Abstract
Subarachnoid hemorrhage (SAH), as a severe brain disease, has high morbidity and mortality. SAH usually induced neurological dysfunction or death and the treatment is far from satisfaction. Here, we investigated the effect of low dose of LPS pretreatment and underlying molecular mechanism in rat SAH model. Firstly, SAH model was induced by prechiasmal cistern injection method (SAH1) and common carotid artery-prechiasmal cistern shunt method (SAH2), respectively, to select the more suitable SAH model. At 6, 12, 24, 48, and 72 h after SAH, brain injury including neurological dysfunction, blood–brain barrier disruption, brain edema, and cell apoptosis were detected. And the expression of MMP9, HMGB1/TLR4, and caspase3 in cortex were also explored. Then, SB-3CT, an inhibitor of MMP9, was administrated to investigate the exact function of MMP9 in the brain injury at 24 h after SAH. Moreover, low dose of LPS was used to verify whether it had nerve protection after SAH and the mechanism involving in MMP9 and caspase 3 was investigated. Our results showed SAH1 seems to be the most suitable SAH model. In addition, MMP9 activated by HMGB1/TLR4 may promote or aggravate brain injury, while inhibiting MMP9 via SB-3CT exerted a neuroprotective effect. Moreover, LPS improved the neurological dysfunction, reduced Evans blue extravasation and brain edema, and inhibited cell apoptosis of cortex in rats with brain injury induced by SAH. Importantly, LPS pretreatment increased the expression level of TLR4, and decreased the level of MMP9 and caspase3. Therefore, the present study revealed that low dose of LPS pretreatment could provide neuroprotective effects on brain injury caused by SAH via downregulating MMP9 and caspase3 and activating TLR4 signal pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subarachnoid hemorrhage (SAH) has high morbidity and mortality. Average 20 ~ 30% patients with SAH died before admission or in a short time after admission. For the survival patients, there is a higher incidence to suffer from neurological and cognitive dysfunction [1–4]. Previous studies showed that patients died from SAH had widespread ischemic brain injury through necropsy [5], and cerebral blood flow (CBF) was obviously reduced in the acute phase after SAH [6–12]. In addition, the disturbances in intracranial pressure and cerebral metabolism may be the reason for brain injury. In clinic, the study of pathophysiology mechanism of SAH on human is certainly limited, so animal model was used for this study.
Previous animal experiments showed that most of death induced by SAH happened immediately after the first bleeding, and early brain injury (EBI) was considered as the main reason of the death [13]. EBI, a concept proposed recently, means the direct damage to whole brain within 72 h after SAH. As EBI happened, it involved in all pathophysiological events of brain before the happening of late-onset vasospasm (3 days to 2 weeks) including cell death or apoptosis, destruction of blood–brain barrier, brain edema, microvascular dysfunction, acute cerebral vasospasm, and so on [14]. Cell apoptosis, an important pathological process in the EBI, can lead to cytotoxic brain edema that further aggravated brain injury after SAH [15, 16]. Blood–brain barrier damage is also an important pathological process of EBI, and extracellular matrix is one of the most important factors leading to blood–brain barrier damage [13, 17]. Increasing evidence suggests that many factors involved in the neural cell apoptosis induced by brain injury [18], such as matrix metalloproteinase-9 (MMP-9), but its certain role and relative signal pathway in the neural cell apoptosis after SAH were unclear.
Matrix metalloproteinases (MMPs), a kind of endopeptidase dependent on zinc ion, consist of at least 25 members at present. MMPs participated in the tissue remodeling and maintained the stability of the internal environment. Furthermore, it can regulate cell activity through degradation of extracellular matrix, regulation of cell adhesion, and transduction of the cell surface protein. MMP9, a member of the MMP families, was secreted in various cells of brain, including endothelial cells, hippocampal neurons, microglia, astrocytes, oligodendrocytes, and inflammatory cells. Previous studies have found that MMP9 can degrade many kinds of extracellular matrix after activation which probably mediated cell apoptosis after brain injury [18–20]. Recently, it was reported that high mobility group box-1 (HMGB1) can upregulate MMP9 through Toll-like family of receptor 4 (TLR4), thus aggravating brain injury and inflammation in the model of cerebral ischemia [21], and lipopolysaccharide (LPS), a component of gram-negative bacteria, regulated immune system by activating TLR4. However, there is no full correlation between LPS and HMGB1, the linkage among LPS, HMGB1 and MMP9 is not clear in the SAH model. Moreover, some preliminary studies indicated that low dose of LPS can induce cerebral protection in brain ischemia [22, 23], but role of LPS in SAH and related mechanism needs to be explored.
In the present study, we firstly compared prechiasmal cistern injection method (SAH1) with common carotid artery-prechiasmal cistern shunt method (SAH2) in order to choose a suitable model. Then, reverse transcription polymerase chain reaction (RT-PCR) and western blotting were used to detect the mRNA and protein expression of MMP9, respectively. Moreover, SB-3CT, an inhibitor of MMP9, was used for investigating the function of MMP9 in EBI induced by SAH. Finally, low dose of LPS was used to verify whether LPS has neuroprotective effects on SAH associated the molecular changes of TLR4, MMP9, and caspase3. The findings of MMP-9 and its relative signal pathway after SAH can provide molecular target for clinical treatment.
Materials and Methods
Animal and Grouping
Male Sprague-Dawley (SD) rats, weighing 250–300 g, were purchased from animal center of Sichuan University. Rats have free access to water and food and were housed in plastic cages with 12 h light/dark cycle following guidelines of the US National Institutes of Health. And before operation, they were fasted for 12 h and deprived from water for 8 h. Animal care and all experimental protocols were approved by the guidelines of the Institutional Medical Experimental Animal Care Committee of Sichuan University, West China Hospital, China.
Firstly, 60 adult male SD rats were randomly divided into three groups as described in Table 1 to select the most suitable method to establish the model. Secondly, 200 and 85 adult male SD rats were randomly divided into six groups as described in Table 2 to determine the role of MMP9 in the EBI after SAH using inhibitor SB-3CT and the role of LPS pretreatment.
