Abstract
Self-complementary adeno-associated viral vector 9 (scAAV9) has been confirmed to be an efficient AAV serotype for gene transfer to the central nervous system (CNS). Neurotrophic factors have been considered to be therapeutic targets for amyotrophic lateral sclerosis (ALS). In the present study, we intramuscularly injected scAAV9 encoding human insulin-like growth factor 1 (hIGF1) into an hSOD1G93A ALS mouse model. We observed that scAAV9-hIGF1 significantly reduced the loss of motor neurons of the anterior horn in the lumbar spinal cord and delayed muscle atrophy in ALS mice. Importantly, IGF1 significantly delayed disease onset and prolonged the life span of ALS mice. In addition, scAAV9-hIGF1 protected motor neurons from apoptosis through upregulation of D-amino acid oxidase (DAO), which controls the level of D-serine. Moreover, to further verify these results, we used CRISPR-Cas9 system to target the central nervous system knockdown of IGF1. This experiment supported the continued investigation of neurotrophic factor gene therapies targeting the central nervous system as a potential treatment for ALS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressing, fatal neurodegenerative disease characterized by motor neuron death in the spinal cord, brain stem, and motor cortex. Patients die of this disease due to severe muscle atrophy, paralysis, and ultimately respiratory failure [1, 2]. Approximately 9/10 of ALS cases are sporadic, whereas the remaining 1/10 of the ALS cases are familial. Mutations in the human SOD1 gene account for approximately 1/5 of familial ALS cases [3]. Glutamate excitotoxicity is implicated in its selective loss of motor neuron pathogenesis [4], and the accumulation of D-serine due to the reduced activity of D-amino acid oxidase (DAO) likely exacerbates motor neurons via NMDA receptors [5, 6]. D-serine is elevated in both the spinal cord of SOD1G93A models of ALS and sporadic cases of ALS [6–8]. However, effective approaches to prevent motor neuron death remain largely unidentified. However, effective approaches to prevent motor neuron death remain largely unidentified. Riluzole, the only FDA-approved treatment for ALS, exerts only modest neuroprotective effects, as it has been reported to extend survival by only a few months [9].
Experiments in vivo and in vitro confirmed that IGF1 has protective effect on the central and peripheral nervous systems and is important for the survival of motor neurons in the spinal cord. IGF1 has previously been shown to have a substantial effect on the SOD1G93A ALS disease model, but its effect in human clinical trials based on a recombinant form of IGF1 has shown controversial results [10–12]. Notably, the failure of these authors to provide a continuous and adequate supply of IGF1 to the central nervous system might explain these disappointing results [13]. Thus, identifying a practical and effective method that can be applied from animal experiments to human trials is urgently needed. The intramuscular injection of viral vectors has some success for therapeutic gene transfer to motor neurons in animal models [14–16]. Kaspar et al. successfully slowed transgenic ALS mice disease through induction of IGF1 production from motor neurons following the retrograde delivery of AAV2 injected into the muscle [17]. Other studies have also observed that IGF1, delivered through AAV2 or AAV4 vectors injected into the central nervous system (CNS), showed a beneficial effect in ALS mice, increasing survival, and delaying the failure of motor functions [18, 19]. However, these therapies showed low transduction rates in spinal cord motor neurons [17, 20]. The recent discovery that self-complementary adeno-associated viral vector 9 (scAAV9) vectors cross the BBB and efficiently transfect motor neurons has been a significant breakthrough in the field of neurodegenerative diseases [21]. Here, we verified whether scAAV9 could achieve gene transfer to the motor neurons of adult ALS mice via intramuscular injection.
In the present study, we administered scAAV9, encoding human insulin-like growth factor 1 (hIGF1), to an hSOD1G93A mouse model of ALS. We used intramuscular injection for multiple muscles, including respiratory muscles. This method was easily accessible and not invasive, while motor system could be protected, and was attractive for human gene therapy [22]. We observed that intramuscular delivery of scAAV9-hIGF1 remarkably slowed the onset of disease progression and increased survival compared to AAV9-GFP-treated mice. However, the protection mechanism remains unclear. It has been reported that IGF1 protects motor neurons through an anti-apoptotic mechanism [17] and attenuates the release of NO and TNF-α from glial cells [19]. In the present study, we observed that IGF1 partially upregulated DAO resulting in the degradation of D-serine and the reduction of glutamate excitatory toxicity. These findings provided the basis for intramuscular scAAV9 delivery as a practical and effective method for gene delivery to motor neurons in adult mice and supported the continued investigation of IGF1 gene therapy targeting the CNS as a potential treatment for ALS patients.
Materials and Methods
Animal Models
Transgenic mice (B6SJL-TgN [SOD1-G93A] 1Gur) were obtained from Jackson Laboratories. The hSOD1 transgene copy number was confirmed using real-time PCR [23] (see Supplementary Materials). Animals were breeding under controlled conditions (12-h light/dark cycle, 60 ± 10% relative humidity, 22 ± 1 °C). Animals were breeding under controlled conditions (12-h light/dark cycle, 60 ± 10% relative humidity, 22 ± 1 °C) [24].
