Abstract
Stem cell biology has played a pivotal role in the field of disease modeling, regenerative medicine, and tissue engineering. The scope of stem cell research has been further extended to address the issues associated with toxicity and biosafety. However, its role in the field of neurotoxicity (NT) and the emerging field of developmental neurotoxicity (DNT) is somewhat underrepresented and needs thorough investigation. Several challenges have hindered the progress of NT and DNT studies, and there is a dire need for human-specific high-throughput in vitro system(s) as a tool with better predictivity, reliability, and reproducibility. The unique proliferation and pluripotency of stem cells makes them a tremendous resource for human material, allowing the prediction of drug toxicity and metabolic effects of chemicals. Recognizing the growing importance of NT and DNT and the application of stem cell biology, in this review article, we provide the diversified approaches of stem cell research which can be effectively applied to the NT and DNT studies and provide an update of the recent progress made so far. We further provide a futuristic approach towards novel stem cell-based strategies for NT and DNT testing. We have further discussed the current technologies, role of induced pluripotent stem cells, the application of three-dimensional (3D) cultures and role of stem cell-derived organs in the NT and DNT studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stem cell biology has been a captivating area of research for biologists across the globe, and it finds many applications in the field of disease modeling, tissue engineering, and regenerative medicine. However, its scope in the field of neurotoxicity and developmental neurotoxicity has not been thoroughly investigated as yet. Neurotoxicity (NT) and developmental neurotoxicity (DNT) are the emerging fields of neuroscience, but their progress has been limited by the various challenges being faced. These challenges arise due to the limited availability of screening models of human origin. The human brain tissue which has been exposed to drugs, disease, and chemicals is difficult to procure [1], and human volunteers are not easily available. Animal models are less predictive in nature and require validation in human beings. Conventional cell culture-based studies are two dimensional (2D) in nature and are not the true representative of a complex organ system such as the human brain. In this regard, stem cell technology can be effectively applied in the field of drug and chemical testing for the assessment of NT and DNT. With stem cells, there is a possibility to perfectly replicate a functional environment such as that within the brain, while also allowing easy testing in terms of neurophysiological and biochemical parameters. Neurotoxicity is generally defined as any adverse effect on the central nervous system (CNS) or the peripheral nervous system (PNS) caused by any physical, chemical, or biological agent [2]. Developmental neurotoxicity refers to the adverse effects of xenobiotics on the nervous system associated with exposure during development [3, 4].
Stem cells have been extensively used for the various toxicological studies and many review articles have been written already. We include some examples below so as to highlight the importance of stem cells in the field of toxicology. Laustriat et al. [5] briefly reviewed the unique properties of pluripotent stem cells as drug discovery models and how they would bring about a revolution in the preliminary stages of toxicological and pathological modeling. They further explored the application of human embryonic stem cells (hESCs) as powerful disease models and discussed that PGD-hESCs, i.e., the human embryonic stem cells obtained from embryos identified during preimplantation genetic diagnosis provide models of relevance for studying the pathological mechanisms of a genetic disease in a much better way than genetically engineered hESCs. Their review also gave an insight into the concept of personalized medicine using patient-specific-induced pluripotent stem cells. Toxicology testing was discussed with emphasis being on stem cell-derived hepatocytes and alternate stem cell-derived in vitro skin models for cosmetic testing. Another review published by Kellyn S Betts [6] explored the capability of stem cells in the field of developmental neurotoxicity. This publication dealt with the limitations of human embryonic stem cells and presented neural stem cells and neurospheres as the simplest three-dimensional (3D) models for DNT testing. It is well known that human embryonic stem cells are faced by many ethical facets and, thus, in this regard, the human cord blood can serve as a rich source of fetal stem cells.
The group of Kyung-Sun Kang and James E. Trosok [4] concisely reviewed the various aspects of stem cell biology and its relevance in the field of toxicology. They provided a precise overview of the nature of stem cells and the role of cell-cell interaction in the biology of cancer cells and stem cells and also familiarized us with the genetic concept which is instrumental in the process of differentiation and lineage specification. With the emphasis being on the fundamentals of stem cell biology, their employment in the field of toxicology was briefly explored. It clearly emerged that apart from embryonic and fetal stem cells, adult stem cells can be effectively used to screen and model organ-specific toxicities as these cells express the organ-specific markers along with the stemness markers (Oct 4) and have better lineage specificities. Adult stem cells will thereby form 3D organoids more efficiently and thus serve as good models for toxicity testing. Kornelia Szebenyi et al. [7] gave us a concise picture of the then prevalent hurdles in the field of toxicological and pharmacological screening and the limitations of the alternate in vitro models. This group precisely summarized the then published studies and briefly discussed the application of human pluripotent stem cells (hPSCs) in the generation of neuronal tissues for disease-specific studies such as the formation of electrophysiologically active functional dopaminergic neurons from hESCs by manipulating the signaling pathways and treatment with FGF-8 and sonic hedgehog. This study further dealt with the aspects of cardiac pharmacology in detail and gave the application of stem cells in this regard. In 2011, Sison-Young et al. [8] came up with a detailed review encompassing pluripotent stem cells for toxicity modeling. This review gave us ample insight into the very basics of stem cell toxicology and provided a detailed account of cardiotoxicity and hepatotoxicity. They also briefly touched upon the field of neurotoxicity. To further add to literature, Zeljko J. Bosnjak et al. [1] came up with a review which dealt with the developmental neurotoxicity of general anesthetics and alcohol and discussed the potential applicability of stem cell-derived in vitro models in the form of human neuronal cell lines. Since then, extensive progress has been made in the field of in vitro stem cell toxicology, and the field of induced pluripotent stem cells (iPSCs) which was then in its infancy has progressed tremendously. Organ-specific toxicities have been well studied and are well reported; however, in the current review, we shall provide an update on the application of stem cells in the field of neurotoxicity and developmental neurotoxicity.
