in vertebrates, with α- and β-synuclein proteins primarily found in brain tissues and observed mainly in presynaptic terminals, and γ-synuclein in the peripheral nervous system and retina [31]. High levels of α-synuclein (SNCA) expression have been linked to a collection of neurodegenerative disorders, including PD and PLA2G6-associated parkinsonism [32,33,34]. Links between PLA2G6 and SNCA expressions have previously been shown in flies and mice, with PLA2G6 being the most downregulated gene in flies overexpressing α-synuclein [35], and α-synuclein axonal and cytoplasmic aggregates being found in Pla2g6-knockout (KO) mice [36]. Therefore, the expression of synuclein proteins was also investigated in our pla2g6-deficient versus wt embryos to examine whether this association also exists in the zebrafish CNS. Although no α-synuclein orthologue has been found in zebrafish, three different synucleins have been characterized in the zebrafish: sncga (zebrafish γ-synuclein A), sncgb (zebrafish γ-synuclein B), and sncb (zebrafish β-synuclein). While the expression of sncgb was restricted to the notochord and was not detected in embryos older than 2 dpf, both sncga and sncb expressions were detected at the early stages of development and found to be restricted to the brain and retina at 72 and 45 hpf, respectively [37]. Because β-synuclein (sncb) is mainly expressed in presynaptic terminals, its expression was determined by WISH and qPCR. High levels of sncb expression were observed across the habenula, forebrain, midbrain, and the hindbrain of morphant embryos (Fig. 2c, d), indicating the existence of an altered sncb expression in pla2g6-deficient embryos.
Pla2g6 Downregulation Causes Motor Neuron Loss and Altered Patterning
It is well-known that motor neuron impairment is a common feature in many NBIA disorders [9]; thus, whether pla2g6 deficiency affects motor neuron development was investigated by injecting our pla2g6-x14 MO in Olig2+ zebrafish [Tg(olig2:EGFP)] embryos. Olig2+ is required for oligodendrocyte and motor neuron specification in the spinal cord, as well as for the development of somatic motor neurons in the hindbrain [38]. Confocal imaging in 5-day-old living Olig2+ wt and morphant revealed a loss of motor neurons and perturbations in their localizations in pla2g6 morphants when compared to wt larvae. Motor neuronal loss was highly visible in both mesencephalon (midbrain) and rhombencephalon (hindbrain) (Fig. 3a, b), while altered motor neuron patterning was mainly apparent in the midbrain of pla2g6-deficient embryos (Fig. 3a), likely suggesting a role of pla2g6 in motor neuron development.
Downregulation of pla2g6 results in impaired cranial motor neuron development. a, b Confocal z-stacks projections of 5-dpf, alive wt (left) and morphant (right) Tg(olig2:EGFP) embryos. A dorsal view, anterior to the left, is shown. Significant loss of motor neurons was observed in both mesencephalon (midbrain; a) and rhombencephalon (hindbrain; b) of pla2g6-deficient embryos. Abnormal patterning of the motor neurons was also appreciable in the mesencephalon of the morphant embryos (a). Scale bar 25μm
Pla2g6 Deficiency Results in Altered Cranial Motor Neuron Development
To further explore the role of pla2g6 in motor neuron development, 1- and 3-day-old wt and morphant embryos were stained with anti-acetylated tubulin antibody that labels neuronal processes/axons/nerve fascicles through the brain and spinal cord [39]. Confocal imaging disclosed that motor neurons and axonal tracks were already visible throughout the brain and spinal cord at both 24 and 72 hpf, where significant axonal loss was observed in morphant embryos (Figs. 4 and 5). This axonal loss was highly pronounced in the forebrain of 24 hpf, as well as forebrain and midbrain of 72 hpf morphant embryos (Figs. 4a, b, c, and 5a, b), being more prominent at 72 hpf (Fig. 5a, b). Defasciculation of axons was highly appreciable at both 24 and 72 hpf (Figs. 4 and 5), while ectopic growth of axons and abnormal organization of axonal projections were mainly noticeable at 72 hpf (Fig. 5a, b, white arrows). Spinal cord deformation was observed in morphant embryos at 24 hpf (Fig. 4a, d, orange arrows), while defasciculation of the trigeminal ganglia, being significantly different in both length and width, was observed at 24 (Fig. 4b, e) and 72 hpf (Fig. 5c). The observed decrease in acetylated tubulin staining in the spinal cord also indicates loss of peripheral neurons (Fig. 4a, d).
