Abstract
Parvalbumin (PV) interneurons are critically involved in the cognitive processes. Based on prior investigations that environmental enrichment reverses impaired cognition after anesthetic exposure, we proposed that environmental enrichment protects PV interneurons and thereby improves sevoflurane-induced cognitive impairments. Six-day-old C57BL/6 male mice were exposed to 3 % sevoflurane or 30 % oxygen/air 2 h daily for 3 days from postnatal day 6 (P6) to P8. The mice were randomly allocated to an enriched environment for 2 h daily between P8 and P90 or a standard environment. Western blotting and immunofluorescence were used for determining PV expression in the prefrontal cortex and hippocampus. In another set of experiments, cognitive tests were assessed by the open field test (P41), Morris water maze test (P54–60), and fear conditioning tests (P42–43 and P89–90). Exposure of neonatal mice to sevoflurane resulted in a reduced freezing response in the contextual test at P43 but not P90. The PV expression in these mice was decreased at P9, P14, P28, and P42, but not at ≥P60. No colocalization of caspase-3 and 5-bromo-2-deoxyuridine or caspase-3 and PV was observed, suggesting that caspase-independent pathways may be involved in the mediation of sevoflurane-induced down-regulation of PV. The sevoflurane-exposed mice that were placed in an enriched environment exhibited normal behavior and had PV interneurons that did not differ from those in the control mice at P42–43. Neonatal sevoflurane exposure induces a reduced freezing response in the contextual test at P43 and developmental delays in PV interneurons in the prefrontal cortex and hippocampus. Placement of the sevoflurane-exposed mice in an enriched environment can prevent these abnormalities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sevoflurane is currently a common inhalational anesthetic for general anesthesia induction and maintenance, especially for obstetric and pediatric patients [1–4]. Several lines of evidence from animal studies suggest the clinically relevant concentration of sevoflurane exposure can induce robust neuroapoptosis in the neonatal brain and cause subsequent long-term neurobehavioral abnormalities later in life [1–4]. Several mechanisms, including the compromise of mitochondrial integrity and neuroapoptosis [5], a decrease in the brain-derived neurotrophic factor [6], the enhancing of gamma-aminobutyric acid type A receptor-mediated excitation [7], an increase in the levels of stress hormones [8], activation of the inflammatory signaling pathways [9], and inhibition of neurogenesis [10] have been proposed, but the mechanisms underlying sevoflurane-induced neurocognitive impairment remain to be elucidated.
Parvalbumin (PV) interneurons are a subset of inhibitory GABAergic neurons that control the excitability of post-synaptic pyramidal neurons [11]. Abnormalities in PV interneurons are apparent in several neuropsychological diseases, including epilepsy [12], schizophrenia [13], Alzheimer’s disease [14], and depression [15]. Disturbances in the functional maturation of PV interneurons during the peri-adolescent period may trigger the occurrence of schizophrenia, while maternal separation of male rats leads to a reduction of PV expression in the prefrontal cortex (PFC) and causes subsequent working memory impairments in adolescence [16]. PV is yet to be expressed by P7 and PV interneurons undergo final differentiation within postnatal day 21; hence, their maturation could be susceptible to environmental insult during this critical period [17]. Although a single sevoflurane exposure to neonatal rats causes the loss of pyramidal neurons in the hippocampus [2], whether sevoflurane exposure during brain development, especially during the brain growth spurt, affects PV interneurons is poorly understood.
In the current study, we investigated whether the developmental trajectories of PV interneurons in the PFC and hippocampus are altered after neonatal sevoflurane exposure in mice. Because previous studies have shown that an enriched environment can normalize PV interneurons under many pathological states [18, 19], the second aim of the present study was to assess whether an enriched environment can reverse cognitive impairments and normalize the neurochemical profile of PV interneurons associated with sevoflurane exposure.
Materials and Methods
Animals and Housing
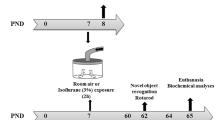
The present study was performed in accordance with the ARRIVE guidelines and the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health (Bethesda, MD, USA), and was approved by the Ethics Committee of Jinling Hospital, Nanjing Medical University, China. C57BL/6 dams (n = 42) with litters containing male pups (n = 324) were purchased from Nanjing University, Nanjing, China, and were housed under controlled illumination (12-h light/dark, lights on at 7:00 a.m.) and temperature (24 ± 1 °C) with free access to food and water. To minimize the influence of litter variability, no more than two pups from each litter were used for each experimental subgroup. Furthermore, we used only male offspring to exclude the influence of estrogen on the biochemical data and neurocognitive function. Six-day-old male C57BL/6 mice were randomly assigned to one of the following four groups: control + standard environment group (n = 98), control + enriched environment group (n = 64), sevoflurane + standard environment group (n = 98), or sevoflurane + enriched environment group (n = 64). The flow chart of the experimental protocol is displayed in Fig. 1a.