Production of the AAV9 Virus
All self-complementary rAAV constructs were designed, produced, and used as previously described [25, 26]. The vectors were produced by infection of HEK293T cells with the helper virus-free three plasmids and under the control of the CMV promoter. ScAAV9 vectors were produced by the trans-encapsidation of the rAAV vector genome flanked by inverted terminal repeats from AAV2 with the capsids of AAV9. The vector was purified via two cesium chloride density-gradient ultracentrifugation steps and subsequently dialyzed against HEPES buffer. The vector titers were determined by real-time PCR, and the final concentration is expressed as the viral genome per milliliter (1 × 1012 vg/ml). The viral particles were divided it into small portions and stored at −80 °C until further use.
In Vivo AAV Injections
At 60 and 90 days of age, littermate- and copy number-matched mice were randomly divided into scAAV9-hIGF1 and AAV9-GFP treatment groups. The mice received bilateral intramuscular (masseter, musculus biceps brachii, musculus triceps brachii, intercostal muscle, abdominal muscle, gastrocnemius, biceps femoris muscle, and quadriceps) injections with AAV9 vectors (10 μl per muscle, n = 15) using a Hamilton syringe. ScAAV9-sgRNA-IGF1-Cas9 or scAAV9-sgRNA-LacZ-Cas9 was intrathecally injected into littermate- and copy number-matched mice at 30 days of age (1 μl/g, 1 μl = 1 × 109 vg) [25].
Assessment of Motor Function and Survival
The motor function of the mice was assessed weekly using a rotarod device (2–80 rpm Rota-Rod 47,600; Ugo Basile, Comerio, Italy). The rotarod device was operated at a constant speed of 15 rpm, and the mice were placed into the device and provided three attempts to remain for a maximum of 180 s per trial. The longest latency was recorded. If the mice could not stay on the rod for 180 s, then disease onset was recorded. The end stage was scored as the date at which the animal when placed on its back in a supine position was unable to right itself within 30 s [27]. Footprint analyses were performed at 110 days of age [28]. A behavioral score system from 1 to 5 was used [29], with 5 healthy without any symptoms of paralysis, 4 slight signs of destabilized gait and paralysis of the hind limbs, 3 obvious paralysis and destabilized gait, 2 fully developed paralysis of the hind limbs, animals only crawl on the forelimbs; and 1 fully developed paralysis of the hind limbs.
Enzyme-Linked Immunosorbent Assay of hIGF1
After 3 weeks of delivery, the lumbar spinal cord was rapidly harvested on ice and immediately homogenated. Enzyme-linked immunosorbent assays (ELISA) for human IGF1 were performed using an ELISA Kit (Abcam, ab100545). The assays were performed following the reference manual.
Immunohistochemical Staining
At 110 days of age, the mice were anesthetized, and the excised muscles were rapidly removed, frozen, and stored at −80 °C. The mice were sacrificed by transcardial perfusion with 0.3% saline, followed by ice-cold 4% paraformaldehyde. The lumbar region (L1–L5) of spinal cord was isolated and postfixed overnight in 4% paraformaldehyde. Twenty-five-micrometer sections were cut using a Vibratome Microtome (Leica VT 1000S, Germany).
For general morphology, the cryostat muscle sections were stained with hematoxylin and eosin (H&E) according to a standard protocol, and nicotinamide adenine dinucleotide hydrogen (NADH) staining was used to assess muscle fibrosis.
For 3,3-Diaminobenzidine (DAB) immunohistochemical (IHC) staining, lumbar spinal cord sections were blocked with 10% horse serum and incubated overnight at 4 °C with the following primary antibodies: rabbit anti-Iba1 (1:400, Wako), mouse anti-SMI32 (1:500, Millipore), and mouse anti-GFAP (1:500, Millipore). The following day, tissues were washed with PBS three times, incubated with secondary antibody, and biotinylated thirdly antibody for 30 min at RT. Sections were then washed for three times in TBS, incubated for 2 min with DAB solution at RT, and terminated with distilled water. The anterior horns of the lumbar enlargement spinal cord were examined. The numbers of SMI32-positive (motor neurons, >20 μm), GFAP-positive (astrocyte), and Iba1-positive (microglial) cells in the ventral horn area were estimated by using Image J software.
All images were captured using an Olympus BX51 microscope equipped with a DP72 digital camera system (Olympus, Tokyo, Japan).
Immunofluorescent Staining
Immunofluorescent staining of the sections was performed using goat anti-hIGF1 at 1:20 (Abcam, Cambridge, MA), mouse anti-GFAP at 1:500 (Millipore), rabbit anti-Iba1 at 1:200 (Wako), mouse anti-SMI32 (1:500, Millipore) and mouse anti-NeuN (1:100, Millipore), rabbit anti-DAO at 1:100 (Abcam, Cambridge, MA), anti-D-serine at 1:400 (Abcam, Cambridge, MA), terminal deoxynucleotidyl transferase (TdT) and TdT-mediated dUTP nick-end labeling (TUNEL) (C1089, Beyotime, China), and rabbit anti-caspase3 at 1:100 (Bioworld) as the primary antibodies. The tissues were subsequently incubated with the appropriate fluorescein isothiocyanate secondary antibodies (1:200, Jackson ImmunoResearch, Westgrove, PA) and Hoechst (1:100, Invitrogen). All images were captured on a laser-scanning confocal microscope.