Zebrafish axonal projections visualized by acetylated α-tubulin staining at 24 hpf. a–e Acetylated α-tubulin is represented with green fire blue color in both wt (upper panels) and morphant (lower panels) embryos. Dorsal, anterior to the top (a, d), as well as lateral, anterior to the right (b, c), views of the confocal z-stacks projections of 1 dpf embryos are shown. a Significant axonal loss across the nervous system was observed in morphants when compared to wt embryos (orange arrows). b Compact trigeminal ganglion was observed in wt embryos, while disorganized trigeminal ganglion was appreciable in pla2g6-deficient embryos (orange arrows). c Significant axonal loss was appreciable in the forebrain of pla2g6 morphants versus wt embryos. d Dorsal views of the embryos revealed the clear signals of spinal cord deformation (orange arrows) in the pla2g6-deficient embryos. Scale bar is 50 μm for ×20 and ×10 images [a (upper panel) and a and d (lower panel)] and 25 μm for ×40 images [b, c, d (upper panel) and b and c (lower panel)]. e Zoomed images of the trigeminal ganglia in wt (upper panel) and morphants (lower panel) revealed that this was significantly different in both length and width (**P = 0.01). Five wt and four pla2g6 morphant embryos were used for the analyses
Zebrafish axonal projections visualized by acetylated α-tubulin staining at 72 hpf. a–c Acetylated α-tubulin is represented with green fire blue color for both wt (upper panels) and morphant (lower panels) embryos. Anterior, right-lateral views of confocal z-stacks projections of 3 dpf embryos are shown. a Axonal loss and abnormal distribution of axonal projections were both observed in the forebrain and midbrain of pla2g6 morphants when compared to wt embryos. b Zoomed confocal images of the forebrain and midbrain revealed the existence of a significant axonal loss and axonal disorganization in pla2g6 morphant embryos. c Zoomed confocal images of the trigeminal ganglia confirmed the existence of a disorganized trigeminal ganglia (orange arrows) in the morphants versus wt. Scale bar is 50 μm for ×20 images (a) and 25 μm for ×40 images (b, c). Zoom was 2.0 for all images
PLA2G6 mRNA Expression Rescued the Phenotypic Defects Observed in pla2g6-Deficient Zebrafish Embryos
The specificity and effectiveness of our pla2g6-x14 MO was evaluated by co-injecting it with hPLA2G6 mRNA. Morphological phenotypes were fully recovered in about 40% (5 ng/μl) to 60% (10 ng/μl) of the injected embryos and partially recovered in about 70% (5 ng/μl) to 85% (10 ng/μl) of the injected embryos in 5-day-old larvae (Fig. 6a). Full recovery of pla2g6 mRNA and the expression of DA markers were also obtained, clearly confirming that the phenotype observed was due to the effects of pla2g6 downregulation (Fig. 6b, c). A slight increase in pla2g6 expression was observed in wt compared with rescue embryos; however, these differences were not statistically significant (wt versus rescue (5 ng/μl), P = 0.87; wt versus rescue (10 ng/μl), P = 0.67). Although the expression of DA markers was slightly lower in embryos co-injected with 10 ng/μl of mRNA than those with 5 ng/μl of mRNA, these differences were not statistically significant (th1, P = 0.99; th2, P = 0.90; and dat, P = 0.33), and no cytotoxic effect was found after co-injecting with either 5 or 10 ng/μl of RNA (lethality 48.5% (5 ng/μl) versus 52.7% (10 ng/μl), P = 0.99, four independent experiments).
Co-injection of our pla2g6 MO and hPLA2G6 mRNA rescued the morphological phenotypes caused by pla2g6 downregulation. a Morphological defects were fully (green color) recovered in about 40% (5 ng/μl) to 60% (10 ng/μl) of the injected embryos. Injection of hPLA2G6 mRNA alone was used as control. b The pla2g6 expression in the morphant embryos reached similar values to those observed in wt pla2g6 expression. c The expression of dopaminergic markers, including th1, th2, and dat, was likewise rescued by co-injecting either 5 or 10 ng/μl of hPLA2G6 mRNA. All experiments were carried out in 5-day-old larvae
Genes Implicated in Neurological Diseases, Brain Functions, and Signaling Pathways Were Found Differentially Expressed in pla2g6-Deficient Embryos
To investigate whether gene regulation contributes to the variable phenotypic expression of PLA2G6 disease and gain insights into the biological processes underlying disease, transcriptome-wide changes upon the loss of pla2g6 expression were determined by RNA-Seq. RNA-Seq was performed in both wt and morphant embryos at the developmental stages where pla2g6 was most significantly downregulated (18 and 72 hpf) (Fig. 1c). At 18 hpf, only 22 genes were found to be differentially expressed (DE) in morphant versus wt embryos (P < 0.05), with only 15 genes (12 upregulated and 3 downregulated) remaining significantly DE after qPCR validation (Table 1; Fig. 7a). Despite the fact that not many genes were found DE, some of the DE genes (DEGs) were known to play relevant roles in the pathogenesis of PD. For instance, the expressions of hsp90 and hsp70, which are known to be increased in PD patients’ brains [40] and α-synuclein mutant transgenic mice [41], and mfn2, which interacts with Parkin and Pink1 to regulate mitophagy [42], were also altered in our pla2g6 morphants.
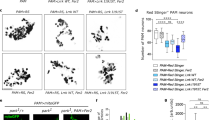
DEGs in morphant versus wt embryos were shown to be implicated in PD-associated pathological pathways. a, b Heatmap (left) and qPCR validation of the DEGs (right) at 18 (a) and 72 hpf (b). Genes in the heatmap were ranked by log2 fold change. Red squares: upregulated genes, blue squares: downregulated genes. Only genes with a P < 0.001 (RNA-Seq) and predicted to be implicated in neurological disorders, nervous system development, glucose metabolism disorders, cell-to-cell signaling, and cell death and survival are shown. x-axis: wt expression (solid back column) versus morphant (solid red column); y-axis: gene expression relative to the reference genes (B2M and GAPDH). Values represent the means ± SEM. ***P < 0.0001, **P < 0.001, *P < 0.05. c Only 21 of the top 43 canonical pathways that were most highly ranked based on their P value (P < 0.05) in the RNA-Seq data are shown. x-axis: significant canonical pathways; y-axis: –log of P values
At 72 hpf, 255 genes were found DE between morphant and wt embryos (P < 0.05), with 147 genes found to be significantly upregulated and 108 genes significantly downregulated (Supplementary Table 2). Only genes found to be enriched in nervous system development and neurological diseases were selected for qPCR validation. These included those directly associated with a neurological disease (n = 21) as well as two genes (grin1 and jun) that were highly involved in the development of the nervous system with functions in its morphology, motor neuron cell death, glucose metabolism, basal ganglia, and movement disorders. Only 16 (1 upregulated and 15 downregulated) genes remained significantly DE after qPCR validation (Table 2; Fig. 7b). A variety of DE genes were implicated in other brain disorders, which might explain the variable phenotypic expression of the PLA2G6 disease. This is the case of atp1a3, mutations of which cause a dominant form of rapid onset dystonia-parkinsonism [43]; npc1, mutations of which are associated with a lipid storage disorder that is characterized by a progressive neurodegeneration [44]; slc2a1, associated with glucose deficiency syndrome and paroxysmal dystonia with spasticity and ataxia [45, 46]; kif5c, known to be responsible for cortical dysplasia and brain malformations [47]; and agrn, whose changes in expression affect amyloid β (Aβ) homeostasis in mice models of Alzheimer’s disease (AD) [48].