a Schematic timeline of the experimental procedure; b photographs of SE and EE; and c effects of sevoflurane exposure on body weight gain in mice. Data are expressed as mean ± SEM. OF, open field; FC, fear conditioning; MWM, Morris water maze; SE, standard environment; EE, enriched environment; WB, western blotting; IF, immunofluorescence
Anesthesia
Mice in the sevoflurane groups received 3 % sevoflurane (30 % oxygen/air) for 2 h a day for three consecutive days in the anesthetizing chamber where the animals were kept warm on a plate to maintain a rectal temperature of 37 ± 0.5 °C, as described in our previous study [9]. Mice in the control group received 30 % oxygen/air at the same flow rate in a similar chamber. The mice breathed spontaneously, and the sevoflurane and oxygen concentrations were measured continuously (GE Datex-ohmeda, Tewksbury, MA, USA). The mice were returned to their mothers after return of the righting reflex upon termination of the sevoflurane exposure.
Experimental Design
The enriched environment in the present study was performed as described in previous study with minor modifications [18, 19]. The mice in the enriched environment groups were put into the enriched environment (60 cm × 50 cm × 40 cm) every day for 2 h from P8–P90, while the mice in the standard environment groups were moved to the new cage (30 cm × 25 cm × 15 cm) with bedding but no toys for 2 h/day throughout the experiment (Fig. 1b). To maintain novelty, the objects in the cage were changed two to three times per week to provide challenging stimulation. The mice were allowed to stay with mothers from P8 to P21 and were then weaned from P22 to P90 in all groups. Afterward, the mice underwent one of the three experimental protocols: (1) euthanized at P9 (short-term effects of sevoflurane exposure on PV expression), P14 (critical developmental period of PV interneuron), P28 (maturation of PV interneuron), P42 (adolescence), P60 (early adulthood), and P90 (adulthood) to determine whether sevoflurane exposure affected PV interneurons; (2) euthanized 7 h after the last sevoflurane exposure to investigate whether sevoflurane exposure-induced developmental neuroapoptosis affected PV interneurons; and (3) sacrificed 7 h after the last sevoflurane exposure to define whether sevoflurane exposure affected neurogenesis. For this purpose, P8 mice were i.p. injected with 5-bromo-2-deoxyuridine (BrdU, 50 mg kg−1, 2×, 4.5 h apart; Sigma-Aldrich, St. Louis, MO, USA) just prior to the last sevoflurane exposure, and killed for brain tissue collection 9 h later. We selected this time interval on the basis of the following two reasons: it is within one cell cycle and it is near the peak time after sevoflurane exposure.
Arterial Blood Gas Analysis
Before blood sampling, all mice received 3 % sevoflurane in 30 % oxygen for 5 min to prevent pain and hyperpnea. Arterial blood sampling from the left cardiac ventricle was performed as previously described with minor modifications [9]. Arterial blood gas analysis was performed immediately after the last sevoflurane exposure or 30 % O2 exposure (P8) with an arterial blood gas analyzer (GEM Premier 3000, Instrumentation Laboratory, Guangzhou, China).
Behavioral and Cognitive Tests
The behavioral and cognitive alternations were assessed by the open field test (P41), Morris water maze test (P54–60), and fear conditioning tests (P42–43 and P89–90, respectively). All behavioral procedures were conducted at daytime from 2:00 PM to 5:00 PM in a sound-isolated room. All behavioral tests were recorded by the same investigator, who was blinded to the animal grouping as previously described [9, 20]. Those animals were not subjected to further laboratory analysis.
Open Field Test
The open field test was performed to detect locomotor activity at P41. Each mouse was released in the center of the arena and its habituation to an open field was tested systematically within the same plastic chamber (40 cm × 40 cm × 45 cm) for 5 min. The total distance traveled and the time spent in the center of the open field were recorded. The arena was cleaned with 75 % ethanol to avoid the presence of olfactory cues.
Fear Conditioning Test
Considering sevoflurane exposure can result in persistent cognitive impairments as assessed in the fear conditioning test [21], we performed this test at P42 to 43 and then again at P89 to 90. This test was examined in a black plastic chamber, with a stainless steel grid floor. The mice were allowed to explore for 3 min for habituation, then a 30 s, 80 dB, 1 kHz tone (CS), which co-terminated with a 2 s, 0.75 mA foot shock (US) was delivered through stainless steel bars by a constant current generator. A contextual test was performed in the conditioning chamber for 5 min without any stimulation 24 h after the conditioning trial. A cued test was performed by presentation of the cue (80 dB noise, 3 min duration) in another context with distinct visual and tactile cues. The cued test was conducted 2 h after the contextual test.
