Abstract
The embodied mammalian brain evolved to adapt to an only partially known and knowable world. The adaptive labeling of the world is critically dependent on the neocortex which in turn is modulated by a range of subcortical systems such as the thalamus, ventral striatum, and the amygdala. A particular case in point is the learning paradigm of classical conditioning where acquired representations of states of the world such as sounds and visual features are associated with predefined discrete behavioral responses such as eye blinks and freezing. Learning progresses in a very specific order, where the animal first identifies the features of the task that are predictive of a motivational state and then forms the association of the current sensory state with a particular action and shapes this action to the specific contingency. This adaptive feature selection has both attentional and memory components, i.e., a behaviorally relevant state must be detected while its representation must be stabilized to allow its interfacing to output systems. Here, we present a computational model of the neocortical systems that underlie this feature detection process and its state-dependent modulation mediated by the amygdala and its downstream target the nucleus basalis of Meynert. In particular, we analyze the role of different populations of inhibitory interneurons in the regulation of cortical activity and their state-dependent gating of sensory signals. In our model, we show that the neuromodulator acetylcholine (ACh), which is in turn under control of the amygdala, plays a distinct role in the dynamics of each population and their associated gating function serving the detection of novel sensory features not captured in the state of the network, facilitating the adjustment of cortical sensory representations and regulating the switching between modes of attention and learning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acetylcholine (ACh) is a neuromodulator that in the neocortex mediates the detection, selection, and further processing of stimuli [1, 2] and is typically associated with attention and synaptic plasticity [1, 3,4,5]. The main source of cortical acetylcholine is the nucleus basalis of Meynert (NBM) in the basal forebrain. The NBM mainly comprises cholinergic and gamma-aminobutyric (GABA) neurons. Cholinergic neurons in the NBM receive their main excitatory drive from the amygdala and from ascending activating systems [6,7,8]. While the amygdala is known to be involved in building associations that are key for fear conditioning, the ascending activating systems (like the Locus Coeruleus with norepinephrine) are principally related to bottom up mechanisms for arousal and wakefulness. In contrast, excitatory projections from prefrontal and insular cortex [9, 10] would convey top-down control of acetylcholine. On the other hand, the NBM’s gabaergic neurons are the principal target of the gabaergic neurons of the central nucleus of the amygdala (CeA) and the core of the nucleus accumbens (NAc) [11, 12], linking nonspecific disinhibition of the NBM to amygdalar projections. The principal targets of cholinergic afferents from the NBM are then in the cortex, these preserving a topological organization, with posterior NBM projecting to temporal-lateral cortex, the intermediate region to the laterodorsal and frontoparietal cortex and the anterior part of the NBM to the medial cortical region and the frontoparietal ocular cortex and the amygdala [13]. Cholinergic modulation of the neocortex depends principally on two distinct cholinergic receptor types: metabotropic muscarinic and ionotropic nicotinic receptors. The activation of muscarinic receptors in the neocortex of the rat has a global excitatory effect on both excitatory and inhibitory neurons [14]. In contrast, binding with nicotinic receptors has a disinhibitory influence on the neural substrate, by targeting preferentially neurons that inhibit other interneurons [15]. Hence, the global effects of acetylcholine on a population of cortical neurons must depend on the balance and distribution of these two opponent types of cholinergic receptors [16]. Here, we will investigate this relationship in the context of sensory processing in classical conditioning. In particular, we will investigate the role of the differential drive of cortical interneurons by acetylcholine on the behavioral state-dependent gating and representation of sensory states.
The neocortex is a complex six-layered structure, typically comprising 80% excitatory and 20% inhibitory neurons. Different gabaergic interneurons can be distinguished depending on dendritic arborization (e.g., basket cells, Martinotti cells, chandelier cells) [17], spiking patterns (e.g., fast spiking, regular spiking) [18], or the expression of neuropeptides and calcium-binding proteins (e.g., parvalbumin (PV), somatostatin (SST), vasoactive intestinal polypeptide, serotonin) [19,20,21,22]. Not too surprisingly, these classifications overlap, to the point that, PV expressing (+) interneurons (PVi+) in the neocortex have fast-spiking dynamics and are principally characterized as basket cells, although not exclusively. SST expressing interneurons (SSTi+) in contrast have regular-spiking dynamics with broad arborizations in the form of Martinotti and chandelier cells, among others. Together with the less well-understood serotonin expressing interneurons, these three inmunochemically identified interneuron classes account for the majority of the gabaergic neurons in the neocortex [23].