RNA-Seq data were also used to perform downstream analyses by integrating diverse types of biological data through the IPA software. Data from DEGs at both 18 and 72 hpf were combined for these analyses. First, we found that the top enriched canonical pathways (P < 0.05, Fisher’s exact test right-tailed), where the significantly DEGs were implicated, included pathways already known or suggested to play a major role in the pathogenesis of PD. These included, but were not limited to, mitochondrial dysfunction, protein ubiquitination pathway, and nNOS (nitric oxide synthases), neuregulin, CREB, GABA receptor, mTOR, and clathrin-mediated endocytosis signaling pathways (Fig. 7c). Interestingly, four of these pathways, including nNOS, neuregulin, CREB, and GABA receptor signaling pathways, are known to be implicated in key neurological functions, such as neurotransmission and nervous system signaling. NO signaling is essential for the long-term potentiation in the hippocampus and the long-term depression in the cerebellum [49]; neuregulins play important roles in the development, maintenance, and repair of the nervous system [50]; CREB activation leads to neuronal excitation and proliferation as well as long-term memory formation [51]; and GABA, which is the main inhibitory neurotransmitter in the mammalian CNS, plays an important role in the synaptic transmission termination [52]. A complete list of the top enriched canonical pathways can be found in Supplementary Table 3. Second, casual molecular networks were formed by using functional relationships between gene products based on their known connections in peer-reviewed literature. Five different biological networks were identified when taking into account all significant DEGs at 18 hpf and those enriched in the nervous system development and neurological diseases at 72 hpf (Table 3 ), while 16 different biological networks were identified when considering all significant DEGs (Table 4). Top cellular functional categories include cellular assembly and organization, cell death and survival, cell morphology, cell-to-cell signaling and interaction, nervous system development and function (Tables 3 and 4), as well as lipid metabolism and molecular transport (Table 4). Third, the specific biological functions in which DEGs were involved were determined by using the Diseases and Functions tool (IPA, Qiagen). DEGs were found to act in about 500 different diseases and functions, including neurological diseases, development and functions of the nervous system, cell death and survival, as well as cellular function, maintenance, signaling, and interaction (Supplementary Table 4). Among these, it is worth highlighting that DEGs are implicated in phenotypic manifestations that have already been reported in PLA2G6-associated disease, such as movement, muscular and mood disorders, basal ganglia disease, neuropathy, dyskinesia, optic atrophy, and epileptic seizures. Some of the top functions within the nervous system development include the morphology and development of neurons, dendritic growth and branching, neuritogenesis, and the morphogenesis and branching of neuritis. Other top functions are involved in cell-to-cell signaling and interaction (i.e., synaptic transmission and depression), glucose metabolism disorder, and cell death and survival (Supplementary Table 4; Fig. 8).
Genes implicated in neurological diseases as well as neuronal functions and signaling pathways were found to be DE in pla2g6-deficient embryos versus wt. a–e Disease and biological function pathways predicted by IPA, where genes are represented as nodes and the biological relationship between two nodes is represented as an edge. a DEGs associated with neurological disease network. b DEGs associated with cell signaling network. c DEGs associated with glucose metabolism network. d DEGs associated with cell death and survival network. e DEGs associated with cell death and survival network. Red: Upregulated genes, green: downregulated genes, blue lines: inhibition, orange lines: activation, yellow lines: inconsistent findings with the state of downstream molecule, and gray lines: no predicted effect
Discussion
We used the zebrafish system to examine the effects of pla2g6 deficiency in the nervous system by combining genetic and imaging approaches. Pla2g6 deficiency leads to several morphological defects, including parkinsonism-related phenotypes, and decreased expression of dopaminergic markers th1, th2, and dat. The observed DA cell loss was highly appreciable in areas where DA neurons are mostly expressed, including the OB, Pr, DC, and CH (Fig. 2b). These results clearly support that pla2g6 deficiency leads to parkinsonism-related phenotypes since progressive DA cell loss in the substantia nigra pars compacta (SNc) is known to be responsible for the main motor manifestations in PD patients [53]. Diencephalic DA cell loss was also observed in parkin-, pink1-, lrrk2-, and fbxo7-deficient zebrafish embryos [20, 21, 54,55,56]. The fact that the expression of dat was decreased in morphant embryos versus wt, like that observed in PD patients versus controls, might suggest a dysfunction in the presynaptic dopamine reuptake as dopamine is reabsorbed into the presynaptic cells via dat [57].