Animal Model
Prechiasmatic Cistern Injection Method (SAH1)
Rats were anesthetized by intraperitoneal injection of 3.6% chloral hydrate (1 ml/100 g), then placed at a prone position and fixed. After skin in forehead were disinfected by 75% alcohol, the center of the frontal was performed a craniotomy. Afterwards, the skull was drilled using dental drill according to the following coordinates: 7.5 mm before anterior fontanelle and with 30 degrees by stereotactic apparatus. The needles were inserted into 10 mm and reached before 2 ~ 3 mm of the bottom of skull prechiasmatic, and before blood injection; the hole of skull needs to be sealed using bone wax. Then, 200 μl of blood, drawn from femoral artery of the experimental rats themselves, were slowly injected into the brain within about 12 s. Subsequently, the needle was drawn and the hole of the bone was sealed using bone wax. The treatment of the sham group was identical to SAH1 method except that autologous blood was replaced by equal saline solution.
Common Carotid Artery-Prechiasmal Cistern Shunt Method (SAH2)
Rats were anesthetized by intraperitoneal injection of 3.6% chloral hydrate (1 ml/100 g), kept in the supine position, and fixed on the board. After the skin in the neck was disinfected using 75% alcohol, it was cut along the middle line of neck. Then, surface membrane was torn using forceps, the adipose tissue was bluntly separated, and the muscles in the neck were exposed. Then, fascia above the right sternocleidomastoid was cut using microscopic camber and the muscles were bluntly separated in order to expose the right common carotid artery. Then, rats were removed to anatomical microscope, and common carotid artery was continuously separated to internal and external carotid artery, then external carotid artery was ligated. After common carotid artery and internal carotid artery was temporarily clamped using vascular clip, external carotid artery was cut in the proximal of vascular clip. Then 3–0 single strands of nylon line was inserted and pushed up into the internal carotid artery until the place of clamp, then the clamp was removed, and the nylon line was continuously pushed forward until it met resistance. When the insertion depth was about 18 ~ 20 mm, it was continuously inserted further 3 mm and punctured the bifurcation between cerebral artery and middle cerebral artery. Finally, the piercing line was stayed out until it stayed for 15 s.
Treatments
Treatment 1
SAH1 method was used for building SAH model in the following research. After the SAH model was completed for 2 ~ 5 h, SB-3CT (25 mg/kg), an inhibitor of MMP9, was injected intraperitoneally. Sham group was performed for SAH1 method except that autologous blood was replaced by equal saline solution. In the placebo group, SB-3CT was replaced by 10% DMSO saline.
Treatment 2
Before rats were treated with SAH, they were injected with LPS (200 mg/kg, Escherichia coli serotype 055; B5, Sigma) intraperitoneally. The selection of this dose was according to the fever and systemic inflammatory response induced by LPS [24]. Preliminary experiments showed that rats in the control group were injected higher doses of LPS which led to the high mortality of rats within 24 h. Sham group was performed for SAH1 method except that autologous blood was replaced by equal saline solution. In the placebo group, LPS was replaced by 10% DMSO saline.
Functional Analysis
Takashi Method
Takashi method was regarded as the scoring criteria of SAH [14]. The neurological scores of rats were performed at 6, 12, 24, 48, and 72 h after SAH, respectively. Aggregate score was 18, 0–7 was severe SAH, 8–12 was moderate SAH, and 13–18 was mild SAH. Three trained neurology and neurosurgery doctors joined in the score evaluation, and they did not participate in the building of model.
Yamaguchi Method
Yamaguchi method was seen as the scoring criteria of SAH [25]. The neurological scores of rats were processed 24 h after SAH, respectively. Three trained neurology and neurosurgery doctors joined in score, and they did not participate in the building of model.
Detection of Regional Cerebral Blood Flow (rCBF)
The rCBF was measured using laser Doppler blood flow meter (moorVMS-LDF2). Briefly, the laser Doppler blood flow meter was placed in the epidural frontal cortex. Then, using dental drill, a hole was made in the place as the following coordinates: 3 mm before coronal and 2 mm lateral to the suture line, and probe was fixed using Histoacryl tissue glue and perpendicular to the surface of the endocranium. At last, probe was connected with laser Doppler blood flow meter in order to measure the change of rCBF after SAH.
Determination of Blood–Brain Barrier Permeability by Evan’s Blue Dye Extravasation
Blood–brain barrier permeability was assessed by measuring the content of Evans blue (EB) in the brain at 6, 12, 24, 48, and 72 h after SAH as previously reported [26]. Briefly, 60 min before the end of each time points, the rats were anesthetized and injected with EB dye (4%, 2.5 ml/kg) into the left femoral vein over 2 min. At the end of each time point, rats were re-anesthetized and transcardially perfused with ice-cold PBS. A microplate spectrophotometer (FLUOstar Optima, BMG, Durham, NC, USA) was used for determining the amount of extravasated EB in the brain (excitation at 620 nm and emission at 680 nm). Vascular leakage was quantified and expressed as microgram per gram of brain tissue based on the previous report [26, 27]. The Evan’s blue dye extravasation was also observed under a fluorescence microscope.
Measurement of Brain Edema
Rats were sacrificed at 6, 12, 24, 48, and 72 h after SAH, and the whole brain was removed. Then, the wet weight of brains was rapidly weighed. After they were placed in an oven with 105 °C for 24 h, their dry weight were measured [15]. The water content of brain = (wet weight−dry weight)/wet weight × 100%.
Hematoxylin-Eosin (HE) Staining
After brains of rats with different treatments were harvested, they were made into paraffin sections. Firstly, the paraffin sections were put into xylene I for 5 min, xylene II for 3 min, 100% ethanol for 3 min, 95% ethanol for 3 min, 90% ethanol for 1 min, 80% ethanol for 1 min, and 70% ethanol for 1 min, respectively, in order to make them dewax. Secondly, the sections were washed for 15 min in hematoxylin staining, for about 5 s in separation staining and several seconds in blue liquid, respectively. Then, after they were washed in 70% ethanol for 3 min, in 80% ethanol for 3 min, and in 90% ethanol for 3 min, they were dyed in eosin for 5 min. Finally, after they were washed twice in 100% ethanol for 3 min and twice in xylene for 5 min, they were dried and sealed using neutral balsam.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP-Biotin Nick End Labeling Assay
The paraffin sections of brains with different treatments were performed for terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) staining. First of all, the sections were dewaxed just the same as previously mentioned. Secondly, they were washed twice in distilled water, each time for 5 min, and thrice in PBS, each for 5 min. Afterwards, the tissues were treated with proteinase K, then they were put in the oven at 37 °C for 30 min. Then, they were washed thrice in PBS, each time for 5 min. After the preparation of TUNEL reaction mixture, the treatment group was added into dUTP mixture including 50 μl TdT and 450 μl fluorescein label, while the negative group was just added fluorescein labeled dUTP. After the glasses were dried, specimen were added 50 μl TUNEL reaction mixture, then they were put in dark and wet box at 37 °C for 1 h. Finally, after sections were washed thrice in PBS, each time for 5 min, they were sealed with DAPI staining and observed under fluorescent microscope.