Morris Water Maze Test
Spatial learning and memory function of the mice was evaluated by the Morris water maze at P54–60. The water maze pool (120 cm in diameter) contained opaque water and a platform (10 cm in diameter) was placed in one quadrant of the pool with the top of the platform 1 cm below the water surface. For spatial training sessions, mice were given four trials per day to locate the hidden platform for six consecutive days. Each trial began with the mouse being released facing the side wall at one of the four quadrants and given 60 s to locate the hidden platform, the quadrant where the platform was previously situated was considered as the target quadrant (T) and the remaining quadrants were defined as opposite (O), right (R), and left (L), respectively. The mouse was allowed 60 s to find the platform upon which they sat for 30 s. If the mouse did not find the platform within 60 s, it was gently guided there and allowed to stay for 30 s. Twenty-four hours after the last training session, the platform was removed from the pool and the mouse was placed in the opposite quadrant, and a 60-s probe trial was performed.
Western Blotting Analysis
Mice were anesthetized by i.p. injection of sodium pentobarbital (50 mg kg−1) and the PFC and hippocampus were harvested at P9, P14, P28, P42, P60, and P90 for western blotting analysis. The samples were homogenized in ice-cold lysis buffer (1 % Nonidet P-40, 0.1 % sodium deoxycholate, 0.1 % SDS, 66 mM EDTA, 10 mM Tris-HCl, pH 7.4) supplemented with protease inhibitor cocktail, and centrifuged at 13,000×g for 10 min at 4 °C. The supernatant was saved and its protein concentration was determined by Bradford assay. Forty micrograms of proteins per lane was separated on SDS-PAGE gels. The separated proteins were then transferred to polyvinylidine fluoride membranes. After blocking with 5 % skimmed milk in Tris-buffered saline with Tween (TBST), the membranes were incubated with goat anti-PV (1:1000; Abcam, Cambridge, UK) and mouse anti-GADPH (1:1000; Cell Signaling, Boston, MA, USA) overnight at 4 °C room. After washing in TBST three times, horseradish peroxidase (HRP) conjugated secondary antibodies (rabbit anti-goat and goat anti-mouse [Bioworld Technology, Inc., USA]) diluted 1:5000 were used to incubate the membranes for 1 h at room temperature. The protein bands were detected by enhanced chemiluminescence, exposed onto X-ray film, and quantified with Image J software (National Institutes of Health, Bethesda, MD, USA). The results from animals under various experimental conditions then were normalized by the mean values of the corresponding control animals.
Immunofluorescence
The mice were anesthetized with 2 % sodium pentobarbital (50 mg kg−1, i.p., Sigma) and transcardially perfused with 30 mL of saline followed by 4 % paraformaldehyde in phosphate-buffered saline (pH 7.4). Brains were immediately removed, postfixed in the same 4 % paraformaldehyde for 2 h, and dehydrated in 30 % sucrose at 4 °C overnight. The brains were embedded in optimum cutting temperature compound, cut in 10-μm-thick sections on a freezing microtome, and mounted on glass slides. Slices were blocked with 1 % bovine serum albumin for 1 h at room temperature followed by incubating the following primary antibodies: rabbit anti-PV (1:600; Abcam, Cambridge, UK), mouse-anti Brdu (1:200; Sigma, St. Louis, MO, USA), mouse anti-PV (1:600; Millipore, MA, USA), and rabbit anti-caspase-3 (1:200; Cell Signaling, Boston, MA, USA) in 1 % bovine serum albumin at 4 °C overnight. After three washes with phosphate-buffered saline, sections were incubated with the secondary antibodies, including goat anti-rabbit IgG-FITC (1:300; Santa Cruz Biotechnologies, Dallas, TX, USA) and goat anti-mouse IgG-cy3 (1:600; Bioworld, MN, USA) for 1 h at room temperature. After washing out the secondary antibody, sections were incubated with 4′, 6-diamidino-2-phenylindole (DAPI) for nuclear staining. The fluorescent images were captured by a confocal microscope (Olympus, Melville, NY, USA).
Statistical Analysis
Data are presented as mean ± standard error of measurement (SEM). The Statistical Program for Social Sciences software (Version 16.0; SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The normality assumption test was performed using the Shapiro-Wilk test with the P value set at 0.05. Single comparisons were tested using the unpaired t test. Multiple comparisons used one-way, two-way, or repeated-measures of analysis of variance (ANOVA) followed by a Bonferroni test. Bivariate relationship was evaluated by the Pearson correlation coefficients. A P value of <0.05 was regarded as a statistically significant difference.
Results
There was no significant difference in body weight gain among the groups throughout the experiment (P > 0.05, two-way ANOVA; Fig. 1c). In addition, repeated exposure of neonatal mice to sevoflurane did not significantly change the pH, arterial oxygen (PO2), or carbon dioxide tension (PCO2) values compared with the control group (all P > 0.05, unpaired t test).