Accumulation of α-synuclein in neurons constitutes the neuropathological hallmark of PD, being phosphorylated α-synuclein, in the form of large aggregates, the major component of Lewy bodies (LBs) [58]. Although the mechanistic pathways by which α-synuclein contributes to PD pathogenesis are not yet well-understood, α-synuclein binds to lipid membranes, particularly synaptic vesicles and mitochondrial inner membranes, where it is thought to regulate the release of synaptic vesicles, and thus, contribute to vesicular trafficking impairments when aggregated. In addition, small presynaptic α-synuclein aggregates are associated with dendritic spine degeneration, leading to defects in neurotransmitter (NT) release [33, 58,59,60] and, thus, affecting synaptic trafficking. Taken together, we examined the expression and distribution of presynaptic β-synuclein (sncb) in pla2g6-deficient embryos versus wt and identified elevated levels of sncb in the main brain areas (Fig. 2c). This finding correlates with the identification of α-synuclein accumulation in both PLA2G6 patients and Pla2g6-knockout (KO) mice [34, 61] and might suggest a relevant role of presynaptic synuclein aggregation in the pathogenesis of PLA2G6-associated disease, like that previously suggested for PD pathogenesis. The increased sncb expression in the habenula is very interesting, since the lateral habenula (LHb) seems to control both motor and cognitive behaviors by its inhibitory effects on midbrain DA neurons [62]. This suggests a role of LHb in the dopamine deficiency seen in PD and related disorders, which will be of great relevance for therapeutic interventions.
Further, the observation of altered motor neuron patterning in the midbrain and significant motor neuron loss in the hindbrain of pla2g6-deficient larvae suggests a role of pla2g6 in cranial motor neuron development, which, in turn, might explain some of the PLA2G6-associated clinical phenotypes, such as cerebellar atrophy, spasticity, ataxia, and neuropathy [63]. Axonal degeneration has been described in both PLA2G6 patients and Pla2g6-KO mice, where it is thought to be driven by the degeneration of the mitochondrial inner and presynaptic membranes [64]. Like in humans and mice, we also observed that pla2g6 dysfunction leads to significant axonal loss as well as axonal defasciculation and disorganization in both 1 and 3 dpf pla2g6-deficient embryos, which suggests that pla2g6 function might be essential for axonal differentiation. Disorganized axonal tracts were also observed in pink1 and lrrk2 zebrafish models [21, 55], indicating, once again, the existence of a clinical overlap between PLA2G6 disease and familiar and idiopathic PD. Spinal cord deformation, previously reported in pink1-deficient morphants [55], was also seen in our pla2g6-deficient embryos. The axonal neurodegeneration seen throughout the CNS might probably affect both neuronal differentiation and signaling.
By investigating the transcriptome-wide changes and integrating diverse types of biological data to determine the biological pathways and networks implicated in disease pathogenesis, we identified altered expression levels in genes already known to participate in the pathophysiology of PD. For instance, hsp70 and hsp90, which belong to the heat-shock-protein family of chaperones with critical roles in a variety of cellular processes, such as promoting the folding of newly synthesized proteins and the refolding of partially folded proteins, and assisting with the removal of misfolded proteins, have been found to be increased in the SNc of PD patients and to colocalize with α-synuclein in LBs. In addition, there is considerable amount of data derived from cellular and animal studies that support the protective roles of hsp70 and hsp90 against α-synuclein aggregation and toxicity [65,66,67]. Interestingly, hspa1l/hsp70i (Hsp70), hsp90aa1 (Hsp90), hspb1 (Hsp27, Hsp25), and hspb2 (Hsp27) expression is increased in our pla2g6-deficient morphants and three (hsp90aa1, hspb1, and hspb2) are predicted to inhibit cell death, likely suggesting a protective role of these three genes in the pathogenesis of PLA2G6-associated disease (Fig. 8 and Supplementary Table 2). This protective role is probably associated with the role of Hsp and Hspb proteins in facilitating the normal folding of proteins and intracellular handling of misfolded proteins, consistent with what has been observed in Parkin p.C298G mutants, in which Hspb1, Hspb2, Hspb4, and Hspb7 suppress protein aggregation via autophagy or proteasomal degradation [68]. Hspb1 is also predicted to activate cell survival, further supporting its protective role in neuronal cell death. Moreover, Hspb1, defects of which cause Charcot-Marie-Tooth disease type 2F (CMT2F) and distal hereditary motor neuropathy (dHMN) [69], prevents Tau and Aβ aggregations and Aβ-induced toxicity in both cellular and animal AD models [70]. Consistent with this, agrn, which regulates Aβ and was downregulated in our pla2g6-deficient morphants, was predicted to promote cell survival (Figs. 7 and 8). In addition, the expression of mfn2, mutations of which cause a human hereditary motor and sensory neuropathy [71] and a canine form of fetal-onset neuroaxonal dystrophy resembling human INAD caused by PLA2G6 mutations [72], was increased in 18 hpf pla2g6 morphants and decreased in 72 hpf pla2g6 morphants. Mfn2, which encodes for mitofusin and contributes to the dynamic balance between mitochondrial fusion and fission while determining mitochondrial morphology [73], was recently shown to mediate Parkin recruitment to damaged mitochondria in a PINK-dependent manner, while Parkin and PINK1 act in a common pathway, influencing mitochondrial integrity and promoting mitochondrial fission. Reduced Mfn2 activity also suppresses the mitochondrial phenotype found in drosophila Pink1 and parkin mutants [74,75,76]. Accordingly, we hypothesize that the axonal loss observed in our pla2g6-deficient zebrafish embryos might be driven by a mitochondrial dysfunction, as seen in Pla2g6-KO mice [64], due to the impairments within the parkin-pink1-mfn2 pathway. Moreover, severe loss of DA neurons in the striatum has been observed in Mfn2-KO mice, meaning that mfn2 is required for the striatal axonal projections of DA neurons [77]. In our data, changes in agrn and mfn2 expressions are predicted to inhibit neuronal development, dendritic growth branching, and formation, morphogenesis, and branching of neurites, further supporting their relevant roles in the development of the nervous system.