Recent work has shown that PVi+ also preferentially express the M1 muscarinic acetylcholine receptor (mAChR) [24], while SSTi+ express nicotinic acetylcholine receptors (nAChR) [24,25,26,27]. Moreover, while the effect of acetylcholine on the neuron level is increasing the inward current, M1 receptors are greatly involved in depolarizing both excitatory and inhibitory PVi+ neurons; nicotinic receptors have shown a disinhibitory effect on the global excitation of the cortex under release of ACh. Despite the implications of these differentiated effects are not disentangled yet, they suggest a general role of acetylcholine in the regulation of gain control of signal versus noise ratios. Under these assumptions, we introduce a model of cholinergic modulation of cortical activity and we evaluate how this interaction, strongly dependent on the behavior-state of the animal, shifts between different modes of gain control.
In this paper, we study the effects of cholinergic modulation on the neocortex through a biophysically constrained computational model at the level of cortical circuits comprising excitatory and two types of inhibitory neurons. The following sections are organized as follows. In the “Materials and Methods” section, we discuss the computational model and its choices. The “Results” section characterizes the impact of having one or two inhibitory populations with different dynamics and studies its implications under cholinergic neuromodulation. Finally, we discuss the obtained results and their implications in understanding cortical function, attention, and learning.
Materials and Methods
Neuron Model
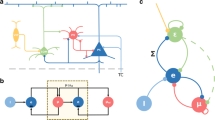
We employed computational modeling techniques to study how acetylcholine modulates the network dynamics in the cortical structures described above (Fig. 1). Our model includes an excitatory population and two inhibitory populations. Auditory stimuli are simulated as incoming spike trains to a population of excitatory cortical neurons. This excitatory population projects topographically to both inhibitory populations and to neighboring excitatory neurons following a distance rule as suggested in [28, 29]. The dynamics of the PVi+ population is modeled as fast-spiking cells, while the SSTi+ population contains low-threshold, regular-spiking cells (see the choice of parameters in Table 1).
Sensory pathway in auditory conditioning: an auditory stimulus is pre-processed in the auditory pathway through superior olive (SC), inferior colliculus (IC), and the medial geniculate nucleus of the thalamus (MGN) until it reaches the primary auditory cortex (A1). Our model of a small fraction of the cortex (top-right) is composed of three cell populations, one excitatory containing 80% of the cells, and two inhibitory containing the remaining 20%. These inhibitory populations correspond to the PVi+ and SSTi+ responsive inhibitory interneurons. Both inhibitory populations receive excitatory input principally from the excitatory population, composed of pyramidal cells. Finally, they project back to it with inhibitory connections. Moreover, unconditioned stimuli (US), indicative of surprising or aversive events are relayed through the nucleus tractus solitarius (NTS) to the amygdala (Am). The amygdala, in turn, uses cortical information to predict future USs. Either the predictions or the US itself stimulate the nucleus basalis of Meynert. The NBM releases ACh in the neocortex and the amygdala (Am), therefore promoting the acquisition of new sensory features and its predictive component. Finally, the amygdala, which is receiving contextual information in the typical form of a conditioned stimulus (CS), predictive of the US, learns the association between the cortical predictive components of and the NBM stimulation, facilitating future learning events, now cued by the CS
Dynamics of neurons in our model were calculated by a mean field approximation of leaky integrate and fire equations as follows:
The firing rate x i of a cell was computed as a leaky integrator:
where α is a decay term, σ(·) a two-term nonlinear function:
with a scaling (k) and a threshold (θ) and I i is the total input firing rate to the neuron x i :
where N is the total number of neurons, G inh a regulatory parameter to influence the driving effect of one population to another (typically G inh = 1 for excitatory inputs and G inh < 0 for inhibitory populations) and w ij the weight of the connection from neuron j to neuron i, (w ij > = 0).