RT-PCR
Total RNAs were extracted from brains of rats with different treatments using Trizol agent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instruction and reversely transcribed into cDNA. Then, MMP9, HMGB1, caspase3, and TLR4 were amplified through different mixtures including 12.5 μl 2 × PCR Master Mix, 0.5 μl 10 mM upstream primer, 0.5 μl 10 mM downstream primer, 10.5 μl PCR water, and 1 μl cDNA temple. And the primers used in this experiment were described in Table 3. Then, the mixtures were reacted at 94 °C for 5 min, cycled 35 times at 94 °C for 1 min, 55 °C for 1 min, 72 °C for 1 min, and finally at 72 h10 min. At last, PCR products were run in 1% agarose gel electrophoresis and taken pictures.
Western Blot
Brain tissues (250 g) were added into 500 μl precooling protein extraction reagent which contained 98% RIPA lysis buffer (Beyotime, Jiangsu, China) and 2% cocktail pill (Roche), and homogenized on ice using in situ homogenate machine. After the mixture was ice-bathed for 30 min and blended every 10 min, the mixture was quashed for 5 s every 5 s, total 10 times in Ultrasonic Cell Breaking Machine. The misture was centrifuged at 12000g for 15 min at 4 °C; then, the supernatant was collected, and the concentration of protein was curtained by BCA protein assay lit (Beyotime Institute). After that, the precipitated proteins (80 μg) were separated on a SDS-PAGE gel at 350 mA for 2 h and transferred to PVDF membranes at 350 mA for 2 h. SDS-PAGE consisted of 10 ml 15% separation gel and 6 ml 5% spacer gel. Ten milliliters 15% separation gel consisted of 2.3 ml ddH2O, 5 ml 30% polyacrylamide, 2.5 ml 1.5 mol/l Tris (PH 8.8), 0.1 ml 10% sodium dodecyl sulfate (SDS), 0.1 ml 10% ammonium persulfate (AP), and 0.004 ml TEMED. Six milliliters 5% spacer gel consisted of 4.1 ml ddH2O, 1.0 ml 30% polyacrylamide, 0.75 ml 1.5 mol/l Tris (PH 6.8), 0.06 ml 10% SDS, 0.06 ml 10% AP, and 0.006 ml TEMED. After the transferring was finished, the PVDF membranes were washed in 1 × TBS, then placed in 5% nonfat milk and slowly swayed for 2 h on horizontal pendulum table. Then, the PVDF membranes were incubated with primary antibodies of MMP9 (Goat, Santa Cruz, 1:500), HMGB1 (Rabbit, Abcam, 1:500), caspase3 (Rabbit, ZSGB-BIO, 1:500), TLR4 (Rabbit, Santa Cruz, 1:500), and β-actin (Mouse, Cell signaling, 1:2000) overnight at 4 °C, respectively. After the PVDF membranes were rapidly washed three times in 1 × TBST for 5 min on horizontal pendulum table, the PVDF membranes were seperatedly incubated with the secondary antibody Abexcel (Donkey anti goat, Goat anti-rabbit and Goat anti-mouse, anti-rabbit, Abcam, 1:1000) for 2 h with bobble at room temperature on horizontal pendulum table. Afterwards, the PVDF membranes were washed three times in 1 × TBST for 5 min on horizontal pendulum table, and scanned in Alpha Innotech (BioRad) with ECL.
Statistical Analysis
Data were analyzed using SPSS 19.0 software. All data were expressed as means ± standard deviation (SD) and compared by one-way ANOVA or repeated-measure ANOVA. The parametric Spearman’s correlation coefficient was applied to evaluate the strength of the relationship between the EB content and relative expression of MMP9 at different time points after SAH. Possibility less than 0.05 (P < 0.05) was regarded as statistical difference.
Results
Successful Selection of Suitable SAH Model of Rats
Comparison of the Lethality Rate, Neurological Score, rCBF, and Water Content of Brain Between SAH1 and SAH2 Group
Firstly, the lethality rate in SAH1 group was 16.7% (3/18), while it reached 61.1% (11/18) in SAH2 group (Fig. 1a). Secondly, Takashi score showed rats in the sham group had no nerve dysfunction and all obtained 18 scores. However, in SAH1 group, most rats revealed neurobehavioral abnormality at 12 h after SAH, and neurobehavior gradually recovered 24 h after SAH. In SAH2 group, rats revealed obviously neurobehavioral abnormality after SAH, while it gradually recovered after 48–72 h SAH, with a few residual neurological dysfunctions (Fig. 1b). Thirdly, LDF showed after SAH, the volume of LDF in SAH1 and SAH2 group quickly fell to 35.8 ± 19.3, 30.4 ± 11.7% of baseline, respectively, and 90 min later, the volume of LDF in SAH1 and SAH2 group were 82.2 ± 25.6 and 69.2 ± 27.5% of baseline which still have not returned to the baseline (Fig. 1c). When compared between groups at 5, 10, and 45 min after SAH, the volume of LDF in SAH1 group was higher than that in SAH2 group (P < 0.05) (Fig. 1c). In the sham group, CBF decreased rapidly after injection of saline, then returned to normal level within 3 min (Fig. 1c).