No significant difference was observed in the total locomotor activity (F 3, 44 = 0.257, P = 0.856, one-way ANOVA; Fig. 2a) and the time spent in the center (F 3, 44 = 0.03, P = 0.993, one-way ANOVA; Fig. 2b) among the four groups. The freezing time of mice exposed to sevoflurane was significantly reduced in the contextual test when compared with those of controls at P43 (F 3, 44 = 4.828, P = 0.005, one-way ANOVA; Fig. 2c). However, there was no significant difference in the freezing time to tone between the groups at P43 (F 3, 44 = 0.247, P = 0.863, one-way ANOVA; Fig. 2d). An enriched environment significantly reversed the decreased freezing time to context of the sevoflurane-treated mice. However, no difference in the freezing time to context (F 3, 44 = 0.084, P = 0.968, one-way ANOVA; Fig. 2e) or the freezing time to tone (F 3, 44 = 0.303, P = 0.823, one-way ANOVA; Fig. 2f) was observed among the four groups at P90. There was no difference in the escape latency (F 3, 44 = 2.104, P = 0.113, repeated-measures of ANOVA; Fig. 2g) and the time spent in the target quadrant (F 3, 44 = 1.014, P = 0.395, one-way ANOVA; Fig. 2h) among the four groups.
Sevoflurane exposure-induced cognitive impairments were reversed by enriched environment. a The total locomotor activity and b the time spent in the center in the open field test (n = 12). c The freezing time to context and d the freezing time to cue at P43 in the fear conditioning test (n = 12). e The freezing time to context and f the freezing time to cue at P90 in the fear conditioning test (n = 12). g Escape latency and h the time spent in the target quadrant in the Morris water maze (n = 12). Data are expressed as mean ± SEM. SE, standard environment; EE, enriched environment
Sevoflurane exposure decreased PV expression at P9 (PFC: t = 5.88, P < 0.001; hippocampus: t = 7.571, P < 0.001, unpaired t test; Fig. 3a, b), P14 (PFC: t = 6.424, P < 0.001; hippocampus: t = 5.807, P < 0.001, unpaired t test; Fig. 3a, b), P28 (PFC: F 3, 16 = 15.749, P < 0.001; hippocampus: F 3, 16 = 5.828, P = 0.007, one-way ANOVA; Fig. 3c, d), and P42 (PFC: F 3, 16 = 13.206, P < 0.001; hippocampus: F 3, 16 = 3.592, P = 0.037, one-way ANOVA; Fig. 5c, d) compared with those of controls, whereas an enriched environment was able to reverse the decreased PV expression for the sevoflurane-treated mice at both P28 and P42. However, the decreased PV expression recovered at P60 (PFC: t = 0.535, P = 0.608; hippocampus: t = 0.48, P = 0.644, unpaired t test; Fig. 3g, h) and thereafter at P90 (PFC: t = 0.885, P = 0.402; hippocampus: t = 0.613, P = 0.701, unpaired t test; Fig. 3g, h).
Effects of sevoflurane and EE on PV levels in the PFC and hippocampus by western blotting. a Representative images of PV in the PFC and hippocampus by western blotting at P9 and P14 and b band density analysis of PV levels in the PFC and hippocampus at P9 and P14 (n = 5), *P < 0.05 vs. control. c Representative images of PV in the PFC and hippocampus by western blotting at P28 and d band density analysis of PV levels in the PFC and hippocampus at P28 (n = 5), *P < 0.05 vs. sevoflurane+SE. e Representative images of PV in the PFC and hippocampus by western blotting at P60 and f band density analysis of PV levels in the PFC and hippocampus at P60 (n = 5). g Representative images of PV in the PFC and hippocampus by western blotting at P90 and h band density analysis of PV levels in the PFC and hippocampus at P90 (n = 5). Data are expressed as mean ± SEM. SE, standard environment; EE, enriched environment; PV, parvalbumin; PFC, prefrontal cortex; Hip, hippocampus
Consistent with the western blotting data, the immunofluorescence results also revealed sevoflurane exposure induced a significant decrease in PV expression at P9 (PFC: t = 9.892, P < 0.001; CA1: t = 6.608, P < 0.001; CA3: t = 12.408, P < 0.001, unpaired t test), P14 (PFC: t = 8.44, P < 0.001; CA1: t = 4.448, P < 0.001; CA3: t = 6.897, P < 0.001, unpaired t test; Fig. 4a, b), P28 (PFC: F 3, 28 = 8.671, P < 0.001; CA1: F 3, 28 = 8.53, P < 0.001; CA3: F 3, 28 = 19.921, P < 0.001, one-way ANOVA; Fig. 3c, d), and P42 (PFC: F 3, 28 = 8.413, P < 0.001; CA1: F 3, 28 = 12.18, P < 0.001; CA3: F 3, 28 = 17.46, P < 0.001, one-way ANOVA; Fig. 5a, b) when compared with those of controls. An enriched environment was sufficient to normalize the decreased PV expression for the sevoflurane-treated mice at both P28 and P42. In addition, the linear regression analysis showed that PV expression in the PFC and hippocampus was positively correlated with the freezing time to context at P42 (P < 0.001; Fig. 5e, f). However, we found that sevoflurane or EE did not affect the number of PV interneurons in the PFC and hippocampus (data not shown).