The Need for Neurotoxicity and Developmental Neurotoxicity
The brain is one of the most evolutionary advanced complex organs in the entire living system. The entire process of brain development is highly complicated and follows a series of well orchestrated cellular and molecular events. The brain starts developing by the end of the third gestational week and continues up till late adolescence [9]. The mature brain comprises more than hundred billion neurons and nearly more than 60 trillion neuronal connections [9]. These numbers are a mere reflection of the brain’s complexity and clearly indicate the relevance of any toxic insult incurred on the same. The central nervous system (CNS) comprises a number of different cell types such as the oligodendrocytes, microglia, astrocytes, and the neurons with each cell type assigned with a specific role and function [10]. Different neurotransmitters released by the various neurons influence the physiology of the entire CNS. The complex interactions between these various cell types are pivotal in maintaining the functionality of the brain. Neurons are considered the functional units of the brain and interact with nearly all the other cell types. Astrocytes help the neurons to migrate to the right positions during brain development and help in synaptic assembling. The astrocytes play a protective role for the neurons in an adult brain and help in the maintenance of the trophic and ionic balance. The neuronal pathology and physiology is further regulated by the astrocytes as they regulate the calcium levels of the brain. Myelination is governed by the oligodendrocytes which help in electrical insulation and allows for rapid transmission along the axons. Myelination prevents ion leakage and results in decreased cell membrane capacitance which leads to the generation of neuronal impulse. Microglia, too, have a protective role [9]. The astrocytes and microglia show increased activity during neuronal damage and secrete a number of neurotoxic and pro-inflammatory factors such as free radicals and cytokines [9]. It is sometimes stated that neurons are representatives of a human being’s age, and their progressive loss is directly correlated with aging. Thus, any premature neuronal loss due to the effect of a toxicant will have serious implications and clearly cannot be ignored. However, an accurate model for neurotoxicity and developmental neurotoxicity must comprise the various cells of the CNS and be a true representative of the complex in vivo milieu.
Hepatotoxicity, cardiotoxicity, and renal toxicity are well documented fields; however, the respective organs involved are comparatively less complex and easier to look into. There is sufficient literature supporting the use of stem cells in modeling hepatotoxicity [11–14] and cardiotoxicity [15–18] as well. However, when the brain is challenged by any form of toxic insult, the consequence though severe may not be easily detected. There are negligible reflective markers in the blood which further make the clinical diagnosis challenging. As the famous proverb goes that “prevention is better than cure,” it is very important that the reason for neuronal damage and neuronal degradation is pointed out on time. It is a well documented fact that the developing brain in fetuses and children is highly vulnerable to chemical exposure and is more susceptible than an adult brain [6, 19]. The doses of chemicals that are harmless for a mature central nervous system have the potential to cause severe neurotoxicity to the developing nervous system [20]. The main reason behind this is that the blood brain barrier is not entirely functional and well developed; oxygen levels are low (hypoxic conditions) and mitosis rates are high [21]. The brain development process comprises many controlled and well orchestrated steps such as differentiation, proliferation, migration, synaptogenesis, neuronal network formation, and neuritogenesis [22]. In order to study and characterize the various aspects of DNT, we first need accurate models that not only mimic the development process but also are true representatives of the actual brain development in utero and in vivo [4, 20]. DNT is still an underrepresented field. Based on the OECD guideline 426, rodent data is available for approximately 200 compounds, but there is a need for human epidemiological data, which is available for only a minimal number of substances [23].