Comparison of the lethality rate, neurological score, rCBF, and water content of brain in the Sham, SAH1 and SAH2 group. a Lethality rate in each group. b Neurological score among three groups at 6, 12, 24, 48, and 72 h post operation. c RCBF in each group during 100 min after SAH. d The morphology of the brain observed in each group. e Water content of the brain in each group at 24 h after SAH. Single asterisk indicates P < 0.05; double asterisk indicates P < 0.01 compared with the sham group. hpo hours post operation, RCBF regional cerebral blood flow
The morphological detection showed in SAH1 group, subarachnoid blood volume was relatively consistent. And bleeding, observed in the skull base of subarachnoid, mainly focused on the former cycle around Willis, while it is less or no bleeding observed on latter cycle (Fig. 1d (SAH1)). In SAH2 group, subarachnoid blood volume was different; part of rats had relatively less hemorrhage which mainly distributed in Willis, brain stem, and around cerebellum (Fig. 1d (SAH2-1/SAH2-2)). The other rats had relatively more hemorrhage, which can diffuse between hemispheres (Fig. 1d (SAH2-1/SAH2-2)). There was no subarachnoid hemorrhage in the sham group (Fig. 1d (Sham)). Finally, the results of brain water content in different groups showed that water content of the brain in SAH1 and SAH2 group was both higher than that in the sham group (P < 0.05), and it was higher in SAH2 group than that in SAH1 group (P < 0.05) (Fig. 1e).
Comparison of HE and TUNEL Staining in SAH1 and SAH2 Group
The results of HE staining showed that in the sham group, neurons of cerebral cortex were neatly arranged, and all neurons shared intact structure, normal morphology, with red staining in cytoplasm and blue in nucleus (Fig. 2a (Sham)). In the SAH1 group, ischemic changes were characterized by contracted neurons, eosinophilic cytoplasm, disappearance of Nissl’s Body and nucleus pyknosis (Fig. 2a (SAH1)). In SAH2 group, cerebral cortex revealed obvious edema, brain tissue became loosed, the staining exhibited shallow, and intercellular space became broaden and turned into vacuoles degeneration (Fig. 2a (SAH2)). In addition, TUNEL staining showed that there were few apoptotic cells in sham group, while they were found extensively in SAH1 and SAH2 groups, while the number of apoptotic cells in SAH2 group was less than that in SAH1 group (Fig. 2b). Taken above evidences together, we chose SAH1 to induce the SAH model for studying involved mechanisms.
The results of HE staining and TUNEL staining. a HE staining in the sham, SAH1 and SAH2 group. b TUNEL staining in the sham, SAH1 and SAH2 group. Red fluorescence represents TUNEL staining for apoptosis; blue fluorescence represents DAPI staining for the cell nucleus. Merged indicated the positive apoptotic cells. Bar = 10 μm in a, 50 μm in b
Expressional Changes and Function of MMP9 in SAH-Induced EBI
Water Content of Brain, Blood–Brain Barrier Permeability, mRNA and Protein Expression of MMP9 at Different Timepoints After SAH
In order to choose the best time for studying the function of MMP9, water content of brain, blood–brain barrier permeability, and mRNA and protein expression of MMP9 at 6, 12, 24, 48, and 72 h after SAH were observed. Results showed that water content of brain began to rise at 12 h after SAH, peaked at 24 h after SAH, and was still high at 72 h after SAH, compared with sham group (P < 0.05) (Fig. 3c). In addition, blood–brain barrier permeability in the cortex was assessed by the content of extravagated EB. The results showed extravasation of EB dramatically increased at 12, 24, 48, and 72 h post SAH, compared with that of the sham group (P < 0.05, Fig. 3d). RT-PCR and western blot showed that in SAH group, the mRNA level of MMP9 began to rise at 12 h after SAH, reached the highest at 24 h after SAH, then decreased gradually, but was still higher at 72 h after SAH than that in the sham group (P < 0.05, Fig. 3a). The results of western blotting were similar with that of RT-PCR (P < 0.05, Fig. 3b). Moreover, in SAH rats, the relative expression of MMP9 correlated well with the extravasation of EB at 24 h (r 0.985, P < 0.001), 48 h (r 0.915, P < 0.001), and 72 h (r 0.786, P = 0.007) (Fig. 3e). Through the above results, 24 h after SAH, was selected for further study.
Alternations in the MMP9 expression and water content after SAH. a, b The mRNA and protein level of MMP9 at different time points in the SAH and sham group. c Water content of brain at different time points in the SAH and sham group. d Evans blue content in the cerebral cortical tissue in the groups of sham, 6, 12, 24, 48, and 72 h post SAH. Asterisk indicates P < 0.05 compared with sham group. e The parametric Spearman’s correlation coefficient was applied to evaluate the strength of the relationship between the relative expression of MMP9 in the cortex and the EB dye content at 24, 48, and 72 h after SAH. Evans blue r24h = 0.985 and r48h = 0.915, r72h = 0.786, P < 0.05 in each cases. f The results of Yamaguchi score in different groups at 24 h after SAH. Asterisk indicates P < 0.05 compared with the sham group. Octothorpe indicates P < 0.05 compared with the SAG + Vehicle group
SB-3CT Administration Relieved Neurological Dysfunction, Downregulated the Expression of MMP9, Reduced EB Extravasation, Alleviated Brain Edema, and Suppressed Cell Apoptosis of Cortex in Rats at 24 h After SAH
SB-3CT, an inhibitor of MMP9, was used in order to investigate the function of MMP9. In the sham group, rats all had no neurological dysfunction and obtained 0. Rats in SAH + SB-3CT group had better appetite and activity than those in SAH + DMSO (Vehicle) and SAH group (P < 0.05), but there was no evident statistical difference on the improvement of neurological deficits between SAH + SB-3CT and SAH+ Vehicle group or SAH group (P > 0.05) (Fig. 3f).
RT-PCR showed that the mRNA expression of MMP9 in SAH group was apparently higher than that in the sham group (P < 0.05). Whereas, SB-3CT administration significantly decreased the mRNA expression of MMP9, compared with that in SAH + Vehicle group, but the level of MMP9 was still higher than that of the sham group (P < 0.05) (Fig. 4b). The results of western blotting were similar with that of RT-PCR (Fig. 4a).