Effects of sevoflurane and EE on PV expression in the PFC and CA1 and CA3 regions of hippocampus by immunofluorescence staining. Representative images of a PV in the PFC, the CA1, and CA3 regions of the hippocampus at P9 and P14 (nuclei counterstained with DAPI, scale bar 100 μm). Fluorescence intensity of b PV in the PFC, CA1, and CA3 regions of the hippocampus at P9 and P14 (n = 8), *P < 0.05 vs. control. Representative images of c PV in the PFC, the CA1, and CA3 regions of the hippocampus at P28 (nuclei counterstained with DAPI, scale bar 100 μm). Fluorescence intensity of d PV in the PFC, CA1, and CA3 regions of the hippocampus at P28 (n = 8), *P < 0.05 vs. sevoflurane+SE. Representative images of e PV in the PFC, the CA1, and CA3 regions of the hippocampus at P60 and P90 (nuclei counterstained with DAPI, scale bar 100 μm). Fluorescence intensity of f PV in the PFC, CA1, and CA3 regions of the hippocampus at P60 and P90 (n = 8), *P < 0.05 vs. control. Data are expressed as mean ± SEM. SE, standard environment; EE, enriched environment; PV, parvalbumin; PFC, prefrontal cortex
Effects of sevoflurane and EE on PV levels in the PFC and CA1 and CA3 regions of hippocampus at P42–43. Representative images of a PV in the PFC, CA1, and CA3 regions of the hippocampus at P42 (nuclei counterstained with DAPI, scale bar 100 μm). Fluorescence intensity of b PV in the PFC, CA1, and CA3 regions of the hippocampus at P42 (n = 8). c Representative image of a single western blotting and d band density analysis of PV levels in the PFC and hippocampus at P42 (n = 5). e, f Correlation analysis of the freezing time to context and PV levels in the PFC and hippocampus (n = 16). Data are expressed as mean ± SEM. *P < 0.05 vs. the sevoflurane+SE group. SE, standard environment; EE, enriched environment; PV, parvalbumin; PFC, prefrontal cortex; Hip, hippocampus
The immunofluorescence data did not show colocalization of caspase-3 in the majority of PV-positive neurons in the PFC (Fig. 6a), indicating the abnormalities in PV interneurons are not due to the apoptotic effects of sevoflurane exposure. Also, there was no colocalization of caspase-3 or BrdU in the PFC (Fig. 6b), suggesting sevoflurane exposure did not target the proliferating neurons during this stage of development.
Double-immunofluorescence staining to detect colocalization of PV and caspase-3 or caspase-3 and BrdU in the PFC. a Representative images of PV (green) and caspase-3 (red) in the PFC (nuclei counterstained with DAPI (blue), scale bar 30 μm, n = 8). b Representative images of caspase-3 (green) and BrdU (red) in the PFC (nuclei counterstained with DAPI, scale bar 30 μm, n = 8). PV, parvalbumin; Brdu, 5-bromo-2-deoxyuridine
Discussion
In this study, we found that repeated exposure of neonatal mice to sevoflurane resulted in unaltered behavior in the open field and Morris water maze tests. These findings are in agreement with reports from other laboratories suggesting that mice could be more resistant to the developmental effects of general anesthetics when compared to neonatal rats [22]. Despite obvious resistivity of neonatal mice to the developmental effects of sevoflurane, we did find a significant impairment in freezing response in the contextual test at P43 that was accompanied by neurochemical disturbances in PV interneurons in the PFC and hippocampus. Development of these abnormalities was precluded in mice periodically exposed to the enriched environment providing indirect evidence that impairments in PV expression and freezing response in the contextual test may be causally linked. Importantly, our findings indicate that these sevoflurane-induced developmental effects in neonatal mice are transient in nature. The transient effects of sevoflurane could be another reason why we were not able to detect behavioral changes in the Morris water maze at P54. Thus, another study in mice using similar protocol, anesthesia with 3 % sevoflurane at P6, found spatial learning and memory impairments in the Morris water maze test performed at P30–P36 [3].