Developmental neurotoxicity so far has been best studied in in vivo models and involves the administration of potent neurotoxins/chemicals/xenobiotics/compounds to pregnant animals at specific stages of fetal and embryonic development [7]. Neurotoxicity too has been thoroughly investigated using in vivo models mainly. High expense incurred and the need for animal sacrifice limits the scope of in vivo research. Extrapolation of in vivo results to that of human beings is further restricted by substantial species differences [7]. Literature supports the fact that drugs and chemicals effectively tested for cardiotoxicity and hepatotoxicity failed during clinical trials [7]. Keeping these limitations in mind, there is a dire need for well characterized in vitro models of human origin which will effectively serve as test systems for neuronal disease modeling, neuronal developmental process, and, most importantly, as screening tools for neurotoxicity and developmental neurotoxicity. They not only provide a quick and easy extrapolation of data but also lessen the moral burden of animal sacrifice. To retrieve live brain tissues from humans is not only difficult but also practically unfeasible too [1]. In this context, human stem cell-based developmental neurotoxicity models could be one of the best alternatives [7, 24]. In the forthcoming sections, we shall discuss the application of stem cells in the field of neurotoxicity and developmental neurotoxicity.
The Relevance of Stem Cells
Stem cells have a remarkable ability for proliferation, self renewal, and differentiation [25]. When provided with select growth factors and culture conditions, they can be differentiated into the various cell lineages [26]. Depending upon the source of origin, stem cells are categorized as adult stem cells (ASCs), fetal stem cells (FSCs), and embryonic stem cells (ESCs) [27]. ESCs are derived from the blastocyst of fertilized egg and being pluripotent in nature can give rise to the cells of all the three germ layers of the body, namely the endoderm, mesoderm, and ectoderm. As compared to FSCs and ASCs, the differentiation potential of ESCs is best but various ethical obligations/norms adverse immune responses elicited, and tumor formations limit their widespread use. Fetal stem cells with their intermediate proliferation and differentiation abilities find widespread application though ethical issues still persist. Our research group has also been using human cord blood-derived stem cells which are fetal in nature for in vitro toxicology studies as well as developmental neurotoxicity studies. These stem cells are advantageous because they do not form tumors.
Adult stem cells (ASCs) are multipotent and are derived from the various tissues in the body such as the central nervous system, bone marrow, skeletal muscle, adipose tissue, and so on. Though the adult stem cells have a limited potential for growth and differentiation, they find a widespread application in autologous transplantations and personalized medicine. Despite being very few in number and difficult accessibility, ASCs are preferable also because they do not pose many ethical challenges. Neural stem cells (fetal as well as adult) find a wide range of applications in NT and DNT testing studies [28, 29]. Classical in vitro toxicology studies employ immortalized cell lines and primary cultures. The major limitation with cell lines is that they are genetically transformed and thus may not represent the normal cell types whereas primary cultures such as neuronal cultures are post mitotic in nature and difficult to maintain. Primary cultures have a very limited lifespan and easily lose their tissue-specific characteristics over time. In this case, stem cells are one of the best alternatives because they provide a virgin, nontransformed source of cells which can be differentiated into any lineage and serve as potent in vitro models [25]. They can be maintained in culture for long durations and can thus serve as good models for chronic toxicities as well [30].
Differentiating Stem Cells: Classical In Vitro Tool for NT and DNT Testing
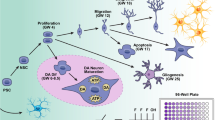
It is well documented that stem cells with their multi lineage differentiation capacities can be made to differentiate into specific neuronal lineages and will thereby sufficiently mimic cells of the developing brain [31, 32]. Successful differentiation of stem cells into neuronal lineages [33–36] is widely reported, and these cells are not only functional but electrophysiologically active too [37]. In our so far published papers, we have successfully tried to establish stem cell-based in vitro model systems for the study of the various aspects of NT and DNT [21] and have proven that human umbilical cord blood-derived stem cells (hUCBSCs) such as hematopoietic stem cells and mesenchymal stem cells when differentiated into neuronal subtypes serve as one of the classical tools for the same [38]. These were employed to assess the developmental neurotoxicity potential of monocrotophos (MCP), a well known developmental neurotoxin and further used to unravel the signaling cascades and mechanistic aspects involved. . Prior to this our research group also assessed the MCP-induced neurotoxicity in rat pheochrocytoma cells-PC12 cell line, an established in vitro neuronal model. The activation of caspase cascade in response to MCP exposure in PC12 cells depicted the apoptosis inducing capacity of MCP in neurons. The observations of this study and the study conducted using MCP-exposed hUCBSCs were on similar lines thus establishing the idea of differentiating stem cells being potent in vitro tools for NT and DNT testing [39, 40]. Our data also proves that early differentiating neurons are metabolically more active and more vulnerable to xenobiotics/compounds and polycyclic aromatic hydrocarbons as compared to the fully differentiated mature neuronal cells [41, 42]. The conversion of human cord blood CD34+Thy1+ stem cells into neuronal subtypes under the influence of specific growth conditions and response of these differentiating cells to MCP has been depicted in Fig. 1.