Alternations in the morphology and molecular after SB-3CT administration. a, b Protein and mRNA expression of MMP9 in cortex at 24 h after treatment in different groups. c Water content of brain at 24 h after treatment in different groups. d TUNEL staining of cortex at 24 h after treatment in different groups. Red fluorescence represents TUNEL staining for apoptosis; blue fluorescence represents DAPI staining for the cell nucleus. Merged indicated the positive apoptotic cells. Bar = 50 μm in d. e Evans blue content in the cerebral cortical tissue in the sham, SAH, SAH + Vehicle, and SAH + SB-3CT groups. Asterisk indicates P < 0.05 compared with the sham group; octothorpe indicates P < 0.05 compared with SAH + Vehicle group
Brain water content in SAH group were higher than that in the sham group (P < 0.05), and it was evidently decreased in SAH + SB-3CT group compared with that in SAH group (P < 0.05) (Fig. 4c). TUNEL staining confirmed that there was almost no positive cell apoptosis in sham group, but some apoptotic cells exhibiting red fluorescence in cortex and sub-cortex ganglion in both SAH group and SAH + SB-3CT group, and the number of apoptotic cells in SAH + SB-3CT group was evident less than that seen in SAH group (Fig. 4d). Additionally, there was a significant decrease in the permeability of the BBB indicated by EB extravasation in the SAH + SB-3CT group when compared with the SAH and SAH + Vehicle groups (P < 0.05).
Pretreatment of LPS Mitigated Neurological Dysfunction, EB Extravasation, Brain Edema, and Cell Apoptosis of Cortex in Rats with EBI Induced by SAH
Yamaguchi score indicated that rats in the sham group had no neurological dysfunction and obtained 0. SAH resulted in a significant neurological dysfunction, while rats in SAH + LPS group got better appetite and activity than that in SAH + Vehicle and SAH group (P < 0.05), but still worse than sham group (P < 0.05) (Fig. 5a). In addition, water content of brain in SAH group obviously increased compared with that in the sham group (P < 0.05), while it was evidently decreased in SAH + LPS group (P < 0.05), but was still a little higher than that in sham group (P > 0.05) (Fig. 5b). Moreover, after LPS pretreatment, EB dye content significantly decreased in the SAH + LPS group compared with the SAH and SAH + Vehicle groups (P < 0.05). TUNEL staining found the number of apoptotic cells in SAH + LPS group was less than that in SAH + Vehicle group (Fig. 5d).
Alternations in the behavior and morphology after pretreatment with LPS in SAH rats. a The results of Yamaguchi score at 24 h after treatment in different groups. b Water content of brain at 24 h after treatment in different groups. c Evans blue content in the cerebral cortical tissue in the sham, SAH, SAH + Vehicle, and SAH + LPS groups. d TUNEL staining at 24 h after treatment in SAH + Vehicle and SAH + LPS groups. Red fluorescence represents TUNEL staining for apoptosis; blue fluorescence represents DAPI staining for the cell nucleus. Merged indicated the positive apoptotic cells. Bar = 50 μm in c. Asterisk indicates P < 0.05 compared with the sham group; octothorpe indicates P < 0.05 compared with SAH + Vehicle group
LPS Pretreatment Increased mRNA Expression of TLR4, and Decreased mRNA Expression of MMP9 and Caspase3 at 24 h After Treatment
The mRNA expression of MMP9, HMGB1, TLR4, and caspase3 were detected at 6, 12, 24, 48, and 72 h in rats and 24 h after SAH was chosen to detect the effect of LPS pretreatment on the gene expression. The mRNA expression of MMP9 in cortex at different time points after treatment has been shown in Fig. 3a, as described above. Furthermore, 24 h after LPS treatment, the mRNA expression of MMP9 in SAH + LPS group was obviously lower than that in SAH group, but was still higher than that in the sham group (P < 0.05) (Fig. 6a).
Molecular changes in mRNA level after LPS pretreatment. a The mRNA expression of MMP9 in cortex at 24 h after treatment in different groups. b The mRNA expression of HMGB1 in cortex at different time points. c The mRNA expression of HMGB1 in cortex at 24 h after treatment in different groups. d The mRNA expression of TLR4 in cortex at different time point. e The mRNA expression of TLR4 in cortex at 24 h after treatment in different groups. f The mRNA expression of caspase3 in cortex at different time points. g The mRNA expression of caspase3 in cortex at 24 h after treatment in different groups. Asterisk indicates P < 0.05 compared with the sham group; octothorpe indicates P < 0.05 compared with SAH + Vehicle group
The mRNA expression of HMGB1 in cortex began to rise at 6 h after treatment, continuously increased at 12, 24, and 48 h after SAH, and reached the highest at 72 h compared with that in the sham group (P < 0.05) (Fig. 6b). Moreover, the mRNA expression of HMGB1 in cortex in SAH + LPS group was slightly higher than that in SAH group, but there was no obvious statistical difference (P > 0.05) (Fig. 6c).
The mRNA expression of TLR4 in cortex began to increase at 6 h after LPS treatment, continuously rose at 12, 24, and 48 h after SAH, and reached the highest at 72 h compared with sham group (P < 0.05) (Fig. 6d). Comparatively, the mRNA expression of TLR4 in SAH + LPS group was higher than that in SAH group (P < 0.05) (Fig. 6e).
The mRNA expression of caspase3 in cortex began to enhance at 6 h after SAH, then reached the highest at 48 h and began to decrease at 72 h, but it was evidently higher than that in the sham group (P < 0.05) (Fig. 6f). However, the mRNA expression of caspase3 in cortex in SAH + LPS group decreased obviously than that in SAH group, but was still higher than that in sham group (P < 0.05) (Fig. 6g).
LPS Increased Protein Expression of TLR4, Decreased Protein Expression of MMP9 and Caspase3 at 24 h After Treatment
The protein expression of MMP9 in cortex at 24 h in SAH group was obviously increased as compared with that in the sham group (P < 0.05). After LPS injection, it was evidently decreased in SAH + LPS group, compared with that in SAH group, but it was still higher than that in sham group (P < 0.05) (Fig. 7a).
Molecular changes in protein level after LPS treatment at 24 h after treatment in different groups. a, b, c, d showed the protein expressional levels of MMP9, HMGB1, TLR4, and caspase3, respectively, indicated by western blotting. Asterisk indicates P < 0.05 compared with the sham group; octothorpe indicates P < 0.05 compared with SAH + Vehicle group
The protein expression of HMGB1 in cortex at 24 h after treatment in SAH group was obviously enhanced than that in the sham group (P < 0.05), and it was increased in SAH + LPS group compared with that in SAH group, but it has no obvious statistical difference (Fig. 7b).