Western Blot Analysis
The 110-day lumbar spinal cord tissue samples were homogenized by sonication in lysis buffer (P1250, APPLYGEN, China) at 4 °C. The homogenate was centrifuged at 10,000g for 10 min at 4 °C in a microcentrifuge. The protein extracts were separated on 12% SDS-PAGE gels and subsequently transferred onto PVDF membranes (Immobilon-P, Millipore Bedford, MA, USA). The PVDF membranes were incubated overnight at 4 °C with the following specific primary antibodies: anti-β-actin (1:1000, Protein Tech Group Inc., Chicago, USA), anti-AKT and anti-P-AKT (each at a 1:1000 dilution, Immunoway), anti-DAO (at a 1:1000 dilution, Abcam), rabbit anti-caspase3 and anti-caspase9 (each at 1:1000, Bioworld), and anti-mIGF1 (1:1000, Protein Tech Group Inc., Chicago, USA). After overnight incubation, the blots were washed and incubated with secondary antibodies (1:10,000, Rockland Immunochemicals, USA). The specific bands were detected using an Odyssey Infrared Imaging System (LI-COR, Lincoln, NE, USA). The relative intensities of the bands were normalized against β-actin.
Quantitative Real-Time PCR
The lumbar spinal cord DNA was isolated using a DNA Extraction Kit (Generay Biotechnology, Shanghai, China), and the lumbar region of spinal cord was homogenized using TRIzol reagent (Qiagen, Hilden, Germany) to isolate total RNA. The messenger RNA was reverse transcribed using the SuperScript Kit (Invitrogen) and oligo dT primers. The expression was analyzed by quantitative real-time PCR using the FastStart SYBR Green Master. Amplification was performed under the following conditions: heat inactivation (10 min at 95 °C, 1 cycle), followed by 40 cycles at 95 °C for 30 s, 60 °C for 60 s, and 72 °C for 30 s. The following sequences were used: hSOD1 5′-CATCAGCCCTAATCCATCTGA-3′ (forward) and 5′-CGCGACTAACAATCAAAGTGA-3′ (reverse); primers for genes mIL2 5′-CTAGGCCACAGAATTGAAAGATCT-3′ (forward) and 5′-GTAGGTGGAAATTCTAGCATCATCC-3′ (reverse), DAO 5′-TTAAACAGCGTCCGTGACCA-3′ (forward) and 5′-TTGCACAACCCCAGTGGATT-3′ (reverse), and GAPDH 5′-CATCTCCTCCCGTTCTGCC-3′ (forward) and 5′-GTGGTG-CAGGATGCATTGC-3′ (reverse) were used as references.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism software. The survival analysis was conducted via Kaplan-Meier analysis, which generates a χ 2 value to examine significance. All other statistical tests not involved in the survival analysis were performed using Student’s t test or one-way analysis of variance, and Bonferroni’s or Tukey’s post hoc tests were performed for each set of data. The results are expressed as the means ± SEM with a P threshold of 0.05.
Results
The Single Intramuscular Administration of AAV9 Mediated Efficient and Persistent Gene Delivery in Adult hSOD1G93AALS Mice
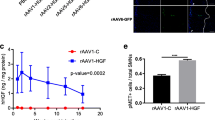
We first verified whether recombinant AAV9 or scAAV9 mediated the transduction of the spinal cord after intramuscular (i.m.) delivery in adult hSOD1G93AALS mice. In this aim, AAV9 expressing green fluorescent protein (GFP) under the control of the CMV promoter (1 × 1010 vg/muscle) was bilaterally injected into the gastrocnemius of 60-day-old ALS mice. After 21 days, the cryosections showed that AAV9 exhibits retrograde axonal transport from the nerve terminals in the injected gastrocnemius to the axons and neurons in the anterior horn of lumbar spinal cord (Fig. 1a). Unexpectedly, at the end stage, the western blot analysis revealed the presence of GFP in protein extracts from the lumbar spinal cord (Fig. 1b). Subsequently, we examined the intramuscular injection of scAAV9-hIGF1 into the bilateral gastrocnemius (1 × 1010 vg/muscle) of 60-day-old ALS mice and evaluated hIGF1 expression at 21 days after injection. Positive hIGF1 staining was observed at the lumbar spinal cord using a confocal microscope (Fig. 1c, d). The levels of hIGF1 expression in the lumbar spinal cord and gastrocnemius of mice injected with AAV9-GFP or scAAV9-hIGF1 were determined using an enzyme-linked immunosorbent assay (Fig. 1e). The results demonstrated that AAV9 could transfer genes to motor neurons through axonal retrograde transport to the connected motor neuron (MN) cell bodies.