Synaptic Connectivity Within the Cortical Structure
Connectivity among the different populations is defined by an exponentially scaled distance rule following [28, 30] which has been proposed as a system for increasing network sparsity while increasing the variety of receptive fields in [31]. We use an exponential distance rule where the probability of forming a connection C ij is defined by the distance that separates two neurons in a plane d ij is:
where c and b are the two parameters of the exponential rule. Must be noticed that the modulation of parameter c in eq. 4 is proportional to the probability of forming that connection, whereas b scales this probability in function of the distance d ij . Notice that P(C | d) can be changed to different distance rules, keeping the general benefits of distance-based connectivity matrices. In specific, PVi+ cells have been shown to be densely, locally connected [32] while SSTi+ cells appear to have similar constraints [33]. In order to model this dense synaptic arborization of the inhibitory population, we defined a second-order distance rule that allows enforcement of a soft constrain in the formation of locally dense synapses. The parameters of our model were fit to the physiological data presented in [17]. The distance rule that we proposed is presented in the next equation, and the parameters are detailed in Table 2.
with c regulating the cost of forming any connection and b regulating the cost of extending a synapse up to a certain distance.
Simulation of Cholinergic Neuromodulation
Cholinergic modulation can regulate the excitability of inhibitory cells by means of different mAChRs and nAChRs. Few studies have aimed to understand the coupled effects of these two receptors on different brain areas, instead explaining their independent contributions in neocortical modulation. Research on muscarinic receptors has shown that they are mainly expressed in PVi+ but not SSTi+ cells [25,26,27]. Among the different mAChR subtypes, the M1 family is preferentially expressed in the soma of neocortical cells. Exposing neocortical cells with mAChR agonists results in the depolarization of PVi+ cells [14] and a global inhibitory effect of the excitatory neurons on the culture [34]. In contrast, nAChRs are mostly present in regular-spiking interneurons, being their main neocortical representative SSTi+ cells. The molecular effects of ACh on nAChRs is gating inward Ca2+ currents in target cells, again depolarizing the cell and increasing its excitability. Despite this molecular description of the mechanisms regulating nAChRs, global effects of nAChR agonists in vitro has shown a disinhibitory effect on the excitatory pyramidal population. Therefore, we consider two main effects of cholinergic interactions on our model. First, ACh in mAChRs will excite the PVi+ population. Second, it will inhibit the SSTi+ population, thus unbalancing global and local inhibition. Therefore, we center our simulation at the functional level of description for simplicity, allowing for different mechanistic descriptions to be considered in future work (e.g., SSTi+ disinhibition through global inhibition of PVi+ cells).
Results
We describe below our simulation results regarding cholinergic modulation of neocortical circuits. The section first describes how the dynamics of the excitatory population are modulated by each inhibitory subpopulation and the imbalance emerging from the application of the effects of acetylcholine and then simulates and studies the specific effects that acetylcholine has in a complex, interconnected cortical network.