Lastly, among the top 276 canonical pathways identified by IPA (Supplementary Table 3), many were found to be implicated in functions already known to be associated with the pathogenesis of PD (i.e., mitochondrial dysfunction, autophagy, protein ubiquitination pathway, and signaling mechanisms), further supporting an overlap between PD and PLA2G6 pathophysiologies, as well as cell signaling. These results are concordant with recent advances in PD genetics and experimental model systems, where impairments in both intracellular trafficking and signaling play a pivotal role in the pathogenesis of PD and related neurodegenerative diseases [59], which is not surprising since the striatum is the major glutamatergic and DA innervation and striatal DA cell loss is the main pathological phenotype observed in PD [78].
Conclusion
We here demonstrated that the zebrafish is a nice model system to mimic neurodegenerative diseases, since we were able to recapitulate PLA2G6-asssociated human phenotypes in zebrafish embryos and showed that there is an existent clinical and pathological overlap between PLA2G6-associated disease and PD. Our data suggest that pla2g6 is essential for cranial motor development, its deficiency leads to axonal degeneration probably due to the impairments in mitochondrial morphology and function, and the presynaptic dysfunction seen in the manifesting disease may likely be driven by abnormal sncb aggregation. Our RNA-Seq data and analyses, which nominate disease-associated gene networks and pathways, also explain and support the pivotal role of pla2g6 function in neurodegeneration. Overall, these data suggest that increased knowledge of pla2g6 function is crucial for the development of therapeutic targets and the prevention of the disease manifestation and progression.
References
Gallati S (2014) Disease-modifying genes and monogenic disorders: experience in cystic fibrosis. Appl Clin Genet 7:133–146. https://doi.org/10.2147/TACG.S18675
Gropman AL, Adams DR (2007) Atypical patterns of inheritance. Semin Pediatr Neurol 14(1):34–45. https://doi.org/10.1016/j.spen.2006.11.007
Krebs CE, Paisan-Ruiz C (2012) The use of next-generation sequencing in movement disorders. Front Genet 3:75
Khateeb S, Flusser H, Ofir R, Shelef I, Narkis G, Vardi G, Shorer Z, Levy R et al (2006) PLA2G6 mutation underlies infantile neuroaxonal dystrophy. Am J Hum Genet 79(5):942–948. https://doi.org/10.1086/508572
Morgan NV, Westaway SK, Morton JE, Gregory A, Gissen P, Sonek S, Cangul H, Coryell J et al (2006) PLA2G6, encoding a phospholipase A2, is mutated in neurodegenerative disorders with high brain iron. Nat Genet 38(7):752–754. https://doi.org/10.1038/ng1826
Paisan-Ruiz C, Bhatia KP, Li A, Hernandez D, Davis M, Wood NW, Hardy J, Houlden H et al (2009) Characterization of PLA2G6 as a locus for dystonia-parkinsonism. Ann Neurol 65(1):19–23. https://doi.org/10.1002/ana.21415
Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G (2011) Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev 111(10):6130–6185. https://doi.org/10.1021/cr200085w
Turk J, Ramanadham S (2004) The expression and function of a group VIA calcium-independent phospholipase A2 (iPLA2beta) in beta-cells. Can J Physiol Pharmacol 82(10):824–832. https://doi.org/10.1139/y04-064
Gregory A, Hayflick S (1993) Neurodegeneration with brain iron accumulation disorders overview. In: Pagon RA, Adam MP, Ardinger HH et al. (eds) GeneReviews(R). University of Washington, Seattle
Paisan-Ruiz C, Guevara R, Federoff M, Hanagasi H, Sina F, Elahi E, Schneider SA, Schwingenschuh P et al (2010) Early-onset L-dopa-responsive parkinsonism with pyramidal signs due to ATP13A2, PLA2G6, FBXO7 and spatacsin mutations. Mov Disord 25(12):1791–1800. https://doi.org/10.1002/mds.23221
Budick SA, O’Malley DM (2000) Locomotor repertoire of the larval zebrafish: swimming, turning and prey capture. J Exp Biol 203(Pt 17):2565–2579
Kastenhuber E, Kratochwil CF, Ryu S, Schweitzer J, Driever W (2010) Genetic dissection of dopaminergic and noradrenergic contributions to catecholaminergic tracts in early larval zebrafish. J Comp Neurol 518(4):439–458. https://doi.org/10.1002/cne.22214
Yamamoto K, Ruuskanen JO, Wullimann MF, Vernier P (2010) Two tyrosine hydroxylase genes in vertebrates. New dopaminergic territories revealed in the zebrafish brain. Mol Cell Neurosci 43(4):394–402. https://doi.org/10.1016/j.mcn.2010.01.006
Schweitzer J, Lohr H, Filippi A, Driever W (2012) Dopaminergic and noradrenergic circuit development in zebrafish. Dev Neurobiol 72(3):256–268. https://doi.org/10.1002/dneu.20911
Kaslin J, Panula P (2001) Comparative anatomy of the histaminergic and other aminergic systems in zebrafish (Danio rerio). J Comp Neurol 440(4):342–377. https://doi.org/10.1002/cne.1390
Bandmann O, Burton EA (2010) Genetic zebrafish models of neurodegenerative diseases. Neurobiol Dis 40(1):58–65. https://doi.org/10.1016/j.nbd.2010.05.017
Martin-Jimenez R, Campanella M, Russell C (2015) New zebrafish models of neurodegeneration. Curr Neurol Neurosci Rep 15(6):33