Single i.m. AAV9 injection mediates widespread and persistent transduction in adult hSOD1G93A mice. All injections were performed at P60. a AAV9-GFP injected into the bilateral gastrocnemius of hSOD1G93A ALS mice for retrograde transduction of spinal motor neurons. At 21 days after injection, GFP is expressed in muscle, axons, and motor neurons. b At the end stage of hSOD1G93A ALS mice, the western blot analysis revealed the presence of GFP in protein extracts from the lumbar spinal cord. c Human IGF1 (hIGF1) staining in AAV9-GFP- and scAAV9-hIGF1-treated mice throughout lumbar spinal cord. Transverse sections of the lumbar spinal cord treated for hIGF1/SMI32 double immunofluorescence. d Statistical analysis for the number/percentage of double-positive cells of AAV9-GFP- and scAAV9-hIGF1-treated mice. e ELISA of hIGF1 from tissues of treated mice obtained at 21 days after injection. The protein of hIGF1 is expressed in the lumbar spinal cord and muscle. Mean + SEM, n = 3. Scale bar = 100 μm
ScAAV9-hIGF1 Delayed Motor Deficits and Delayed Disease Onset and Prolonged the Life Span in ALS Mice
ALS mice typically develop a progressive neurodegenerative disorder that selectively damages upper and lower motoneurons, resulting in various degrees of weakness and limb musculature atrophy at approximately 90 days of age. In this experiment, we observed that scAAV9-hIGF1 mice could hold its hind limbs outward, while AAV9-GFP mice did not hold its hind limbs outward, and muscle atrophy was observed at 110 days of age (Fig. 2a). ScAAV9-hIGF1 treated mice activities more flexible than AAV9-GFP mice (see Supplementary Materials). Footprint analysis showed significant impairment in the walking patterns of AAV9-GFP-injected mice at 110 days of age (Fig. 2b). Statistical analysis showed that the stride length of the scAAV9-hIGF1 treatment group was larger than that of the AAV9-GFP group (Fig. 2c). Moreover, we examined the body weight and motor function of the mice during disease progression, observing that scAAV9-hIGF1 mice exhibited better motor function and reduced weight loss compared with age-matched AAV9-GFP mice (Fig. 2d).
Delivery of scAAV9-hIGF1 at 60 or 90 days of age delay motor deficits in ALS mice. a The different hind limb-clasping and muscle atrophy phenotypes at 110 days in ALS mice. b Representative footprints of 110 days from AAV9-GFP- and scAAV9-hIGF1-treated mice. c Footprint analysis showed significant differences in the step length of the mice in the two groups. d Plots showing the performance of AAV9-GFP- or scAAV9-hIGF1-treated ALS mice on body weight and rotarod test. *P < 0.05, n = 15
To explore whether scAAV9-hIGF1 treatment could influence disease onset and progression, we measured the motor function using a rotarod test with ALS mice. We observed that disease onset and end stage were significantly delayed in ALS mice treated with scAAV9-hIGF1 compared to mice treated with AAV9-GFP (Fig. 3). Male mice treated for 60 days showed delayed disease onset after 24 days, whereas female mice treated for 60-day treatment showed delayed disease onset after 18 days. The life span of male mice treated for 60 days was extended for 29 days, while that of female mice treated for 60 days was extended 24 days, the life span of male mice treated for 90 days was extended 15 days, and the life span of female mice treated 90 days was extended 14 days.
ScAAV9-hIGF1 Protected Skeletal Muscle from Atrophy and Promoted Motor Neuron Survival in ALS Mice
The muscle staining of cryosections of the gastrocnemius revealed obvious differences in AAV9-GFP mice compared with scAAV9-hIGF1 mice (Fig. 4a). Morphological analysis of gastrocnemius with H&E staining showed more atrophic fibers in AAV9-GFP mice than scAAV9-hIGF1 mice. According to NADH staining, the myofibers in the two groups of mice were classified as type I or type II fibers. Statistical analysis showed that the average muscle fiber area of scAAV9-hIGF1 mice was larger than that of AAV9-GFP mice (Fig. 4b).
ScAAV9-hIGF1 protects skeletal muscle and motor neurons and reduces astroglial and microglial responses in ALS mice. a Effects of scAAV9-hIGF1 on skeletal muscle pathology in hSOD1G93A mice. H&E and NADH staining of gastrocnemius muscle sections in AAV9-GFP- and scAAV9-hIGF1-treated mice at 110 days of age. Scale bar = 100 μm. b Statistical analysis of the average muscle fiber area. c IHC for SMI-32, GFAP, and Iba1 of AAV9-GFP- and scAAV9-hIGF1-treated mice at 110 days of age. Scale bar = 200um. Quantification of SMI-32 (d), GFAP (e), and Iba1 (f) in AAV9-GFP- and scAAV9-hIGF1-injected mice at 110 days of age. There are all significant differences
To determine the effect of scAAV9-hIGF1 on motor neuron survival, we performed immunostaining with SMI32, a motor neuron-specific biomarker in the nervous system, to assess the morphology and survival of motor neurons per slice in the anterior horn of lumbar spinal cord of ALS mice. SMI32 immunostaining showed that IGF1 could protect the motor neurons in the ALS mice (Fig. 4c). Statistical analysis showed an obvious reduction in the loss of SMI32-positive neurons in scAAV9-hIGF1 mice compared with AAV9-GFP mice (Fig. 4d). These results suggested that scAAV9-hIGF1 treatment could protect motor neurons in the ALS mice. Microglial and astrocytic activation were assessed in the anterior horn of the lumbar spinal cord of mice. IHC showed significantly fewer GFAP-positive glial cells in the anterior horn of the lumbar spinal cords of scAAV9-hIGF1-treated ALS mice compared with GFP-treated mice (Fig. 4c, e). IHC also showed that scAAV9-hIGF1 treatment significantly reduced Iba1 levels in the anterior horn of the lumbar spinal cords of ALS mice (Fig. 4c, f). All the experiments were performed using mice at 110 days of age.