Different Inhibitory Populations Regulate Different Aspects of Gain Control in the Neocortex
First, we wanted to functionally characterize the two populations of interneurons. With this objective, we considered a single neocortical layer with 80% of excitatory cells and 20% of inhibitory cells [35]. The choice of parameters for the distance rule of each inhibitory population was based on [17], with PVi+ cells being characterized by shorter ranges but stronger weights than SST, as detailed in Table 1. Hence, we characterized how the firing rate depended on input intensity and background noise levels. The input to the cortical excitatory population was designed as a pure tone in the spectral domain with noise in the rest of the spectrum, as characterized for the primary auditory cortex in [36]. Following observations by [37], our model shows the same properties of gain control observed in the visual cortex (Fig. 2a–c): increases in input intensity make neurons more responsive to the signal, while increases in noise levels make them less responsive to it. This behavior can be understood as an implementation of a gain control mechanism, with a component that regulates the sensitivity to the input signal and another that controls the network’s noise sensitivity. We then tested what was the contribution of each of the two inhibitory populations to this mechanism of gain control. We scaled the weights towards each inhibitory population, ranging from 0%PVi+/200%SSTi+ to 200%PVi+/’%SSTi+. Figure 2d, e shows that the balance of local and global inhibition through PVi+ and SSTi+ populations is critical to the presented mechanisms of gain control. Increased PVi+ activity resulted in a nonlinear decrease in the sensitivity to noise or signal-to-noise ratio (SNR) while it also decreased significantly the sensitivity to high intensity inputs. Considering the observed effects of acetylcholine on the inhibitory populations of the neocortex, our results suggest a reduction of the intensity for strong signals with a small increase of the gain of low intensity inputs when rebalancing inhibitory gains towards increased PVi+ activity. All together, we characterized a mechanism for gain control in the neocortex, where the equilibrium between gains of local and global inhibition controls the signal-to-noise balance.
Gain modulation in cortical networks. a Amplitude-frequency histograms for different input noise and input intensity levels on a representative sample cell. b Curves comparing the variation of firing rate in function of input intensity for different noise levels (bottom) and noise for different input intensity levels (top)
Differences in Connectivity of Inhibitory Populations Select Different Communication Channels in the Neocortex
Next, we tested what would be the effects of the different connectivity ranges on the frequency domain. We applied a Morlet wavelet convolution to the time series of mean neural activity generated by our model exposed to random noise. Figure 3a shows the mean spectral power of the population when long (top) or short (bottom) range inhibition was removed, and the other was left. Whereas removing short-range inhibition diminished only high-frequency oscillations, the absence of long-range inhibition significantly decreased the power spectrum in most of the lower frequency bands. This can be interpreted as a bandpass filtering of the noise of the network. In the presence of longer ranges of inhibition activity is integrated and, subsequently, lowpass filtered, leading to a suppression of activity in the lower bands. The opposite would occur if inhibition is modulating a smaller amount of neurons in a local range: the integration disappears, activity in lower frequencies increases significantly for being disinhibited, while higher frequencies, particularly associated with individual neuron activity is significantly reduced. Figure 3b compares the mean power for high and low frequencies (HF and LF, respectively) when long- and short-range connectivity (SR and LR, respectively) was removed. The involvement of inhibitory networks with oscillatory patterns in brain activity has been suggested before [16, 38] and demonstrated [39, 40]. While the function of this emergent pattern still eludes a unified and comprehensive explanation, oscillatory dynamics have been said to have an important effect on binding by synchrony and the emergence of different pathways for the transfer of information [41,42,43,44]. We show that these oscillations emerge at different frequency bands that are strongly dependent on the network’s anatomical structure itself, suggesting that specific layers of the cortex with different proportions of interneuron subtypes, as well as behavior state-dependent neuromodulation strictly define, block, or promote different communication channels.