Xi Y, Noble S, Ekker M (2011) Modeling neurodegeneration in zebrafish. Curr Neurol Neurosci Rep 11(3):274–282. https://doi.org/10.1007/s11910-011-0182-2
Xi Y, Ryan J, Noble S, Yu M, Yilbas AE, Ekker M (2010) Impaired dopaminergic neuron development and locomotor function in zebrafish with loss of pink1 function. Eur J Neurosci 31(4):623–633. https://doi.org/10.1111/j.1460-9568.2010.07091.x
Flinn L, Mortiboys H, Volkmann K, Koster RW, Ingham PW, Bandmann O (2009) Complex I deficiency and dopaminergic neuronal cell loss in parkin-deficient zebrafish (Danio rerio). Brain 132(Pt 6):1613–1623. https://doi.org/10.1093/brain/awp108
Sheng D, Qu D, Kwok KH, Ng SS, Lim AY, Aw SS, Lee CW, Sung WK et al (2010) Deletion of the WD40 domain of LRRK2 in zebrafish causes parkinsonism-like loss of neurons and locomotive defect. PLoS Genet 6(4):e1000914. https://doi.org/10.1371/journal.pgen.1000914
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Thisse C, Thisse B (2008) High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc 3(1):59–69. https://doi.org/10.1038/nprot.2007.514
Turner KJ, Bracewell TG, Hawkins TA (2014) Anatomical dissection of zebrafish brain development. Methods Mol Biol 1082:197–214. https://doi.org/10.1007/978-1-62703-655-9_14
Trapnell C, Pachter L, Salzberg SL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25(9):1105–1111. https://doi.org/10.1093/bioinformatics/btp120
Roberts A, Pimentel H, Trapnell C, Pachter L (2011) Identification of novel transcripts in annotated genomes using RNA-Seq. Bioinformatics 27(17):2325–2329. https://doi.org/10.1093/bioinformatics/btr355
Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z (2009) GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics 10(1):48. https://doi.org/10.1186/1471-2105-10-48
Xie C, Mao X, Huang J, Ding Y, Wu J, Dong S, Kong L, Gao G et al (2011) KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res 39(Web Server issue):W316–W322
Sina F, Shojaee S, Elahi E, Paisan-Ruiz C (2009) R632W mutation in PLA2G6 segregates with dystonia-parkinsonism in a consanguineous Iranian family. Eur J Neurol 16(1):101–104. https://doi.org/10.1111/j.1468-1331.2008.02356.x
Filippi A, Mahler J, Schweitzer J, Driever W (2010) Expression of the paralogous tyrosine hydroxylase encoding genes th1 and th2 reveals the full complement of dopaminergic and noradrenergic neurons in zebrafish larval and juvenile brain. J Comp Neurol 518(4):423–438. https://doi.org/10.1002/cne.22213
George JM (2002) The synucleins. Genome Biol 3(1):REVIEWS3002
Lee VM, Trojanowski JQ (2006) Mechanisms of Parkinson’s disease linked to pathological alpha-synuclein: new targets for drug discovery. Neuron 52(1):33–38. https://doi.org/10.1016/j.neuron.2006.09.026
Stefanis L (2012) Alpha-synuclein in Parkinson’s disease. Cold Spring Harb Perspect Med 2(2):a009399
Paisan-Ruiz C, Li A, Schneider SA, Holton JL, Johnson R, Kidd D, Chataway J, Bhatia KP et al (2010) Widespread Lewy body and tau accumulation in childhood and adult onset dystonia-parkinsonism cases with PLA2G6 mutations. Neurobiol Aging 33(4):814–823. https://doi.org/10.1016/j.neurobiolaging.2010.05.009
Scherzer CR, Jensen RV, Gullans SR, Feany MB (2003) Gene expression changes presage neurodegeneration in a Drosophila model of Parkinson’s disease. Hum Mol Genet 12(19):2457–2466. https://doi.org/10.1093/hmg/ddg265
Malik I, Turk J, Mancuso DJ, Montier L, Wohltmann M, Wozniak DF, Schmidt RE, Gross RW et al (2008) Disrupted membrane homeostasis and accumulation of ubiquitinated proteins in a mouse model of infantile neuroaxonal dystrophy caused by PLA2G6 mutations. Am J Pathol 172(2):406–416. https://doi.org/10.2353/ajpath.2008.070823
Sun Z, Gitler AD (2008) Discovery and characterization of three novel synuclein genes in zebrafish. Dev Dyn 237(9):2490–2495. https://doi.org/10.1002/dvdy.21569
Zannino DA, Appel B (2009) Olig2+ precursors produce abducens motor neurons and oligodendrocytes in the zebrafish hindbrain. J Neurosci 29(8):2322–2333. https://doi.org/10.1523/JNEUROSCI.3755-08.2009
Devine CA, Key B (2003) Identifying axon guidance defects in the embryonic zebrafish brain. Methods Cell Sci 25(1–2):33–37. https://doi.org/10.1023/B:MICS.0000006851.84998.e0
Uryu K, Richter-Landsberg C, Welch W, Sun E, Goldbaum O, Norris EH, Pham CT, Yazawa I et al (2006) Convergence of heat shock protein 90 with ubiquitin in filamentous alpha-synuclein inclusions of alpha-synucleinopathies. Am J Pathol 168(3):947–961. https://doi.org/10.2353/ajpath.2006.050770
Jeon GS, Park SW, Kim DW, Seo JH, Cho J, Lim SY, Kim SD, Cho SS (2004) Glial expression of the 90-kDa heat shock protein (HSP90) and the 94-kDa glucose-regulated protein (GRP94) following an excitotoxic lesion in the mouse hippocampus. Glia 48(3):250–258. https://doi.org/10.1002/glia.20075
Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW (2010) Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet 19(24):4861–4870. https://doi.org/10.1093/hmg/ddq419
de Carvalho AP, Sweadner KJ, Penniston JT, Zaremba J, Liu L, Caton M, Linazasoro G, Borg M et al (2004) Mutations in the Na+/K+-ATPase alpha3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron 43(2):169–175. https://doi.org/10.1016/j.neuron.2004.06.028
Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA et al (1997) Niemann-pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science 277(5323):228–231. https://doi.org/10.1126/science.277.5323.228
Weber YG, Storch A, Wuttke TV, Brockmann K, Kempfle J, Maljevic S, Margari L, Kamm C et al (2008) GLUT1 mutations are a cause of paroxysmal exertion-induced dyskinesias and induce hemolytic anemia by a cation leak. J Clin Invest 118(6):2157–2168. https://doi.org/10.1172/JCI34438
Weber YG, Kamm C, Suls A, Kempfle J, Kotschet K, Schule R, Wuttke TV, Maljevic S et al (2011) Paroxysmal choreoathetosis/spasticity (DYT9) is caused by a GLUT1 defect. Neurology 77(10):959–964. https://doi.org/10.1212/WNL.0b013e31822e0479
Poirier K, Lebrun N, Broix L, Tian G, Saillour Y, Boscheron C, Parrini E, Valence S, Pierre BS, Oger M, Lacombe D, Genevieve D, Fontana E, Darra F, Cances C, Barth M, Bonneau D, Bernadina BD, N’Guyen S, Gitiaux C, Parent P, des Portes V, Pedespan JM, Legrez V, Castelnau-Ptakine L, Nitschke P, Hieu T, Masson C, Zelenika D, Andrieux A, Francis F, Guerrini R, Cowan NJ, Bahi-Buisson N, Chelly J (2013) Mutations in TUBG1, DYNC1H1, KIF5C and KIF2A cause malformations of cortical development and microcephaly. Nat Genet 45 (6):639–647, doi: https://doi.org/10.1038/ng.2613.
Rauch SM, Huen K, Miller MC, Chaudry H, Lau M, Sanes JR, Johanson CE, Stopa EG et al (2011) Changes in brain beta-amyloid deposition and aquaporin 4 levels in response to altered agrin expression in mice. J Neuropathol Exp Neurol 70(12):1124–1137. https://doi.org/10.1097/NEN.0b013e31823b0b12
Garthwaite J, Boulton CL (1995) Nitric oxide signaling in the central nervous system. Annu Rev Physiol 57(1):683–706. https://doi.org/10.1146/annurev.ph.57.030195.003343
Falls DL (2003) Neuregulins: Functions, forms, and signaling strategies. Exp Cell Res 284(1):14–30. https://doi.org/10.1016/S0014-4827(02)00102-7
Wu GY, Deisseroth K, Tsien RW (2001) Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc Natl Acad Sci U S A 98(5):2808–2813
Knapp RJ, Malatynska E, Yamamura HI (1990) From binding studies to the molecular biology of GABA receptors. Neurochem Res 15(2):105–112. https://doi.org/10.1007/BF00972199
Surmeier DJ, Obeso JA, Halliday GM (2017) Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci 18(2):101–113. https://doi.org/10.1038/nrn.2016.178
Zhao T, Zondervan-van der Linde H, Severijnen LA, Oostra BA, Willemsen R, Bonifati V (2012) Dopaminergic neuronal loss and dopamine-dependent locomotor defects in Fbxo7-deficient zebrafish. PLoS One 7(11):e48911. https://doi.org/10.1371/journal.pone.0048911
Anichtchik O, Diekmann H, Fleming A, Roach A, Goldsmith P, Rubinsztein DC (2008) Loss of PINK1 function affects development and results in neurodegeneration in zebrafish. J Neurosci 28(33):8199–8207. https://doi.org/10.1523/JNEUROSCI.0979-08.2008
Sallinen V, Kolehmainen J, Priyadarshini M, Toleikyte G, Chen YC, Panula P (2010) Dopaminergic cell damage and vulnerability to MPTP in Pink1 knockdown zebrafish. Neurobiol Dis 40(1):93–101. https://doi.org/10.1016/j.nbd.2010.06.001
Wilson JM, Levey AI, Rajput A, Ang L, Guttman M, Shannak K, Niznik HB, Hornykiewicz O et al (1996) Differential changes in neurochemical markers of striatal dopamine nerve terminals in idiopathic Parkinson’s disease. Neurology 47(3):718–726. https://doi.org/10.1212/WNL.47.3.718
Schulz-Schaeffer WJ (2010) The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol 120(2):131–143. https://doi.org/10.1007/s00401-010-0711-0
Abeliovich A, Gitler AD (2016) Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature 539(7628):207–216. https://doi.org/10.1038/nature20414
Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E et al (2006) Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci 9(2):251–259. https://doi.org/10.1038/nn1632
Sumi-Akamaru H, Beck G, Shinzawa K, Kato S, Riku Y, Yoshida M, Fujimura H, Tsujimoto Y et al (2016) High expression of alpha-synuclein in damaged mitochondria with PLA2G6 dysfunction. Acta Neuropathol Commun 4(1):27. https://doi.org/10.1186/s40478-016-0298-3
Hikosaka O, Sesack SR, Lecourtier L, Shepard PD (2008) Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci 28(46):11825–11829. https://doi.org/10.1523/JNEUROSCI.3463-08.2008
Illingworth MA, Meyer E, Chong WK, Manzur AY, Carr LJ, Younis R, Hardy C, McDonald F et al (2014) PLA2G6-associated neurodegeneration (PLAN): further expansion of the clinical, radiological and mutation spectrum associated with infantile and atypical childhood-onset disease. Mol Genet Metab 112(2):183–189. https://doi.org/10.1016/j.ymgme.2014.03.008
Sumi-Akamaru H, Beck G, Kato S, Mochizuki H (2015) Neuroaxonal dystrophy in PLA2G6 knockout mice. Neuropathology 35(3):289–302. https://doi.org/10.1111/neup.12202
Labrador-Garrido A, Bertoncini CW, Roodveldt C (2011) The Hsp70 chaperone system in Parkinson’s disease, etiology and pathophysiology of Parkinson’s disease. In: PAQR (ed). InTech.