ScAAV9-hIGF1 Upregulated DAO and Degraded D-Serine
Enhanced glutamate toxicity through overproduction of D-serine in glia has been proposed as the mechanism underlying ALS motoneuronal death [30]. It has been identified that DAO plays an essential role in degrading D-serine. We used Affymetrix GeneChip® Mouse Exon 1.0 ST Array to investigate the expression profiles of lumbar spinal cord samples from scAAV9-hIGF1- and AAV9-GFP-treated hSOD1G93A ALS mice, and surprisingly, DAO messenger RNA (mRNA) expression was upregulated in scAAV9-hIGF1-treated mice (Supplementary Materials). We used immunofluorescence to investigate the expression and localization of DAO in the anterior horn of the lumbar spinal cord from scAAV9-hIGF1- and AAV9-GFP-treated mice. Transverse sections of the lumbar spinal cord were co-labeled with antibodies directed against DAO and either NeuN, a neuron-specific nuclear protein (Fig. 5a); Iba1, a marker of microglia (Fig. 5b); or GFAP, a marker of astrocytes (Fig. 5c). Fluorescence microscopy showed that DAO was primarily localized in neuronal cells, and a small amount was detected in glial cells. We observed that DAO expression was upregulated in scAAV9-hIGF1-treated mice. Subsequently, we examined DAO protein expression by western blotting, showing increased DAO protein expression in scAAV9-hIGF1 mice compared with AAV9-GFP mice (Fig. 6a, b). In addition, we performed real-time PCR to examine the level of DAO mRNA. The results showed that DAO mRNA was also upregulated in scAAV9-hIGF1 mice (Fig. 6c). As DAO potentially modulates neurotransmission through D-serine [6] [8], we also examined the quantity of D-serine in the lumbar spinal cord of the two groups of ALS mice, and the results showed that D-serine was reduced in scAAV9-hIGF1-treated mice (Fig. 6d, e).
Immunofluorescence co-localization analysis of DAO distribution in the lumbar spinal cord. The co-localization of NEUN (a), Iba1 (b), and GFAP (c) in the anterior horn of the lumbar spinal cord of AAV9-GFP- and scAAV9-hIGF1-treated mice at 110 days of age. DAO is primarily distributed in neurons and upregulated in scAAV9-hIGF1-treated mice. Scale bar = 100 μm
ScAAV9-hIGF1 upregulated DAO expression and reduced the D-serine level. a Western blot images and b quantitative densitometry of DAO standardized with β-actin in spinal cords of AAV9-GFP- and scAAV9-hIGF1-treated mice at 110 days of age. c Analysis of RT-PCR in lumbar spinal cord in the AAV9-GFP- or scAAV9-hIGF1-treated mice showed that DAO mRNA was significantly upregulated in the scAAV9-hIGF1 group. d Immunofluorescence quantity analysis showed that D-serine decreased in scAAV9-hIGF1-treated mice. e D-serine co-localization with GFAP in the two groups of mice throughout the lumbar spinal cord
ScAAV9-hIGF1 Inhibited Apoptosis in ALS Mice
ScAAV9-hIGF1-treated mice exhibited smaller numbers of cells positive for TUNEL than AAV9-GFP-treated mice at 110 days of age (Fig. 7a, e). In addition, immunofluorescence (IF) showed a decrease in cleaved caspase3 expression in scAAV9-hIGF1-treated mice compared to AAV9-GFP mice (Fig. 7a, f). We also observed that scAAV9-hIGF1-treated mice had higher levels of phosphorylated Akt (P-AKT) (Fig. 7b, g) and significantly reduced amounts of cleaved caspase3 (Fig. 7c, h) and cleaved caspase9 (Fig. 7d, i) compared to AAV-GFP mice.
ScAAV9-hIGF1 inhibited apoptosis in ALS mice. a TUNEL staining and cleaved caspase3 with Hoechst nuclear counterstaining of the lumbar spinal cord in 110-day mice treated with AAV9-GFP or scAAV9-hIGF1. b Western blotting of active phosphorylated Akt (P-AKT) and AKT in the lumbar spinal cord of ALS mice treated with AAV9-GFP and scAAV9-hIGF1. c Western blotting of cleaved caspase3 in the lumbar spinal cord of ALS mice treated with AAV9-GFP or scAAV9-hIGF1. d Western blotting of cleaved caspase9 in the lumbar spinal cord of the two groups of ALS mice. e, f, g, h, i Quantification evaluation of TUNEL (e), cleaved caspase3 (f, h), P-AKT (g), and cleaved caspase9 (i) in the lumbar spinal cord of ALS mice treated with AAV9-GFP or scAAV9-hIGF1. The protein levels of P-AKT were significantly upregulated, while the expressions of TUNEL, cleaved caspase3, and cleaved caspase9 were markedly downregulated. Scale bar = 100 μm
ScAAV9-sgRNA-IGF1-Cas9 Exacerbated Disease via Reduced DAO and Activated the Apoptotic Pathway
The present study was designed to elucidate the effects of low IGF-1 levels induced by scAAV9-sgRNA-IGF1-Cas9 knockdown IGF-1, particularly in the CNS of hSOD1G93A ALS mice. To achieve a better knockdown effect, we used intrathecally injected scAAV9-sgRNA-IGF1-Cas9 or scAAV9-sgRNA-LacZ-Cas9 to mice at 30 days of age (1 μl/g, 1 μl = 1 × 109 vg). Using this model, we examined the hypothesis that IGF-1 deficiency in the CNS decreases DAO levels and activated the apoptotic pathway, thereby aggravating the phenotype of hSOD1G93A ALS mice. Hind limb weakness of IGF-1-deficient mice was observed earlier (Fig. 8a) at 85 days of age. IGF-1-deficient mice showed accelerated weight loss (Fig. 8b) and poorer movement function scores (Fig. 8c) compared to the scAAV9-sgRNA-LacZ-Cas9 control. Western blotting (WB) showed reduced IGF1 levels in scAAV9-sgRNA-IGF1-Cas9 mice (Fig. 10a, d). IF (Fig. 9a, b) and WB (Fig. 10a, c) showed that the expression of DAO was downregulated in the IGF-1 knockdown group. In addition, IF showed that the D-serine level was upregulated in IGF-1 knockdown mice (Fig. 9c, d). We examined the expression of cleaved caspase3 (Fig. 10b, e) and cleaved caspase9 (Fig. 10b, f), and the results showed increased expression in the IGF-1 knockdown group compared to the scAAV9-sgRNA-LacZ-Cas9 control.