Schematic diagram represents the conversion of human cord blood CD34+Thy1+ stem cells into neuronal subtypes under the influence of specific growth conditions. Further, monocrotophos, an organophosphate pesticide and known developmental neurotoxin-induced alterations in the expression (mRNA and protein) of markers associated to stemness, stage-specific neuronal development and injury, have also been studied by our research group
Induced Pluripotent Stem Cells (iPSCs): a Potential Tool to Study NT and DNT
Shinya Yamanaka and his group revolutionized the very basics of embryology and gave a new face to the biology of development. They not only challenged but also proved that the process of differentiation is reversible [43, 44]. In their historical work published in Cell, 2006, they showed that pluripotency could be induced in mouse somatic cells. Four genes encoding transcription factors, i.e., Oct4, Sox2, Klf4 and c-Myc were introduced using retroviral vectors [43, 44]. These were the first induced pluripotent stem cells (iPSCs); later in 2007, human iPSCs were generated using a similar technology [45]. Apart from these four above mentioned transcription factors, Lin28 and Nanog could be used in place of c-Myc and Klf4 to impart pluripotency to human fibroblasts. Embryonic stem cells due to their inherent ability to self renew, divide, and differentiate have been considered as one of the most suitable treatments for intractable diseases like spinal cord injury and Parkinson’s disease [45]. However, ethical conflicts regarding the use of human embryos and immune rejection post transplantation restrict the scope of embryonic stem cells. In this regard, iPSC technology provides a relief and can be employed instead, as a powerful tool for regenerative medicine, disease modeling, age-related studies as well as NT and DNT studies [46, 47]. Disease modeling has been studied so far using in vivo models, but the imprecise recapitulation of human diseases has limited its scope [48]. For example, in the case of amyotrophic lateral sclerosis, many drugs proved to be therapeutic in rodents but failed to elicit the same response in human beings [49], thereby confirming that efficient disease modeling requires cells of human origin [50, 51].
Nearly a decade ago, the iPSCs technology which seemed unlikely and farfetched has made a substantial progress and is being routinely used for the reprogramming of somatic cells to provide for stem cells in bulk and is also being employed for toxicity and drug testing studies [52, 53]. Successful reprogramming of fibroblasts into electro physiologically functional post mitotic neurons has been accomplished [54], and the quest to achieve specific functional neuronal subtypes continues and has been nearly achieved. Successful reprogramming of fibroblasts into functional dopaminergic [55] and glutaminergic neurons [56] has been accomplished. In 2011, the group of Son et al. [57] successfully reprogrammed human and mouse fibroblasts into functional spinal motor neurons but in a way slightly different from the conventional reprogramming being employed for iPSC generation. They chose to transform the fibroblasts into motor neurons with the help of select transcription factors that were forced to express and thus acted on cells intrinsically rather than acting extrinsically as in the case of morphogen-driven reprogramming. Eight well known transcription factors were selected which direct formation of motor neurons during development along with three specification factors (Asc11, Brn2, and Myt11) that form induced neurons from fibroblasts. Repeated experimentation proved that motor neuron induction was efficiently achieved by seven main factors namely Brn2, Hb9, Asc11, Myt11, Lhx3, Is11, and Ngn2. These were named the “induced motor neurons or iMNs”. The resulting iMNs were not only electrophysiologically active but also expressed channels and receptors to generate transmitter-sensitive excitable membranes. They functionally resemble spinal motor neurons in their ability to induce muscle contraction and form synapses with them, and when grafted in the developing chick spinal cord, they efficiently migrated to the ventral horn and sent axonal projections through the ventral root. Sensitivity towards the amyotrophic lateral sclerosis (ALS) degenerative stimulus further confirmed the identity of these induced motor neurons. However, the most striking feature that emerged was that these transforming fibroblasts did not transit or pass through the proliferative neural progenitor state. This paved way discovery that factor-defined reprogramming when supplemented with massive gene expression changes can bring about a more efficient and faster transformation of mouse and human fibroblasts into spinal motor neurons. If efficient, rapid, and reproducible production of specific neuronal subtypes is achieved, accurate target-specific neurotoxicity and developmental toxicity studies will be performed. Induced pluripotent stem cells can thus also function as promising NT and DNT in vitro models can be employed for patient-specific mechanistic studies [58]. They can provide an insight into the gene-environment interactions and help us resolve issues of genetic susceptibility of individuals towards environmental risks. Personalized developmental neurotoxicological and neurotoxicological risk assessment are one of the future goals of the iPSC technology. Few of the most representative studies investigating the NT and DNT of drugs/chemicals using stem cells/iPSCs as biological tool are summarized in Table 1.