Accumulating evidence has suggested drugs that block N-methyl-D-aspartate (NMDA) receptors and/or activation of gamma-aminobutyric acid receptors can cause widespread neuroapoptosis in the developing brain and result in subsequent long-term neurobehavioral abnormalities later in life [1, 23]. Sevoflurane is an inhalation anesthetic commonly used in obstetric or pediatric surgery and has been shown as a NMDA receptor antagonist [1]. It has been proposed that 3 % sevoflurane exposure to P6 mice for 6 h causes long-lasting neurocognitive dysfunction [1]. However, in another study comparing isoflurane to sevoflurane exposure in P7 rats, neither isoflurane nor sevoflurane was associated with impaired cognitive impairments when tested from 31 to 40 days after the anesthetics exposure [24]. Moreover, one recent study suggested that sevoflurane anesthesia even improves cognitive impairments, as reflected by reduced latency to find the hidden platform in both young adult and aged rats treatment when tested 1 week after exposure [25]. These discrepancies might be attributed to the differences in methods of anesthetic exposure, animal species (rats vs. mice), pharmacology (sevoflurane vs. isoflurane), anesthetic concentrations, anesthetic durations, and time to perform the cognition tests. However, the mechanism by which neonatal sevoflurane exposure induces cognitive impairments remains poorly understood.
PV interneurons represent a subpopulation of GABAergic interneurons that have been shown to play a key role in the generation of gamma oscillations, which provide a fundamental mechanism of information processing during sensory perception, motor behavior, and memory formation by coordinating neuronal activity in networks of the hippocampus and neocortex [11]. Dysfunction of PV interneurons, as evidenced by decreased expression of GABA-related genes such as the 67-kDa isoform of glutamate decarboxylase or PV, is closely linked to the cognitive deficits in schizophrenia [26] and Alzheimer’s disease [14]. This apparent phenotype loss (represented by decreased expression of PV) in PV interneurons led to the suggestion that dysfunction of these PV interneurons may have substantial functional repercussions on local inhibitory processes in the hippocampus and neocortex. Interestingly, sevoflurane is reported to cause epileptiform electroencephalographic activity [8], which may reflect a disruption in the normal balance of excitation and inhibition in the brain. The hyperexcitatory events raise significant concerns because they may potentially lead to subsequent cognitive impairments [21]. Because PV interneurons control the excitability of post-synaptic pyramidal neurons, it is possible that reduced PV expression results in diminished cortical inhibitory drive, which ultimately may lead to epileptiform electroencephalogram and consequent cognitive impairments.
Convergent evidence suggests the balance between excitation and inhibition is fundamental for the development of functional neuronal networks throughout the period of postnatal development [27, 28]. In contrast to excitatory neurons, inhibitory interneurons have multiple phenotypes that vary in morphology, neurochemistry, and physiology, and represent only 20 to 30 % of neurons in the cortex [11]. In addition, inhibitory networks play a key role in the experience-dependent refinement of neural networks that last through the first postnatal 4 weeks [29, 30]. In the present study, we show that repeated sevoflurane exposure caused a significantly decreased PV expression. It has been demonstrated that acute ketamine administration induces PV interneuron phenotype loss during the period of highest vulnerability to prenatal stress [31]. Because sevoflurane can function as an NMDA receptor antagonist, it is not surprising that sevoflurane exposure can also result in PV interneuron phenotype loss. Importantly, we report here that this PV interneuron phenotype loss co-occurs with the appearance of cognitive impairments, highlighting a possible association between PV interneuron dysfunction and consequent cognitive impairments associated with sevoflurane exposure.
Because it has been suggested that an enriched environment can normalize PV interneurons under many pathological states [18, 19], we compared mice reared in an enriched environment to those reared in standard housing. An enriched environment is a non-invasive, non-pharmacological intervention that exerts beneficial effects on cognitive impairments in various brain diseases [32], including inhalational anesthetics exposure-induced cognitive impairments [33, 34]. Notably, we found that the sevoflurane-exposed mice that were placed in an enriched environment exhibited almost normal PV expression, while 1 month of an enriched environment was sufficient to reverse sevoflurane exposure-induced cognitive impairments and prevent the decrease in PV expression compared to housing in a standard environment. By contrast, early life stress such as maternal separation leads to a reduction of PV expression in the PFC and causes subsequent working memory impairments in adolescence [16]. Since activity-dependent regulation of gene expression is critical for inhibitory network remodeling through life experiences [29, 30], it is possible that beneficial effects of an enriched environment might be related to its ability to enhance neuronal viability or possibly by providing sufficient compensatory inhibition to the overexcited hippocampal circuitry. Finally, our results revealed that there was no colocalization of caspase-3 and PV interneurons, suggesting that caspase-independent pathways may be involved in mediation of the decreased PV expression induced by sevoflurane [35]. Also, there was no colocalization of BrdU and PV interneuron, indicating the abnormalities of PV interneurons were not due to the effect of sevoflurane on the proliferating cells.