Different inhibitory populations resonate at different frequency channels and differentially control the gain in the network. a Examples of frequency bands for PV− (bottom) and SST− (top) conditions. Dashed lines define the boundaries of the high (HF, 60–100) and low frequency (LF, 0–20) bands. b Long-range and short-range connectivity patterns of inhibition (PV− and SST−, respectively) show significantly different frequency responses to noise, the first provoking low-frequency oscillations and the latter provoking high-frequency oscillations. The statistics are extracted from 10 randomly generated networks (mean ± standard deviation: LR-HF 10.7 ± 1.0 10−3, SR-HF 37.9 ± 3.6 10−3, LR-LF 33.0 ± 6.3 10−3, SR-LF 5.7 ± 1.3 10−3; t test, p < 0.01). c–e Gain plots comparing the effects of the different inhibitory networks on the excitatory firing rate, for SST− (c), PV− (d), and both active (e)
In order to characterize the gain modulation of cortical inhibition by acetylcholine, we tested the impact of modulating the gain of each inhibitory population (Fig. 3c, d). Our results suggest that the mechanisms of gain control detailed in the previous section have a strong dependence on the network connectivity. While increases in the gain of the global inhibitory population (SSTi+) directly reduced the overall activity of the network, independently of the amplitude of current neuronal activity, local inhibition was stronger with stronger inputs. Figure 3e shows how modulating the balance between each inhibitory subnetwork, instead of each of them independently, shifts the network dynamics to that of a dynamic range compressor (DRC), slightly increasing the gain of low intensity signals while reducing the impact of the stronger ones, as the balance between both types of inhibition is perturbed. While DRCs are typically used as compressors, trying to scale the data in a way that no information is lost (i.e., increase the gain of low intensity signals and reduce that of high intensity ones, to remove overshadowing effects), the benefits of such mechanisms of gain control in sensory networks could serve as a mechanism for exploration of the sensory space. More specifically, considering the differentiated effects of acetylcholine on the inhibitory networks of the neocortex, release of acetylcholine could become a mechanism for fostering this kind of sensory exploration as a global disinhibition and a stronger input gain control. This modulation could serve as a mechanism for the exploration of alternative explanations during arousing events or attention processes, where levels of ACh are increased.
Acetylcholine Reduces Variability but Not Mean Activity in the Cortical Network
Next, we wanted to see what the effects of acetylcholine are on this kind of network. We would expect that excitation of the PVi+ population would reduce the intensity of the most salient signals present in the sensory stream. In contrast, we would expect that inhibition of the SSTi+ population would reduce the gain control of the network, thus increasing the sensitivity to noisy or low intensity signals, as suggested by Fig. 3e. Again, we constructed a network with 80% excitatory and 20% inhibitory units. The inhibitory units were split into 50% PVi+ and 50% SSTi+ types, as found in layer IV of the rat neocortex [23]. In this case, we presented a task-irrelevant stimulus (IS) that was constantly present with high intensity, while a task-relevant stimulus (RS) was presented at a lower intensity. Cholinergic modulation can be considered as driven by the arousing state of perceiving an unconditioned aversive stimulus. We considered two cases in which the cholinergic modulation was paired or not paired with the RS. We did not observe a significant increase in mean activity when pairing the RS with the effects of ACh (Fig. 4a, b) but a global decrease in the variance of the RS and not the IS in the conditions of the paired ACh compared to the unpaired cases. This suggests that the nonlinear dynamics of the system have a temporal component that cannot be ignored, probably related to the stability of the system. Previous work has studied the effect of ACh on the auditory cortex of rats, showing similar changes in variance but not in average activity, for the RS [45], suggesting that a global balance between the levels of PVi+ and SSTi+ is essential for observing this effects. We also suggest that acetylcholine may act as a tracer for other modulatory mechanisms affecting synaptic plasticity, such as dopamine. The role of ACh could then be that of allowing the perception of a broader range of the sensory space, while other neuromodulators and mechanisms of synaptic plasticity could potentially select the sensory features that are actually relevant for a given task or situation.
ACh effects on the inhibitory populations modulates standard deviation but not mean excitatory activity. a, b Mean activity of the excitatory population with (M post) or without (M pre) acetylcholine present, for different amounts of noise. The mean activity is shown for neurons responding to the relevant (paired with ACh) or the irrelevant (unpaired) stimuli, or responding to none of them (others). The plots show no major difference on mean activity irrespective of the noise levels (SNR). c, d Same as a, but for the standard deviation of the excitatory population. The neurons not responding to any of the stimuli (others, yellow) are not displayed for clarity
Discussion
We have characterized the anatomical and physiological details of cortical gain control and studied its interactions with acetylcholine. We have further proposed a model that suggests neural mechanisms for the detection and binding of sensory features that are relevant in a specific behavioral state, when ACh is released. Our model suggests that these effects are strongly dependent on two different kinds of inhibitory interneurons. Specifically, acetylcholine could be locally changing the gain control mechanisms in specific cortical areas, strongly influencing information gating and detection. We also observed a reduction of the variance of neurons responsive to relevant stimuli, suggesting a role in biasing synaptic plasticity. Having a reduced variance facilitates synapse formation by correlation-based learning mechanisms. However, the mechanism described does not explain how learning occurs when acetylcholine release is delayed with respect to the presented stimulus. One suggestion would be that acetylcholine plays a role in the consolidation of the memory of that stimulus, that feedback from other cortical or subcortical areas could have re-evoked [46].