Transition from 2D to 3D: Provide Better Possibilities for NT and DNT Studies
It is both interesting and challenging for a biologist to mimic life in vitro. To see the events of the genesis of life unfold in an orderly manner within a laboratory has always captured the imagination of scientists across the globe. Since life is not two-dimensional (2D) but three-dimensional (3D), therefore, a recent revolution in the field of life sciences is the 3D cell culture [59]. Cell culture forms the basis of most of the cell biology, biochemical, drug development, and pharmacokinetic studies. The various in vitro studies are best represented by the standard conventional 2D monolayer cell culture systems. Since, easy handling, maintenance, and simplified growth conditions make 2D cultures the classical in vitro tool. The cost incurred is low and ethical issues are minimal. However useful the 2D cell cultures may be, they can never be the true representatives of an in vivo model [60]. The exposure given with various chemicals is effective only on the upper surface since the cells are in contact with a surface (flasks, petridishes, etc.). However, the biological scenario inside a live animal is very different because no cell or organ acts in isolation and the entire animal body functions in a highly orchestrated manner. When a toxicant enters the living system it will affect the different cells of the body in different ways, these are the terminally differentiated cells of a tissue, the transit amplifying cells, the adult stem cells and the cells of the immune system. Thus any clinical symptom or toxic effect seen is a result of a complex interplay of the various cells and their interaction with the provided toxicant. 2D cell cultures are incapable of recapitulating the structure, function and physiology of live tissues and neither can they mimic the exact dynamic in vivo environment [61, 62].
Biochemical and morphological endpoints have been employed to study the neuro-developmental aspects of the brain such as proliferation, differentiation, migration, and apoptosis but the functional aspects are still under covered and scarcely represented. There is a need for studies related to neuronal network formation, neuronal functionality, and inter- and intracellular signaling involved in NT and DNT testing [22]. Microelectrode arrays (MEAs) serve as one of the potential in vitro tools for characterizing and assessing the functionality of neuronal networks, synaptic plasticity, synaptic transmission, and their response to potent developmental neurotoxins [63–66]. However, one major limitation of the MEAs is that they cannot provide path-specific measurements [67]. The network formation assay (NFA) is another potent in vitro tool employed for NT and DNT studies [68]. Being based on the principle of cell patterning and display of neurite interconnections, it can thus be used to predict and screen potent developmental neurotoxins whose mode of actions and molecular targets are unknown [20]. However, these tools are still confined within the zone of two-dimensional cell culture.
Neurospheres to Organoid Tissue Niche: a new era of 3D Models for NT and DNT Studies
The biggest challenge that still remains is to establish high throughput screening tools so that a number of new and unrecognized potent neurotoxins can be screened and their toxic potentials and mechanisms predicted. In 2007, Johns Hopkins Centre for Alternatives to Animal Testing (Developmental Neurotoxicity TestSmart program) and The European Centre for the validation of Alternative methods and the European Chemical Industry Council joined hands to establish effective DNT models so as to allow for better risk assessment and hazard management [69]. This joint venture successfully established human neurospheres as effective 3D in vitro tools for DNT testing. Ellen Fritsche of the Environmental Health Research Institute in Dusseldorf and the University of Aachen and her group are among the first few to employ neurospheres (created from neuroprogenitor cells) as powerful in vitro 3D cell systems [6]. They have shown that neurospheres when exposed with well known neurodevelopmental toxicants such as methyl-mercury chloride and mercury chloride result in a decreased number of nerve cells produced by them, and the migration process is severely affected as well. Cell migration during brain development is also affected by polybrominated diphenyl ether (PBDE) flame retardants. Human neurospheres mimic many of the biological basic processes of the developing human brain-like differentiation, proliferation, migration, and apoptosis, and their interruption by the various developmental neurotoxins will demonstrate DNT [70, 71]. Though, the neurospheres are the simplest 3D structures that can be easily used as 3D models, but they are faced by several limitations. They are not representative of the whole organisms and are metabolically restricted hence unsuitable for pharmacokinetic studies [6]. Higher cognitive functions, synaptic functionality, and network functions are missing. Neurospheres do not form the cortical layers which are present in the complex developing brain. Despite these limitations, neurospheres are an indispensable 3D in vitro tool because they can be easily grown in culture; as compared to in vivo studies, time taken is very less that is around 4 weeks for the assessment of various neuronal functions, and the self organizing nature of these is instrumental in paracrine functions. There is a complex interplay between cells which can influence each other and make the situation more realistic to that of cells in vivo. However, the quest to achieve better suitable stem cell-derived 3D model continues.
The establishment of in vitro 3D organogenesis nearly a decade ago laid the possibility that the generation of whole organs within a laboratory is not an unachievable process; however, it is not as easy as it seems to unravel the entire process of organogenesis in vitro. In the past decade, substantial progress has been made in the field of 3D tissue culture and artificial tissue engineering [72, 73]. However, these terms cannot be used interchangeably because tissue engineering differs from conventional 3D self-organizing culture in that cells are forcibly made to assume complex structures using artificial scaffolds. Self organizing tissues are far superior because they are governed by the cells’ own internal programs and do not require any artificial support system [72]. The superior quality of self-organizing 3D tissues is seen from the fact that when self-organized human retina was transplanted in SCID mouse testes, teratomas were not formed [74]. One of the effective strategies is to model the events of neurogenesis in laboratory [75].