In summary, our results show that repeated exposure of neonatal mice to sevoflurane resulted in altered PV expression and freezing response in the contextual test later in life, while enriched environment ameliorated all these abnormalities.
References
Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J (2009) Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology 110(3):628–637. doi:10.1097/ALN.0b013e3181974fa2
Feng X, Liu JJ, Zhou X, Song FH, Yang XY, Chen XS, Huang WQ, Zhou LH, Ye JH (2012) Single sevoflurane exposure decreases neuronal nitric oxide synthase levels in the hippocampus of developing rats. Br J Anaesth 109(2):225–233. doi:10.1093/bja/aes121
Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, Sun D, Baxter MG, Zhang Y, Xie Z (2013) Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology 118(3):502–515. doi:10.1097/ALN.0b013e3182834d77
Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO (2009) Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 110(4):796–804. doi:10.1097/01.anes.0000344728.34332.5d
Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z (2012) Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol 71(5):687–698. doi:10.1002/ana.23536
Head BP, Patel HH, Niesman IR, Drummond JC, Roth DM, Patel PM (2009) Inhibition of p75 neurotrophin receptor attenuates isoflurane-mediated neuronal apoptosis in the neonatal central nervous system. Anesthesiology 110(4):813–825. doi:10.1097/ALN.0b013e31819b602b
Edwards DA, Shah HP, Cao W, Gravenstein N, Seubert CN, Martynyuk AE (2010) Bumetanide alleviates epileptogenic and neurotoxic effects of sevoflurane in neonatal rat brain. Anesthesiology 112(3):567–575. doi:10.1097/ALN.0b013e3181cf9138
Cao W, Pavlinec C, Gravenstein N, Seubert CN, Martynyuk AE (2012) Roles of aldosterone and oxytocin in abnormalities caused by sevoflurane anesthesia in neonatal rats. Anesthesiology 117(4):791–800
Ji MH, Qiu LL, Yang JJ, Zhang H, Sun XR, Zhu SH, Li WY, Yang JJ (2015) Pre-administration of curcumin prevents neonatal sevoflurane exposure-induced neurobehavioral abnormalities in mice. Neurotoxicology 46:155–164. doi:10.1016/j.neuro.2014.11.003
Zhu C, Gao J, Karlsson N, Li Q, Zhang Y, Huang Z, Li H, Kuhn HG, Blomgren K (2010) Isoflurane anesthesia induced persistent, progressive memory impairment, caused a loss of neural stem cells, and reduced neurogenesis in young, but not adult, rodents. J Cereb Blood Flow Metab 30(5):1017–1030. doi:10.1038/jcbfm.2009.274
Hu H, Gan J, Jonas P (2014) Interneurons. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science 345(6196):1255263. doi:10.1126/science.1255263
Li KX, Lu YM, Xu ZH, Zhang J, Zhu JM, Zhang JM, Cao SX, Chen XJ, Chen Z, Luo JH, Duan S, Li XM (2011) Neuregulin 1 regulates excitability of fast-spiking neurons through Kv1.1 and acts in epilepsy. Nat Neurosci 15(2):267–273. doi:10.1038/nn.3006
Del Pino I, García-Frigola C, Dehorter N, Brotons-Mas JR, Alvarez-Salvado E, Martínez de Lagrán M, Ciceri G, Gabaldón MV, Moratal D, Dierssen M, Canals S, Marín O, Rico B (2013) Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron 79(6):1152–1168. doi:10.1016/j.neuron.2013.07.010
Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ (2012) Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149(3):708–721. doi:10.1016/j.cell.2012.02.046
Zhou Z, Zhang G, Li X, Liu X, Wang N, Qiu L, Liu W, Zuo Z, Yang J (2015) Loss of phenotype of parvalbumin interneurons in rat prefrontal cortex is involved in antidepressant- and propsychotic-like behaviors following acute and repeated ketamine administration. Mol Neurobiol 51(2):808–819. doi:10.1007/s12035-014-8798-2
Brenhouse HC, Andersen SL (2011) Nonsteroidal anti-inflammatory treatment prevents delayed effects of early life stress in rats. Biol Psychiatry 70(5):434–440. doi:10.1016/j.biopsych.2011.05.006
Yang JM, Zhang J, Yu YQ, Duan S, Li XM (2014) Postnatal development of 2 microcircuits involving fast-spiking interneurons in the mouse prefrontal cortex. Cereb Cortex 24(1):98–109. doi:10.1093/cercor/bhs291
Inácio AR, Ruscher K, Wieloch T (2011) Enriched environment downregulates macrophage migration inhibitory factor and increases parvalbumin in the brain following experimental stroke. Neurobiol Dis 41(2):270–278. doi:10.1016/j.nbd.2010.09.015