IGF-1 deficiency in the CNS aggravated phenotype of hSOD1G93A ALS mice. a The different hind limb-clasping and muscle-wasting phenotypes in 85-day-old ALS mice. AAV9-sgRNA-IGF1-Cas9-treated mice at 85 days showed marked hind limb muscle wasting compared to AAV9-sgRNA-LacZ-Cas9 mice. b Differences in the weight loss between the two groups of the mice. c Different behavioral scores between the two groups of the mice. *P < 0.05, n = 7
Immunofluorescence images and statistical analysis of DAO and D-serine of lumbar myeloid anterior horn at 90 days of age. The results showed that DAO expression was decreased (a, b) and D-serine expression was increased (c, d) in IGF-1-deficient mice, and the IGF-1 deficiency in the CNS accelerated the loss of motor neurons (a) and aggravated astroglial responses (c) in ALS mice. Scale bar = 100 μm
Western blot analysis of DAO and apoptosis proteins of the lumbar myeloid anterior horn at 90 days of age. a Western blot images show the protein levels of DAO and mIGF1 in the lumbar spinal cords of 90-day ALS mice. b Western blot images show the protein levels of cleaved caspase9 and cleaved caspase3 in the lumbar spinal cords of 90-day ALS mice (c, d, e, f). The relative levels of DAO, mIGF1, cleaved caspase9, and cleaved caspase3 were compared between AAV9-sgRNA-LacZ-Cas9- and AAV9-sgRNA-IGF1-Cas9-treated ALS mice
Discussions
Noninvasive and effective gene transfer to motor neurons has long been a challenge for ALS gene therapy. AAV9 vectors have been reported to perform retrograde axonal transport via intramuscular injection for the safe and efficient transduction of central nervous system tissue [31]. Self-complementary vectors are more efficient transducing agents than conventional AAVs [32]. We analyzed the capacity of scAAV9 for gene delivery to motor neurons after intramuscular injection and investigated the feasibility of this approach for hIGF1 gene therapy in adult hSOD1G93A ALS mice. These findings indicated that a single i.m. injection of scAAV9 can induce retrograde axonal transport to MNs in adult mice.
The main delivery routes of the ALS animal model include intravenous injection, subcutaneous injection, intramuscular injection, intracerebroventricular injection, intrathecal injection, and so on. In our laboratory, we examined intravenous injection, intrathecal injection, intracerebroventricular injection, and intramuscular injection. In our experimental findings, intravenous injection use a large dosage, poor targeting, and poor curative effect; intrathecal injection use a small dosage, have a good curative effect, but have a large trauma; and intracerebroventricular injection also use a small dosage, prolonged the survival, but did not delay the onset and have a large trauma. Intramuscular injection is a common administration, and the dosage compared with intravenous injection is small, strong targeting, good effect, and noninvasive.
IGF1 has previously been shown to have a substantial effect on the SOD1G93A ALS disease model, but its effect in human clinical trials for ALS has been consistently negative, suggesting that inappropriate delivery approaches of these agents is part of the problem [12, 33]. Clinical trials have shown that repeated subcutaneous injections of rhIGF1 protein, through blood circulation to the central nervous system, which has a short half-life, and low bioavailability (difficult to pass the blood-brain barrier), cannot be sustained and play a stable role. Gene therapy may solve this problem. The intramuscular injection of scAAV9 for retrograde transmission to motor neurons was confirmed by subsequent experiments in adult SOD1G93A ALS mice. The present study showed that scAAV9-hIGF1 injected into a number of muscles significantly increased the life expectancy of hSOD1G93A ALS mice and improved behavioral performance.
However, the protection mechanism of IGF1 remains unclear. Previous experiments have confirmed that D-serine is elevated in amyotrophic lateral sclerosis [5]. Enhanced glutamate toxicity via overproduction of D-serine in glial cells is a mechanism of ALS motor neuron death, and D-serine was increased in the ALS spinal cord [34]. D-serine is primarily degraded by DAO [35]. In the present study, we applied a series of experiments and observed that the level of DAO in scAAV9-hIGF1-treated mice was significantly higher than that in the AAV9-GFP group. These results provide the first evidence that IGF1 may partially, through the upregulation of DAO expression, reduce glutamate excitability toxicity.