Pluripotent embryonic stem cells (ESC) have been employed to give rise to a self-organizing cerebral cortical structure in vitro. The multilineage capacity of ESCs was exploited and ESC culture was induced to produce cortical neuroepithelial progenitors which further led to the formation of the various layers of the cerebral cortex in a sequential manner—firstly, layer I neurons then VI, V, IV, and II/III, respectively [76]. This was a classical case of neural self-forming tissue but the process was still not the exact mimic of in vivo development because the apical basal order of the layers was reversed. Further studies in the field have led to the entire recapitulation of the neural tube development in vitro using human embryonic and induced pluripotent stem cells [75]. The blood brain barrier (BBB) which is an integral part of the CNS and pivotal in its physiology and function has also been generated by combining different cell types such as the astrocytes, pericytes, neurons, and microglia in co-culture. Here, again, there is a need for humanized in vitro BBB models. However, an in vitro BBB model has been generated using the cells of human origin. The group of Hatherell et al. [77] has also generated a 3D BBB in vitro model using human brain vascular pericytes, cerebral microvascular endothelial cells, and human astrocytes. Another, such in vitro BBB model comprised a co-culture of the rat E-18 cortical cells along with rat brain endothelial cells (RBE4); this model formed an endothelial barrier, possessed tight junctions, and showed neuro-inflammatory responses [78]. 3D cell culture has not only progressed in the recent past but also significant achievements have been made. Neuroepithelial structures have been generated from embryonic stem cells as well as induced pluripotent stem cells. The formation of these structures closely mimics and recapitulates the events of embryonic development [79]. The cerebral cortex of human beings is considered one of the most complicated and complex structures in the course of evolution [80] and its entire recapitulation in vitro is still a challenging task. However, in the recent past, a successful “cerebral organoid culture” has been established by Knobliche and his group which clearly depicts not only the histological but also the developmental features of the brain as well. Eight to ten days later, neuronal differentiation was observed and 20–30 days later, embroyoid bodies grew into well compartmentalized 3D structures with specific layers of the brain. This model of the human brain is well characterized with the presence of structures like the forebrain, midbrain, hindbrain, hippocampus, retina, meninges, and choroid plexus [80]. Such in vitro 3D brain models provide better possibilities for all the developmental neurobiologists across the globe. Once a developing human brain model is established in a laboratory monitoring and analyzing, the various aspects of DNT will be greatly facilitated. With this, there will also be a possibility to carry out the phase-specific testing of compounds and chemicals. We will thus also be able to carry out path specific mechanistic studies as well as stage-specific inhibitions by the various neurotoxins. Few of the most representative studies carried out to derive the organoid structures from stem cells are listed in the Table 2.
Human Brain on Chip: a Step Forward Towards NT and DNT Studies
In recent times, the latest approach is to develop micro-physiological systems which combine various cell types in a specific 3D configuration to stimulate an organ’s biology and function, and this has been termed as “human on chip” models. These models are useful as they provide concise, compact, and reliable information regarding the response of a human system to various substances/chemicals and may serve as sophisticated novel in vitro tools. This project has been initiated by the National Institutes of Health, the US Food and Drug Administration and the Defense Advanced Research Project Agency (DARPA) of the USA. A stepwise strategy is being followed to achieve the same [81]. First, a primary culture of rat brain cells is being employed; they are aggregated to assume a 3D structure and this is being used to monitor the various DNT pathways. To further increase the relevance of the model, the cells of human origin particularly human iPSCs are being used. To create an aggregated human model, iPSC-derived neurons and astrocytes are being combined. However, the various differentiation protocols are still being standardized. The neurons and astrocytes thus obtained are brought in a 3D embryoid body state using special 3D suspension cultures and conditions. Generation of iPSCs is very taxing; however, the fact that iPSCs derived neuronal and glial development recapitulates the brain developmental stages in utero makes it a lucrative possibility [81].
A comparative study of the rodent and human data is essential to establish the species differences so as to ensure better success rates of drug development during clinical trial phases. The failure rate observed is approximately 95–97 % for substances during the clinical trial phase [78]. This is primarily due to the different effects observed in humans which remained unidentified in the preclinical animal tests. Using the conventional in vivo model systems one cannot take the epigenetic background into consideration nor can the effect of medication history on sensitivity be estimated. However, iPSCs derived from neurodevelopmental disorder patients can resolve this issue. In the above mentioned joint project, i.e., the National Institutes of Health, the US Food and Drug Administration and the Defense Advanced Research Project Agency (DARPA) of the USA, the disorders chosen were Rett syndrome, Down syndrome, and tuberous sclerosis. A comparative study will thus provide us with relevant information regarding the effect of disease-relevant mutations on various aspects of DNT [78, 81]. These integrated approaches are an effort to combine the various 2D as well as 3D cell culture approaches and provide compact humanized stem cell based in vitro models which shall provide rapid, reproducible, and reliable insights into the various aspects of NT and DNT.