Komitova M, Xenos D, Salmaso N, Tran KM, Brand T, Schwartz ML, Ment L, Vaccarino FM (2013) Hypoxia-induced developmental delays of inhibitory interneurons are reversed by environmental enrichment in the postnatal mouse forebrain. J Neurosci 33(33):13375–11387. doi:10.1523/JNEUROSCI.5286-12.2013
Ji M, Dong L, Jia M, Liu W, Zhang M, Ju L, Yang J, Xie Z, Yang J (2014) Epigenetic enhancement of brain-derived neurotrophic factor signaling pathway improves cognitive impairments induced by isoflurane exposure in aged rats. Mol Neurobiol 50(3):937–944. doi:10.1007/s12035-014-8659-z
Cornejo BJ, Mesches MH, Coultrap S, Browning MD, Benke TA (2007) A single episode of neonatal seizures permanently alters glutamatergic synapses. Ann Neurol 61(5):411–426
Loepke AW, Istaphanous GK, McAuliffe JJ 3rd, Miles L, Hughes EA, McCann JC, Harlow KE, Kurth CD, Williams MT, Vorhees CV, Danzer SC (2009) The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg 108(1):90–104. doi:10.1213/ane.0b013e31818cdb29
Yonamine R, Satoh Y, Kodama M, Araki Y, Kazama T (2013) Coadministration of hydrogen gas as part of the carrier gas mixture suppresses neuronal apoptosis and subsequent behavioral deficits caused by neonatal exposure to sevoflurane in mice. Anesthesiology 118(1):105–113. doi:10.1097/ALN.0b013e318275146d
Liang G, Ward C, Peng J, Zhao Y, Huang B, Wei H (2010) Isoflurane causes greater neurodegeneration than an equivalent exposure of sevoflurane in the developing brain of neonatal mice. Anesthesiology 112(6):1325–34. doi:10.1097/ALN.0b013e3181d94da5
Callaway JK, Jones NC, Royse AG, Royse CF (2012) Sevoflurane anesthesia does not impair acquisition learning or memory in the Morris water maze in young adult and aged rats. Anesthesiology 117(5):1091–1101. doi:10.1097/ALN.0b013e31826cb228
Jiang Z, Rompala GR, Zhang S, Cowell RM, Nakazawa K (2013) Social isolation exacerbates schizophrenia-like phenotypes via oxidative stress in cortical interneurons. Biol Psychiatry 73(10):1024–1034. doi:10.1016/j.biopsych.2012.12.004
Doischer D, Hosp JA, Yanagawa Y, Obata K, Jonas P, Vida I, Bartos M (2008) Postnatal differentiation of basket cells from slow to fast signaling devices. J Neurosci 28(48):12956–12968. doi:10.1523/JNEUROSCI.2890-08.2008
Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF (1998) Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 282(5393):1504–1508
Donato F, Rompani SB, Caroni P (2013) Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature 504(7479):272–276. doi:10.1038/nature12866
Shinohara Y, Hosoya A, Hirase H (2013) Experience enhances gamma oscillations and interhemispheric asymmetry in the hippocampus. Nat Commun 4:1652. doi:10.1038/ncomms2658
Wang CZ, Yang SF, Xia Y, Johnson KM (2008) Postnatal phencyclidine administration selectively reduces adult cortical parvalbumin-containing interneurons. Neuropsychopharmacology 33(10):2442–2455
Nithianantharajah J, Hannan AJ (2006) Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci 7(9):697–709
Shih J, May LD, Gonzalez HE, Lee EW, Alvi RS, Sall JW, Rau V, Bickler PE, Lalchandani GR, Yusupova M, Woodward E, Kang H, Wilk AJ, Carlston CM, Mendoza MV, Guggenheim JN, Schaefer M, Rowe AM, Stratmann G (2012) Delayed environmental enrichment reverses sevoflurane-induced memory impairment in rats. Anesthesiology 116(3):586–602. doi:10.1097/ALN.0b013e318247564d
Fan D, Li J, Zheng B, Hua L, Zuo Z (2014) Enriched environment attenuates surgery-induced impairment of learning, memory, and neurogenesis possibly by preserving BDNF expression. Mol Neurobiol 53(1): 344−354
Schoch KM, Madathil SK, Saatman KE (2012) Genetic manipulation of cell death and neuroplasticity pathways in traumatic brain injury. Neurotherapeutics 9(2):323–337
Acknowledgments
This study was supported by the grants from the National Science Foundation of China (No. 81271216; 81300946; and 81471105).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Ji, Mh., Wang, Zy., Sun, Xr. et al. Repeated Neonatal Sevoflurane Exposure-Induced Developmental Delays of Parvalbumin Interneurons and Cognitive Impairments Are Reversed by Environmental Enrichment. Mol Neurobiol 54, 3759–3770 (2017). https://doi.org/10.1007/s12035-016-9943-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9943-x