The cellular mechanisms of ALS disease progression have not yet been elucidated. Glial cells play an important role in the occurrence and progression of disease. The basic pathological characteristics of ALS include apoptosis of neurons and proliferation of the glial cells. The study observed that IGF1 in the ALS disease model played a role in the activation of motor neurons in the anti-apoptotic pathway, and have muscle nutrition effect, and so on, which shows that it plays a role in the treatment of ALS. We observed that IGF1 overexpression can delay muscle atrophy and disease onset, prolong survival, inhibit caspase9 activation, and reduce apoptosis in mice, consistent with the results of previous studies. We also observed that IGF1 overexpression upregulated DAO levels, increased D-serine degradation, reduced microglial activation and astrocyte responses, and protected motor neurons. Further, we used a CRISPR/Cas9 system to knock down IGF1 in ALS mice and observed decreased DAO and increased D-serine, and caspase9 activation, whereas IGF1 overexpression showed the opposite results, suggesting that the DAO expression levels were positively regulated by IGF1.
In summary, we showed that a single intramuscular injection of scAAV9-hIGF1 vectors facilitated retrograde transport to motor neurons in hSOD1G93A ALS adult mice. We further showed that the intramuscular injection of scAAV9-hIGF1 significantly alleviated the pathology of ALS mice, demonstrating the therapeutic efficacy of scAAV9-hIGF1 to protect motor neurons from apoptosis through upregulation of DAO. This practical and effective method for delivering the IGF1 gene to the CNS may have potential for use in the therapy of ALS patients.
References
Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC (2011) Amyotrophic lateral sclerosis. Lancet 377(9769):942–955
Seals RM, Kioumourtzoglou MA, Gredal O, Hansen J, Weisskopf MG (2015) ALS and the military: a population-based study in the Danish registries. Epidemiology
Kaur SJ, McKeown S, Rashid S (2015) Mutant SOD1 mediated pathogenesis of amyotrophic lateral sclerosis. Gene
Bruijn LI, Miller TM, Cleveland DW (2004) Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci 27:723–749
Sasabe J, Chiba T, Yamada M, Okamoto K, Nishimoto I, Matsuoka M, Aiso S (2007) D-serine is a key determinant of glutamate toxicity in amyotrophic lateral sclerosis. EMBO J 26(18):4149–4159
Sasabe J, Miyoshi Y, Suzuki M, Mita M, Konno R, Matsuoka M, Hamase K, Aiso S (2012) D-amino acid oxidase controls motoneuron degeneration through D-serine. Proc Natl Acad Sci U S A 109(2):627–632
Sasabe J, Suzuki M, Imanishi N, Aiso S (2014) Activity of D-amino acid oxidase is widespread in the human central nervous system. Front Synaptic Neuroscience 6:14
Paul P, de Belleroche J (2014) The role of D-serine and glycine as co-agonists of NMDA receptors in motor neuron degeneration and amyotrophic lateral sclerosis (ALS). Front Synaptic Neuroscience 6:10
Wokke J (1996) Riluzole. Lancet 348(9030):795–799
Lai EC, Felice KJ, Festoff BW, Gawel MJ, Gelinas DF, Kratz R, Murphy MF, Natter HM et al (1997) Effect of recombinant human insulin-like growth factor-I on progression of ALS. A placebo-controlled study. The North America ALS/IGF-I Study Group Neurology 49(6):1621–1630
Borasio GD, Robberecht W, Leigh PN, Emile J, Guiloff RJ, Jerusalem F, Silani V, Vos PE et al (1998) A placebo-controlled trial of insulin-like growth factor-I in amyotrophic lateral sclerosis. European ALS/IGF-I study group. Neurology 51(2):583–586
Sorenson EJ, Windbank AJ, Mandrekar JN, Bamlet WR, Appel SH, Armon C, Barkhaus PE, Bosch P et al (2008) Subcutaneous IGF-1 is not beneficial in 2-year ALS trial. Neurology 71(22):1770–1775
Raoul C, Aebischer P (2004) ALS, IGF-1 and gene therapy: ‘it’s never too late to mend. Gene Ther 11(5):429–430
Azzouz M, Le T, Ralph GS, Walmsley L, Monani UR, Lee DC, Wilkes F, Mitrophanous KA et al (2004) Lentivector-mediated SMN replacement in a mouse model of spinal muscular atrophy. J Clin Invest 114(12):1726–1731
Baumgartner BJ, Shine HD (1998) Neuroprotection of spinal motoneurons following targeted transduction with an adenoviral vector carrying the gene for glial cell line-derived neurotrophic factor. Exp Neurol 153(1):102–112
Greig JA, Peng H, Ohlstein J, Medina-Jaszek CA, Ahonkhai O, Mentzinger A, Grant RL, Roy S et al (2014) Intramuscular injection of AAV8 in mice and macaques is associated with substantial hepatic targeting and transgene expression. PLoS One 9(11):e112268
Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH (2003) Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science 301(5634):839–842
Dodge JC, Treleaven CM, Fidler JA, Hester M, Haidet A, Handy C, Rao M, Eagle A et al (2010) AAV4-mediated expression of IGF-1 and VEGF within cellular components of the ventricular system improves survival outcome in familial ALS mice. Mol Therapy : J Am Soc Gene Ther 18(12):2075–2084