Summary and Future Perspectives
It is clearly evident from the above discussion that stem cells are an indispensable tool for toxicology studies. In this review article, we have briefly summarized the application of stem cells specifically for the assessment of NT and DNT studies. We have provided a brief overview of the current prevalent trends in the field of in vitro toxicology with stem cells at its core. Stem cell based NT and DNT testing also has its limitations. For example, when two-dimensional in vitro studies are carried out, the complex environment of an organ is lacking. Cell cultures are isolated systems where there is no organ system and immunological system. The blood vasculature is lacking and the cellular interactions are minimal. Thus, these 2D in vitro models can be employed for the preliminary screening of chemicals and drugs; however, the data obtained may not be directly applicable to human beings. As we gradually move towards three-dimensional in vitro culture, we see better possibilities of alternate in vitro models of human origin. Once good NT and DNT models are established, the Herculean task of screening and establishing the role of various neurotoxins and developmental neurotoxins will be made achievable. In the future, we shall be in a better position to correlate the physiology of a toxicant affected organ with the phenotypic expression as well. The long-term goal will be to study not only organ-based toxicity but also the organ-organ interactions. Stem cell-based in vitro models which establish a direct association between DNT/NT and neurodegenerative diseases are currently lacking and can be developed any time soon. Disease correlation with stem cell-derived models for NT and DNT will help us delve deeper and further into the molecular and cellular insights. With this review, we wish to make a small but worthy contribution towards the prestigious archives. Hopefully, in the near future we shall come up with some novel innovative 3D human stem cell-based models and contribute generously towards the emerging field of neurotoxicity and developmental neurotoxicity. The prevention of NT and DNT is possible if suitable predictive tools are made available. Stem cells provide a good source of human material which can be employed for the development of such in vitro models where extrapolation of data to human beings would be directly possible. The prevention and cure of neurotoxicity and developmental neurotoxicity will ensure a healthier and longer lived race of Homo sapiens in times to come.
References
Bosnjak ZJ (2012) Developmental neurotoxicity screening using human embryonic stem cells. Exp Neurol 237(1):207–210
Costa LG, Giordano G, Guizzetti M, Vitalone A (2007) Neurotoxicity of pesticides: a brief review. Front Biosci 13:1240–1249
Coecke S, Goldberg AM, Allen S, Buzanska L, Calamandrei G, Crofton K, Hareng L, Hartung T, Knaut H, Honegger P (2007) Workgroup report: incorporating in vitro alternative methods for developmental neurotoxicity into international hazard and risk assessment strategies. Environ Health Perspect 115(6):924–931
Kang K-S, Trosko JE (2011) Stem cells in toxicology: fundamental biology and practical considerations. Toxicol Sci 120(suppl 1):S269–S289
Laustriat D, Gide J, Peschanski M (2010) Human pluripotent stem cells in drug discovery and predictive toxicology. Biochem Soc Trans 38(4):1051
Betts KS (2010) Growing knowledge: using stem cells to study developmental neurotoxicity. Environ Health Perspect 118(10):A432
Szebényi K, Erdei Z, Péntek A, Sebe A, Orbán TI, Sarkadi B, Apáti Á (2011) Human pluripotent stem cells in pharmacological and toxicological screening: new perspectives for personalized medicine. Pers Med 8(3):347–364
Sison-Young R, Kia R, Heslop J, Kelly L, Rowe C, Cross M, Kitteringham N, Hanley N, Park B, Goldring C (2011) Human pluripotent stem cells for modeling toxicity. Adv Pharmacol (San Diego, Calif) 63:207–256
Stiles J, Jernigan TL (2010) The basics of brain development. Neuropsychol Rev 20(4):327–348
Silver J, Schwab ME, Popovich PG (2014) Central nervous system regenerative failure: role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb Perspect Biol 7(3):a020602. doi:10.1101/cshperspect.a020602
Godoy P, Hewitt NJ, Albrecht U, Andersen ME, Ansari N, Bhattacharya S, Bode JG, Bolleyn J, Borner C, Böttger J (2013) Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol 87(8):1315–1530
Lu J, Einhorn S, Venkatarangan L, Miller M, Mann DA, Watkins PB, LeCluyse E (2015) Morphological and functional characterization and assessment of iPSC-derived hepatocytes for in vitro toxicity testing. Toxicol Sci 147(1):39–54
Kia R, Sison RL, Heslop J, Kitteringham NR, Hanley N, Mills JS, Park BK, Goldring CE (2013) Stem cell‐derived hepatocytes as a predictive model for drug‐induced liver injury: are we there yet? Br J Clin Pharmacol 75(4):885–896