Daturpalli S, Waudby CA, Meehan S, Jackson SE (2013) Hsp90 inhibits alpha-synuclein aggregation by interacting with soluble oligomers. J Mol Biol 425(22):4614–4628. https://doi.org/10.1016/j.jmb.2013.08.006
Ebrahimi-Fakhari D, Saidi LJ, Wahlster L (2013) Molecular chaperones and protein folding as therapeutic targets in Parkinson’s disease and other synucleinopathies. Acta Neuropathol Commun 1(1):79. https://doi.org/10.1186/2051-5960-1-79
Minoia M, Grit C, Kampinga HH (2014) HSPA1A-independent suppression of PARK2 C289G protein aggregation by human small heat shock proteins. Mol Cell Biol 34(19):3570–3578. https://doi.org/10.1128/MCB.00698-14
Evgrafov OV, Mersiyanova I, Irobi J, Van Den Bosch L, Dierick I, Leung CL, Schagina O, Verpoorten N et al (2004) Mutant small heat-shock protein 27 causes axonal Charcot-Marie-Tooth disease and distal hereditary motor neuropathy. Nat Genet 36(6):602–606. https://doi.org/10.1038/ng1354
Abisambra JF, Jinwal UK, Jones JR, Blair LJ, Koren J 3rd, Dickey CA (2011) Exploiting the diversity of the heat-shock protein family for primary and secondary tauopathy therapeutics. Curr Neuropharmacol 9(4):623–631. https://doi.org/10.2174/157015911798376226
Zuchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, Zappia M, Nelis E et al (2004) Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet 36(5):449–451. https://doi.org/10.1038/ng1341
Fyfe JC, Al-Tamimi RA, Liu J, Schaffer AA, Agarwala R, Henthorn PS (2011) A novel mitofusin 2 mutation causes canine fetal-onset neuroaxonal dystrophy. Neurogenetics 12(3):223–232. https://doi.org/10.1007/s10048-011-0285-6
Santel A, Fuller MT (2001) Control of mitochondrial morphology by a human mitofusin. J Cell Sci 114(Pt 5):867–874
Chen Y, Dorn GW 2nd (2013) PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 340(6131):471–475. https://doi.org/10.1126/science.1231031
Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ (2008) The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A 105(5):1638–1643. https://doi.org/10.1073/pnas.0709336105
Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA et al (2006) Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441(7097):1162–1166. https://doi.org/10.1038/nature04779
Lee S, Sterky FH, Mourier A, Terzioglu M, Cullheim S, Olson L, Larsson NG (2012) Mitofusin 2 is necessary for striatal axonal projections of midbrain dopamine neurons. Hum Mol Genet 21(22):4827–4835. https://doi.org/10.1093/hmg/dds352
Kreitzer AC, Malenka RC (2008) Striatal plasticity and basal ganglia circuit function. Neuron 60(4):543–554. https://doi.org/10.1016/j.neuron.2008.11.005
Acknowledgements
We thank Joshua Barber at the Zebrafish core at the Columbia University Medical Center for his assistance with the MO and mRNA microinjections and the staff at the Microscopy Shared Resource Facility at the ISMMS for their training and assistance with the zebrafish imaging.
Funding
This work was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (R01NS079388; CPR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Ethics Approval
All experiments involving animals were performed under the national (United States National Institutes of Health) and Institutional Animal Care and Use Committee at the Icahn School of Medicine at Mount Sinai (ISMMS) guidelines and approved by ISMMS IACUC (Protocol number: LA11-00313).
Electronic Supplementary Material
ESM 1
(XLS 282 kb)
Rights and permissions
About this article
Cite this article
Sánchez, E., Azcona, L.J. & Paisán-Ruiz, C. Pla2g6 Deficiency in Zebrafish Leads to Dopaminergic Cell Death, Axonal Degeneration, Increased β-Synuclein Expression, and Defects in Brain Functions and Pathways. Mol Neurobiol 55, 6734–6754 (2018). https://doi.org/10.1007/s12035-017-0846-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0846-2