Acetylcholine has been associated with plasticity, arousal, and reward, as well as with sensory uncertainty [47] and sustained attention [2]. The observed release of acetylcholine during arousing and rewarding events supports its role in sensory feature detection during behaviorally relevant events. Moreover, the fact that the distinct interneuron populations expressing distinct AChRs are distributed in different proportions across cortical layers and areas indicates a potential role in the regulation of information channels. Greater proportions of PVi+ in intermediate and superficial layers could selectively inhibit thalamic input in layer IV, potentially selecting between feed-forward or feedback information sources. In contrast, frequency modulation in deeper layers of the neocortex has also been suggested to promote feedback communication between different cortical areas [40, 48]. However, data about the proportion of inhibitory cells and acetylcholine receptors is variable for most cortical areas and is spread among different species, limiting the constraints on the model.
Recent findings on memory formation have proposed that memory consolidation could be explained by the combination of different learning mechanisms operating at different time-scales [49]. Our results pose the question of how acetylcholine-mediated plasticity could be accounted in for the formation of long-term memories or even trauma. The facilitative effect of ACh on dopamine release [50] and the involvement of dopamine in memory consolidation in different brain areas [51] pose a clear target for further experiments where ACh-mediated feature selection is followed by plastic changes and memory formation in the cortex.
Future work will then be dedicated to model the effects of simultaneous modulation of inhibition and plasticity on a cortical network, considering and extending the anatomical particularities of inhibition in the neocortex and its layered structure. This is of special relevance to advance in the understanding of mechanisms for learning and education depending on the agent’s behavioral state as well as acceleration of rehabilitation and recuperation of cortical function.
References
Turchi J, Sarter M (1997) Cortical acetylcholine and processing capacity: effects of cortical cholinergic deafferentation on crossmodal divided attention in rats. Cogn Brain Res 6:147–158
Himmelheber AM, Sarter M, Bruno JP (2000) Increases in cortical acetylcholine release during sustained attention performance in rats. Cogn Brain Res 9:313–325
Klinkenberg I, Sambeth A, Blokland A (2011) Acetylcholine and attention. Behav Brain Res 221:430–442
Phillis JW (1968) Acetylcholine release from the cerebral cortex: its role in cortical arousal. Brain Res 7:378–389
Gould R, Nedelcovych M, Dencker D et al (2014) Influence of M1 muscarinic acetylcholine receptor activation on arousal and cognitive performance using electroencephalography and novel touchscreen cognition assessment (845.5). FASEB J 28:845
Jones BE, Cuello AC (1989) Afferents to the basal forebrain cholinergic cell area from pontomesencephalic—catecholamine, serotonin, and acetylcholine—neurons. Neuroscience 31:37–61. doi:10.1016/0306-4522(89)90029-8
Smiley JF, Subramanian M, Mesulam M-M (1999) Monoaminergic–cholinergic interactions in the primate basal forebrain. Neuroscience 93:817–829. doi:10.1016/S0306-4522(99)00116-5
Zaborszky L (1989) Afferent connections of the forebrain cholinergic projection neurons, with special reference to monoaminergic and peptidergic fibers. In: Central cholinergic synaptic transmission. Birkhäuser Basel, pp 12–32. doi:10.1007/978-3-0348-9138-7_2