Dodge JC, Haidet AM, Yang W, Passini MA, Hester M, Clarke J, Roskelley EM, Treleaven CM et al (2008) Delivery of AAV-IGF-1 to the CNS extends survival in ALS mice through modification of aberrant glial cell activity. Mol Ther : J Am Soc Gene Ther 16(6):1056–1064
Hollis ER 2nd, Kadoya K, Hirsch M, Samulski RJ, Tuszynski MH (2008) Efficient retrograde neuronal transduction utilizing self-complementary AAV1. Mol Ther: J Am Soc Gene Ther 16(2):296–301
Duque S, Joussemet B, Riviere C, Marais T, Dubreil L, Douar AM, Fyfe J, Moullier P et al (2009) Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol Ther: J Am Soc Gene Ther 17(7):1187–1196
Towne C, Schneider BL, Kieran D, Redmond DE Jr, Aebischer P (2010) Efficient transduction of non-human primate motor neurons after intramuscular delivery of recombinant AAV serotype 6. Gene Ther 17(1):141–146
Alexander GM, Erwin KL, Byers N, Deitch JS, Augelli BJ, Blankenhorn EP, Heiman-Patterson TD (2004) Effect of transgene copy number on survival in the G93A SOD1 transgenic mouse model of ALS. Brain Res Mol Brain Res 130(1–2):7–15
Ludolph AC, Bendotti C, Blaugrund E, Chio A, Greensmith L, Loeffler JP, Mead R, Niessen HG et al (2010) Guidelines for preclinical animal research in ALS/MND: a consensus meeting. Amyotroph Lateral Sclerosis : Off Publ World Fed Neurol Res Group Motor Neuron Dis 11(1–2):38–45
Guo Y, Wang D, Qiao T, Yang C, Su Q, Gao G, Xu Z (2015) A single injection of recombinant adeno-associated virus into the lumbar cistern delivers transgene expression throughout the whole spinal cord. Mol Neurobiol
Anderton RS, Meloni BP, Mastaglia FL, Boulos S (2013) Spinal muscular atrophy and the antiapoptotic role of survival of motor neuron (SMN) protein. Mol Neurobiol 47(2):821–832
Li M, Ona VO, Guegan C, Chen M, Jackson-Lewis V, Andrews LJ, Olszewski AJ, Stieg PE et al (2000) Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science 288(5464):335–339
Dijkstra JR, Meek MF, Robinson PH, Gramsbergen A (2000) Methods to evaluate functional nerve recovery in adult rats: walking track analysis, video analysis and the withdrawal reflex. J Neurosci Methods 96(2):89–96
Knippenberg S, Thau N, Dengler R, Petri S (2010) Significance of behavioural tests in a transgenic mouse model of amyotrophic lateral sclerosis (ALS). Behav Brain Res 213(1):82–87
Heath PR, Shaw PJ (2002) Update on the glutamatergic neurotransmitter system and the role of excitotoxicity in amyotrophic lateral sclerosis. Muscle Nerve 26(4):438–458
Dayton RD, Wang DB, Klein RL (2012) The advent of AAV9 expands applications for brain and spinal cord gene delivery. Expert Opin Biol Ther 12(6):757–766
McCarty DM, Monahan PE, Samulski RJ (2001) Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther 8(16):1248–1254
Lange DJ, Felice KJ, Festoff BW, Gawel MJ, Gelinas DF, Kratz R, Lai EC, Murphy MF et al (1996) Recombinant human insulin-like growth factor-I in ALS: description of a double-blind, placebo-controlled study. North American ALS/IGF-I study group. Neurology 47(4 Suppl 2):S93–S94 discussion S94-95
Paul P, Murphy T, Oseni Z, Sivalokanathan S, de Belleroche JS (2014) Pathogenic effects of amyotrophic lateral sclerosis-linked mutation in D-amino acid oxidase are mediated by D-serine. Neurobiol Aging 35(4):876–885
Mitchell J, Paul P, Chen HJ, Morris A, Payling M, Falchi M, Habgood J, Panoutsou S et al (2010) Familial amyotrophic lateral sclerosis is associated with a mutation in D-amino acid oxidase. Proc Natl Acad Sci U S A 107(16):7556–7561
Acknowledgements
The authors would like to thank YanSu Guo for the technical support and Yue Bi for the assistance with the microarray analysis. This study was financially supported in part by grants from the Natural Science Foundation of China (H0912-81171210 and C090301-30870882) and the Science and Technological Department of Hebei Province (0647007D).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
HuiQian Lin and HaoJie Hu contributed equally to this work.
Electronic supplementary material
Fig. S1
The hSOD1G93A mice with high copy numbers of the mutant human SOD1 gene. Statistical analysis showed that the hSOD1 DNA copy number of the mutant SOD1 gene showed no difference between groups. (GIF 26 kb)
Fig. S2
Gene chip analysis in the lumbar spinal cord in the AAV9-GFP or scAAV9-hIGF1-treated mice. DAO was significantly upregulated in the scAAV9-hIGF1 group. (GIF 34 kb)
Fig. S4
Double staining of TUNEL and neuron (NEUN), astrocyte (GFAP), and microglia (Iba1). Neuron is the origin of apoptotic cells. (GIF 117 kb)
12035_2016_335_MOESM8_ESM.mp4
Vid. 4 The movie of 30d intrathecal injected AAV9-sgRNA-LacZ-Cas9 and AAV9-sgRNA-IGF1-Cas9 ALS mice at 85 d. (MP4 17,400 kb)
Rights and permissions
About this article
Cite this article
Lin, H., Hu, H., Duan, W. et al. Intramuscular Delivery of scAAV9-hIGF1 Prolongs Survival in the hSOD1G93A ALS Mouse Model via Upregulation of D-Amino Acid Oxidase. Mol Neurobiol 55, 682–695 (2018). https://doi.org/10.1007/s12035-016-0335-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0335-z