The protein expression of TLR4 in cortex at 24 h in SAH group obviously rose than that in sham group (P < 0.05). Comparatively, after LPS injection, it exhibited further increase in SAH + LPS group, compared with that in SAH group (P < 0.05) (Fig. 7c). In addition, the protein expression of caspase3 in cortex at 24 h after treatment in SAH group was obviously enhanced than that in the sham group (P < 0.05), while LPS injection evidently reduced the expression of caspase3 in SAH + LPS group, as compared with that in SAH group, and it was still higher than that in the sham group (P < 0.05) (Fig. 7d).
Discussion
In this study, we got several fruitful findings as follows: (1) prechiasmal cistern injection (SAH1) was more suitable to induce the SAH model, as it is more controllable and reliable than common carotid artery-prechiasmal cistern shunt method (SAH2); (2) SAH causes motor deficits and neuropathological/behavioral deficits, increased the permeability of the BBB, induced the brain edema, and accelerated apoptosis in the cortex, and the possible mechanism is associated with the MMP9 upregulation; (3) an inhibitor of MMP-9, SB-3CT administration, and LPS pretreatment improved neurological dysfunction, reduced blood–brain barrier disruption and brain edema and inhibited cell apoptosis of cortex in rats with SAH, in which, the vital mechanism is possibly associated with activating TLR4, and inhibiting the expression of MMP9 and caspase3 in cortex.
Previously, it has been reported that rats, rabbits, dogs, monkeys, and so on were usually used for producing SAH model [28–31]. Moreover, rats, as a common experimental animal that can reveal similar cerebral artery pathological changes with human, has been well addressed in the reproducing SAH model. Most genes related to human SAH have been proved in the model of rats. In this study, firstly, SAH1 and SAH2 were used for building SAH model in order to choose the more suitable method for studying SAH. Our results showed that SAH2 had high mortality and reached 61.1%, while a low mortality (16.7%) was seen in SAH1. In addition, the monitor on water content of the brain suggested that brain edema in SAH2 group was more serious than that in SAH1 group. Meanwhile, LDF, a nice tool to study hemodynamics after SAH, has been used to monitor the change of CBF real time [32], and we found that LDF in SAH1 and SAH2 was still lower than the baseline within 90 min. Additionally, the changed tendency of cerebral blood flow was different between SAH1 and SAH2, but their cerebral perfusion pressure was similar. In the sham group, CBF restored within 3 min, but intracranial pressure had a substantive rise [9]. Moreover, histologic changes in SAH1 and SAH2 group were both obvious, but cell apoptosis in SAH1 group was more evident than that in SAH2 group. As SAH2 model is difficult to control the subarachnoid hemorrhage and set the control group, it may result in diversity in the experiment, which is difficult to study and keep the experimental reliable and repeatable. Comparatively, SAH1 can keep stable subarachnoid hemorrhage, with easy operability, high repeatability, and low mortality. Using this method could easily set the control group such as DMSO saline, thus we could get the experimental data more consistently and reliably. So we chose SAH1 in the later study, which is also supported by previous studies that SAH1 method is valuable for the research of subarachnoid hemorrhage [9, 33–36]. Together, SAH1 seems to be the more suitable SAH model for studying pathology and pathophysiology and associated mechanisms after SAH.
Pervious study showed that activation of extracellular matrix remodeling genes, including MMP9, were found in the brain after SAH through gene chip, quantitative PCR and protein analysis technology. The results suggested that MMP9 may involve in the process of early extracellular matrix remodeling after SAH [37]. And MMP9 was also shown to have strong proteolytic function especially for small artery basement membrane and can lead to brain edema and secondary hemorrhage through degrading basement membrane components [38]. In the present study, we found that the relative expression of MMP9 in the cortex was activated at the different time points and it was positively correlated with the content of EB dye extravasation at 24, 48, and 72 h after SAH. Moreover, SB-3CT, an inhibitor of MMP9, was used for studying the function of MMP9 in SAH1 induced EBI. After SAH, the mRNA and protein expression of MMP9 were increased early, while SB-3CT addition decreased the mRNA and protein expression of MMP9 as compared with those in SAH and SAH + Vehicle group. What is more, we found that after SAH, rats had various decline in appetite and movement, and even part of them revealed hemiplegia and could not walk. However, after addition of SB-3CT, the appetite and movement of rats improved. In the index of blood–brain barrier disruption and brain edema, water content of the brain and EB extravasation in SAH + SB-3CT were lower than that in SAH + Vehicle group, but still higher than that in sham group. In addition, the number of cell apoptosis was evidently higher in SAH + Vehicle group than that in sham group, while SB-3CT administration significantly decreased cell apoptosis. These results suggested that MMP9 may involve in the process of EBI after SAH, and promotes or aggravates brain injury. Suppression of MMP9 may have protective effects on the EBI after SAH. Previous evidences suggested that MMP9 engaged in pathogenesis of brain injury after cerebral ischemia and may be associated with the occurrence of some degenerative diseases at the same time [39, 40]. Activation of MMP9 can degrade extracellular matrix components, cause blood-brain barrier damage and cell death, and further lead or aggravate vascular and cytotoxic brain edema [13, 41]. In addition, activation of MMP9 resulted in neuronal cell death and hemorrhagic transformation in cerebral ischemia model [42, 43]. Comparatively, our study reported and discussed the role of MMP9 and the treatment of EBI as well as associated mechanisms after SAH.
It has been reported that TLR4, a very important receptor of HMGB1, plays a crucial role in extracellular process [44–47], and release of HMGB1 has been as a sign of cell death [48]. Although previous studies suggested that HMGB1 can upregulate MMP9 through TLR4, thereby aggravate brain injury and inflammatory in cerebral ischemia model [21], there is no full correlation between LPS and HMGB1 in the SAH model, so it is difficult to infer the linkage among LPS, HMGB1, and MMP9 after SAH. In our study, the results revealed that LPS mitigated neurological dysfunction, reduced EB extravasation, and brain edema as well as cell apoptosis of cortex in rats with EBI induced by SAH. Meanwhile, LPS effectively increased the expression of TLR4 but not HMGB1, while it decreased the level of MMP9 and caspase3 at 24 h after treatment. Previous evidences have indicated LPS can activate TLR to regulate immune system, and low dose of LPS can induce cerebral protection in brain ischemia involving in enhancing the tolerance [22, 23, 25, 49], but related mechanism is still unknown, let alone in the SAH model. In this study, we showed the neuroprotective roles of low dose of LPS in the SAH rats may be through enhancing the tolerance, and the mechanism was associated with preactivation of TLR4, and downregulating the expression of MMP9 and caspase3, which was the most novel finding in present study.