Carnes KM, Fuller TA, Price JL (1990) Sources of presumptive glutamatergic/aspartatergic afferents to the magnocellular basal forebrain in the rat. J Comp Neurol 302:824–852. doi:10.1002/cne.903020413
Zaborszky L, Gaykema R, Swanson D, Cullinan W (1997) Cortical input to the basal forebrain. Neuroscience 79:1051–1078. doi:10.1016/S0306-4522(97)00049-3
Gastard M, Jensen SL, Martin JR III et al (2002) The caudal sublenticular region/anterior amygdaloid area is the only part of the rat forebrain and mesopontine tegmentum occupied by magnocellular cholinergic neurons that receives outputs from the central division of extended amygdala. Brain Res 957:207–222. doi:10.1016/S0006-8993(02)03513-8
Grove EA (1988) Neural associations of the substantia innominata in the rat: afferent connections. J Comp Neurol 277:315–346. doi:10.1002/cne.902770302
Liu AKL, Chang RC-C, Pearce RKB, Gentleman SM (2015) Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol 129:527–540. doi:10.1007/s00401-015-1392-5
Kawaguchi Y (1997) Selective cholinergic modulation of cortical GABAergic cell subtypes. J Neurophysiol 78:1743–1747. doi:10.1016/0006-8993(82)90067-1
Yi F, Ball J, Stoll KE et al (2014) Direct excitation of parvalbumin-positive interneurons by M1 muscarinic acetylcholine receptors: roles in cellular excitability, inhibitory transmission and cognition. J Physiol 592:3463–3494. doi:10.1113/jphysiol.2014.275453
Lawrence JJ (2008) Cholinergic control of GABA release: emerging parallels between neocortex and hippocampus. Trends Neurosci 31:317–327. doi:10.1016/j.tins.2008.03.008
Markram H, Toledo-Rodriguez M, Wang Y et al (2004) Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 5:793–807. doi:10.1038/nrn1519
Connors BW, Gutnick MJ (1990) Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci 13:99–104
Kawaguchi Y, Kubota Y (1997) GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex 7:476–486. doi:10.1093/cercor/7.6.476
Kawaguchi Y (1993) Groupings of nonpyramidal and pyramidal cells with specific physiological and morphological characteristics in rat frontal cortex. J Neurophysiol 69:416–431
Kubota Y, Hattori R, Yui Y (1994) Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res 649:159–173
Shepherd GM (2003) The synaptic organization of the brain. Oxford University Press, New York
Rudy B, Fishell G, Lee S, Hjerling-leffler J (2010) Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol 71(1):45–61. doi:10.1002/dneu.20853
Disney AA, Aoki C, Hawken MJ (2007) Gain modulation by nicotine in macaque V1. Neuron 56:701–713. doi:10.1016/j.neuron.2007.09.034
Disney AA, Aoki C (2008) Muscarinic acetylcholine receptors in macaque V1 are most frequently expressed by parvalbumin-immunoreactive neurons. J Comp Neurol 507:1748–1762
Disney AA, Alasady HA, Reynolds JH (2014) Muscarinic acetylcholine receptors are expressed by most parvalbumin-immunoreactive neurons in area MT of the macaque. Brain Behav 4:431–445. doi:10.1002/brb3.225
Demars MP, Morishita H (2014) Cortical parvalbumin and somatostatin GABA neurons express distinct endogenous modulators of nicotinic acetylcholine receptors. Mol Brain 7:75. doi:10.1186/s13041-014-0075-9
Markov NT, Misery P, Falchier A et al (2011) Weight consistency specifies regularities of macaque cortical networks. Cereb Cortex 21:1254–1272. doi:10.1093/cercor/bhq201
Benucci A, Verschure PFMJ, König P (2007) Dynamical features of higher-order correlation events: Impact on cortical cells. Cogn Neurodyn. doi:10.1007/s11571-006-9000-y
Markov NT, Ercsey-Ravasz MM, Gomes ARR et al (2012) A weighted and directed interareal connectivity matrix for macaque cerebral cortex. Cereb Cortex bhs270
Puigbò J-Y, van Wijngaarden J, Low SC, Verschure PFMJ (2016) Synaptogenesis: constraining synaptic plasticity based on a distance rule. In: Proc. of the Int. Conf. Artif. Neural Networks in LNCS 9886:28–35. doi:10.1007/978-3-319-44778-0_4
Packer AM, Yuste R (2011) Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci 31:13260–13271
Adesnik H, Bruns W, Taniguchi H (2012) A neural circuit for spatial summation in visual cortex. Nature 490:226–231. doi:10.1038/nature11526
Gulledge AT (2005) Cholinergic inhibition of neocortical pyramidal neurons. J Neurosci 25:10308–10320. doi:10.1523/JNEUROSCI.2697-05.2005
Douglas RJ, Martin K a C (2007) Mapping the matrix: the ways of neocortex. Neuron 56:226–238. doi:10.1016/j.neuron.2007.10.017
Yang X, Wang K, Shamma SA (1992) Auditory representations of acoustic signals. IEEE Trans Inf Theory 38:824–839
Carandini M, Heeger DJ, Movshon JA (1997) Linearity and normalization in simple cells of the macaque primary visual cortex. J Neurosci 17:8621–8644
Hasselmo ME (2006) The role of acetylcholine in learning and memory. Curr Opin Neurobiol 16:710–715. doi:10.1016/j.conb.2006.09.002
Metherate R, Cox CL, Ashe JH (1992) Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci 12:4701–4711
Lee JH, Whittington MA, Kopell NJ (2015) Potential mechanisms underlying intercortical signal regulation via cholinergic neuromodulators. J Neurosci 35:15000–15014. doi:10.1523/JNEUROSCI.0629-15.2015
Callaway EM (2004) Feedforward, feedback and inhibitory connections in primate visual cortex. Neural Netw 17:625–632. doi:10.1016/j.neunet.2004.04.004
Bastos AM, Vezoli J, Bosman CA et al (2015) Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron 85:390–401. doi:10.1016/j.neuron.2014.12.018
Michalareas G, Vezoli J, Van Pelt S et al (2016) Alpha-beta and gamma rhythms subserve feedback and feedforward influences among human visual cortical areas. Neuron 89:384–397. doi:10.1016/j.neuron.2015.12.018
Gross J (2016) Let the rhythm guide you: non-invasive tracking of cortical communication channels. Neuron 89:247. doi:10.1016/j.neuron.2016.01.001
Froemke RC, Carcea I, Barker AJ et al (2013) Long-term modification of cortical synapses improves sensory perception. Nat Neurosci 16:79–88. doi:10.1038/nn.3274
Weinberger NM (2004) Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci 5:279–290. doi:10.1038/nrn1366
Yu AJ, Dayan P (2005) Uncertainty, neuromodulation, and attention. Neuron 46:681–692. doi:10.1016/j.neuron.2005.04.026
Roopun AK, Lebeau FEN, Rammell J et al (2010) Cholinergic neuromodulation controls directed temporal communication in neocortex in vitro. Front Neural Circuits 4:8. doi:10.3389/fncir.2010.00008
Benna MK, Fusi S (2016) Computational principles of synaptic memory consolidation. Nat Neurosci 19:1697–1706. doi:10.1038/nn.4401
Aosaki T, Miura M, Suzuki T et al (2010) Acetylcholine-dopamine balance hypothesis in the striatum: an update. Geriatr Gerontol Int. doi:10.1111/j.1447-0594.2010.00588.x
Lalumiere RT, Mcgaugh JL (2005) Memory enhancement induced by post-training intrabasolateral amygdala infusions of β-adrenergic or muscarinic agonists requires activation of dopamine receptors: involvement of right, but not left, basolateral amygdala. Learn Mem 12:527–532. doi:10.1101/lm.97405
Acknowledgments
This work has been supported by the European Research Council’s CDAC project: “The Role of Consciousness in Adaptive Behavior: A Combined Empirical, Computational and Robot based Approach” (ERC-2013- ADG 341196); as well as the European Project What You Say Is What You Did WYSIWYD (FP7 ICT 612139).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Puigbò, JY., Maffei, G., Herreros, I. et al. Cholinergic Behavior State-Dependent Mechanisms of Neocortical Gain Control: a Neurocomputational Study. Mol Neurobiol 55, 249–257 (2018). https://doi.org/10.1007/s12035-017-0737-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0737-6