Conclusion
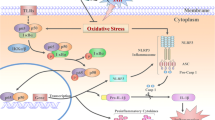
Our results suggest that SAH increased HMGB1, then upregulated the MMP9 expression, which may be the pathological mechanism of EBI induced by SAH. Importantly, low dose of LPS pretreatment can downregulate MMP9 and caspase3 by activating TLR4 for a positive neuroprotection on EBI after SAH, which may provide a novel target for the treatment of EBI induced by SAH (Fig. 8).
Summaries for the role of low dose of LPS pretreatment and related mechanism involving in HMGB1/TLR4/MMP9 signaling pathway and caspase3 in the SAH-induced EBI. Through comparison of SAH1 and SAH2 in the alternations of lethality rat, behavior, and morphology, SAH1 was selected to induce the SAH model in the following experiment. It showed that SAH deteriorated the neurological behavior, aggravated the cell apoptosis, and disrupted the BBB permeability indicated by EB extravasation, by increasing the MMP9 expression, which was indicated by SB-3CT administration. Previous study suggested that HMGB1 can upregulate MMP9 through TLR4, thereby aggravate brain injury and inflammatory in cerebral ischemic model. Therefore, we performed the LPS pretreatment to further demonstrate the role of MMP9 in EBI after SAH. Finally, we concluded that HMGB1 upregulated MMP9 through TLR4 in EBI induced by SAH. Pretreatment of low dose LPS can downregulate MMP9 and caspase3 by activating TLR4, and enhance the tolerance, thereby induced nerve protective effect on EBI after SAH. EBI early brain injury, SAH subarachnoid hemorrhage, BBB blood–brain barrier, EB Evans blue
Change history
06 December 2016
An erratum to this article has been published.
04 September 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s12035-024-04463-3
Reference
Broderick JP, Brott TG, Duldner JE et al (1994) Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 25(7):1342–1347
Edner G, Kagstrom E, Wallstedt L (1992) Total overall management and surgical outcome after aneurysmal subarachnoid haemorrhage in a defined population. Br J Neurosurg 6(5):409–420
Hutter BO, Kreitschmann-Andermahr I, Gilsbach JM (2001) Health-related quality of life after aneurysmal subarachnoid hemorrhage: impacts of bleeding severity, computerized tomography findings, surgery, vasospasm, and neurological grade. J Neurosurg 94(2):241–251
Ljunggren B, Saveland H, Brandt L et al (1984) Aneurysmal subarachnoid hemorrhage. Total annual outcome in a 1.46 million population. Surg Neurol 22(5):435–438
Crompton MR (1964) The pathogenesis of cerebral infarction following the rupture of cerebral berry aneurysms. Brain 87:491–510
Bederson JB, Germano IM, Guarino L (1995) Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke 26(6):1086–1091 discussion 1091-2
Bederson JB, Levy AL, Ding WH et al (1998) Acute vasoconstriction after subarachnoid hemorrhage. Neurosurgery 42(2):352–360 discussion 360-2
Jackowski A, Crockard A, Burnstock G et al (1990) The time course of intracranial pathophysiological changes following experimental subarachnoid haemorrhage in the rat. J Cereb Blood Flow Metab 10(6):835–849
Prunell GF, Mathiesen T, Diemer NH et al (2003) Experimental subarachnoid hemorrhage: subarachnoid blood volume, mortality rate, neuronal death, cerebral blood flow, and perfusion pressure in three different rat models. Neurosurgery 52(1):165–175 discussion 175-6
Schwartz AY, Masago A, Sehba FA et al (2000) Experimental models of subarachnoid hemorrhage in the rat: a refinement of the endovascular filament model. J Neurosci Methods 96(2):161–167
Veelken JA, Laing RJ, Jakubowski J (1995) The Sheffield model of subarachnoid hemorrhage in rats. Stroke 26(7):1279–1283 discussion 1284
Zhao W, Ujiie H, Tamano Y et al (1999) Sudden death in a rat subarachnoid hemorrhage model. Neurol Med Chir (Tokyo) 39(11):735–741 discussion 741-3
Cahill J, Calvert JW, Zhang JH (2006) Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 26(11):1341–1353
Kusaka G, Ishikawa M, Nanda A et al (2004) Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 24(8):916–925
Bazan NG, Rodriguez de Turco EB (1980) Membrane lipids in the pathogenesis of brain edema: phospholipids and arachidonic acid, the earliest membrane components changed at the onset of ischemia. Adv Neurol 28:197–205
Park S, Yamaguchi M, Zhou C et al (2004) Neurovascular protection reduces early brain injury after subarachnoid hemorrhage. Stroke 35(10):2412–2417
Hamann GF, Okada Y, Fitridge R et al (1995) Microvascular basal lamina antigens disappear during cerebral ischemia and reperfusion. Stroke 26(11):2120–2126
Gu Z, Cui J, Brown S et al (2005) A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci 25(27):6401–6408
Guo Z, Sun X, He Z et al (2010) Matrix metalloproteinase-9 potentiates early brain injury after subarachnoid hemorrhage. Neurol Res 32(7):715–720
Guo ZD, Zhang XD, Wu HT et al (2011) Matrix metalloproteinase 9 inhibition reduces early brain injury in cortex after subarachnoid hemorrhage. Acta Neurochir Suppl. 110(Pt 1):81–84
Qiu J, Xu J, Zheng Y et al (2010) High-mobility group box 1 promotes metalloproteinase-9 upregulation through toll-like receptor 4 after cerebral ischemia. Stroke 41(9):2077–2082
Rosenzweig HL, Lessov NS, Henshall DC et al (2004) Endotoxin preconditioning prevents cellular inflammatory response during ischemic neuroprotection in mice. Stroke 35(11):2576–2581
Rosenzweig HL, Minami M, Lessov NS et al (2007) Endotoxin preconditioning protects against the cytotoxic effects of TNFalpha after stroke: a novel role for TNFalpha in LPS-ischemic tolerance. J Cereb Blood Flow Metab 27(10):1663–1674
Hang CH, Shi JX, Tian J, Li JS, Wu W, Yin HX (2004) Effect of systemic LPS injection on cortical NF-kappaB activity and inflammatory response following traumatic brain injury in rats. Brain Res 1026(1):23–32
Pradillo JM, Fernandez-Lopez D, Garcia-Yebenes I et al (2009) Toll-like receptor 4 is involved in neuroprotection afforded by ischemic preconditioning. J Neurochem 109(1):287–294
Hu Q, Chen C, Yan J, Yang X, Shi X, Zhao J, Lei J, Yang L et al (2009) Therapeutic application of gene silencing MMP-9 in a middle cerebral artery occlusion-induced focal ischemia rat model. Exp Neurol 216:35–46
Lenzsér G, Kis B, Snipes JA, Gáspár T, Sándor P, Komjáti K, Szabó C, Busija DW (2007) Contribution of poly (ADP-ribose) polymerase to postischemic blood–brain barrier damage in rats. J Cereb Blood Flow Metab 27:1318–1326
Masuda T, Sato K, Yamamoto S et al (2002) Sympathetic nervous activity and myocardial damage immediately after subarachnoid hemorrhage in a unique animal model. Stroke 33(6):1671–1676
Pradilla G, Thai QA, Legnani FG et al (2005) Local delivery of ibuprofen via controlled-release polymers prevents angiographic vasospasm in a monkey model of subarachnoid hemorrhage. Neurosurgery 57(1 Suppl):184–190 discussion 184-90
Westermaier T, Jauss A, Eriskat J et al (2009) Time-course of cerebral perfusion and tissue oxygenation in the first 6 h after experimental subarachnoid hemorrhage in rats. J Cereb Blood Flow Metab 29(4):771–779
Yin YH, Wang F, Pan YH et al (2009) Effects of dose-response of topical administration of nimodipine on cerebral vasospasm after subarachnoid hemorrhage in rabbits. Am J Med Sci 337(2):123–125
Lindegaard KF (1999) The role of transcranial Doppler in the management of patients with subarachnoid haemorrhage—a review. Acta Neurochir Suppl 72:59–71
Delgado TJ, Brismar J, Svendgaard NA (1985) Subarachnoid haemorrhage in the rat: angiography and fluorescence microscopy of the major cerebral arteries. Stroke 16(4):595–602
Solomon RA, Antunes JL, Chen RY, Bland L, Chien S (1985) Decrease in cerebral blood flow in rats after experimental subarachnoid hemorrhage: a new animal model. Stroke 16(1):58–64
Delgado TJ, Diemer NH, Svendgaard NA (1986) Subarachnoid hemorrhage in the rat: cerebral blood flow and glucose metabolism after selective lesions of the catecholamine systems in the brainstem. J Cereb Blood Flow Metab 6(5):600–606
Ram Z, Sahar A, Hadani M (1991) Vasospasm due to massive subarachnoid haemorrhage—a rat model. Acta Neurochir 110(3–4):181–184
Vikman P, Beg S, Khurana TS et al (2006) Gene expression and molecular changes in cerebral arteries following subarachnoid hemorrhage in the rat. J Neurosurg 105(3):438–444
Castillo J, Leira R, Blanco M (2004) Metalloproteinases and neurovascular injury. Neurologia 19(6):312–320
Zhao BQ, Tejima E, Lo EH (2007) Neurovascular proteases in brain injury, hemorrhage and remodeling after stroke. Stroke 38(2 Suppl):748–752
Kiaei M, Kipiani K, Calingasan NY et al (2007) Matrix metalloproteinase-9 regulates TNF-alpha and FasL expression in neuronal, glial cells and its absence extends life in a transgenic mouse model of amyotrophic lateral sclerosis. Exp Neurol 205(1):74–81
Fukuda S, Fini CA, Mabuchi T et al (2004) Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke 35(4):998–1004
Zalewska T, Ziemka-Nalecz M, Sarnowska A et al (2003) Transient forebrain ischemia modulates signal transduction from extracellular matrix in gerbil hippocampus. Brain Res 977(1):62–69
Rosell A, Cuadrado E, Ortega-Aznar A et al (2008) MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke 39(4):1121–1126
Yu M, Wang H, Ding A et al (2006) HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 26(2):174–179
Park JS, Svetkauskaite D, He Q et al (2004) Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279(9):7370–7377
van Zoelen MA, Yang H, Florquin S et al (2009) Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock 31(3):280–284
Kruger B, Krick S, Dhillon N et al (2009) Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci U S A 106(9):3390–3395
Wang H, Bloom O, Zhang M et al (1999) HMG-1 as a late mediator of endotoxin lethality in mice. Science 285(5425):248–251
Tasaki K, Ruetzler CA, Ohtsuki T et al (1997) Lipopolysaccharide pre-treatment induces resistance against subsequent focal cerebral ischemic damage in spontaneously hypertensive rats. Brain Res 748(1–2):267–270
Acknowledgements
This research was supported by a grant of the National Science Foundation of China (No. 81271326, No.81100910), and supported by the Program for IRTSTYN, together with program Innovative Research Team In Science and Technology in Yunnan province (2016–2019).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Ting-Hua Wang, Liu-Lin Xiong, Shuai-Fen Yang, Shu-Fen Wang and, Jia-Liu contributed equally to this work.
The original version of this article was revised: The author's name Shu-Feng Wang was changed to Shu-Fen Wang per request of authors.
An erratum to this article is available at https://doi.org/10.1007/s12035-016-0320-6.
About this article
Cite this article
Wang, TH., Xiong, LL., Yang, SF. et al. RETRACTED ARTICLE: LPS Pretreatment Provides Neuroprotective Roles in Rats with Subarachnoid Hemorrhage by Downregulating MMP9 and Caspase3 Associated with TLR4 Signaling Activation. Mol Neurobiol 54, 7746–7760 (2017). https://doi.org/10.1007/s12035-016-0259-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0259-7