McGivern JV, Ebert AD (2014) Exploiting pluripotent stem cell technology for drug discovery, screening, safety, and toxicology assessments. Adv Drug Deliv Rev 69:170–178
Mordwinkin NM, Burridge PW, Wu JC (2013) A review of human pluripotent stem cell-derived cardiomyocytes for high-throughput drug discovery, cardiotoxicity screening, and publication standards. J Cardiovasc Transl Res 6(1):22–30
Sinnecker D, Laugwitz K-L, Moretti A (2014) Induced pluripotent stem cell-derived cardiomyocytes for drug development and toxicity testing. Pharmacol Ther 143(2):246–252
Clements M, Millar V, Williams A, Kalinka S (2015) Bridging functional and structural cardiotoxicity assays using human embryonic stem-cell derived cardiomyocytes for a more comprehensive risk assessment. Toxicol Sci 148(1):241–260. doi:10.1093/toxsci/kfv180
Khan JM, Lyon AR, Harding SE (2013) The case for induced pluripotent stem cell‐derived cardiomyocytes in pharmacological screening. Br J Pharmacol 169(2):304–317
Ek CJ, Dziegielewska KM, Habgood MD, Saunders NR (2012) Barriers in the developing brain and neurotoxicology. Neurotoxicology 33(3):586–604
Van Thriel C, Westerink RH, Beste C, Bale AS, Lein PJ, Leist M (2012) Translating neurobehavioural endpoints of developmental neurotoxicity tests into < i > in vitro</i > assays and readouts. Neurotoxicology 33(4):911–924
Kumar V, Jahan S, Singh S, Khanna V, Pant A (2015) Progress toward the development of in vitro model system for chemical-induced developmental neurotoxicity: potential applicability of stem cells. Arch Toxicol 89(2):265–267
De Groot MW, Westerink RH, Dingemans MM (2013) Don’t judge a neuron only by its cover: neuronal function in in vitro developmental neurotoxicity testing. Toxicol Sci 132(1):1–7
Zimmer B, Kuegler P, Baudis B, Genewsky A, Tanavde V, Koh W, Tan B, Waldmann T, Kadereit S, Leist M (2010) Coordinated waves of gene expression during neuronal differentiation of embryonic stem cells as basis for novel approaches to developmental neurotoxicity testing. Cell Death Differ 18(3):383–395
Lee H-y, Inselman AL, Kanungo J, Hansen DK (2012) Alternative models in developmental toxicology. Syst Biol Reprod Med 58(1):10–22
Liu W, Deng Y, Liu Y, Gong W, Deng W (2013) Stem cell models for drug discovery and toxicology studies. J Biochem Mol Toxicol 27(1):17–27
Cananzi M, De Coppi P (2012) CD117+ amniotic fluid stem cells: state of the art and future perspectives. Organogenesis 8(3):77–88
Nam H, Lee K-H, Nam D-H, Joo KM (2015) Adult human neural stem cell therapeutics: current developmental status and prospect. World J Stem Cells 7(1):126–136
Gorba T, Conti L (2013) Neural stem cells as tools for drug discovery: novel platforms and approaches. Expert Opin Drug Discovery 8(9):1083–1094
Bellenchi GC, Volpicelli F, Piscopo V, Perrone‐Capano C, di Porzio U (2013) Adult neural stem cells: an endogenous tool to repair brain injury? J Neurochem 124(2):159–167
Canovas-Jorda D, Louisse J, Pistollato F, Zagoura D, Bremer S (2014) Regenerative toxicology: the role of stem cells in the development of chronic toxicities. Expert Opin Drug Metab Toxicol 10(1):39–50
Hazeltine LB, Selekman JA, Palecek SP (2013) Engineering the human pluripotent stem cell microenvironment to direct cell fate. Biotechnol Adv 31(7):1002–1019
Lu D, Chen EY, Lee P, Wang Y-C, Ching W, Markey C, Gulstrom C, Chen L-C, Nguyen T, Chin W-C (2014) Accelerated neuronal differentiation toward motor neuron lineage from human embryonic stem cell line (H9). Tissue engineering part C: methods
Thompson LH, Björklund A (2015) Reconstruction of brain circuitry by neural transplants generated from pluripotent stem cells. Neurobiol Dis 79:28–40. doi:10.1016/j.nbd.2015.04.003
Okolicsanyi RK, Griffiths LR, Haupt LM (2014) Mesenchymal stem cells, neural lineage potential, heparan sulfate proteoglycans and the matrix. Dev Biol 388(1):1–10
Ferroni L, Gardin C, Tocco I, Epis R, Casadei A, Vindigni V, Mucci G, Zavan B (2013) Potential for neural differentiation of mesenchymal stem cells. In: Mesenchymal stem cells-basics and clinical application I. Springer, Berlin Heidelberg, pp 89–115
Nikoletopoulou V, Tavernarakis N (2012) Embryonic and induced pluripotent stem cell differentiation as a tool in neurobiology. Biotechnol J 7(9):1156–1168
Prè D, Nestor MW, Sproul AA, Jacob S, Koppensteiner P, Chinchalongporn V, Zimmer M, Yamamoto A, Noggle SA, Arancio O (2014) A time course analysis of the electrophysiological properties of neurons differentiated from human induced pluripotent stem cells (iPSCs). PLoS One 9(7):e103418
Kashyap MP, Kumar V, Singh AK, Tripathi VK, Jahan S, Pandey A, Srivastava RK, Khanna VK, Pant AB (2014) Differentiating neurons derived from human umbilical cord blood stem cells work as a test system for developmental neurotoxicity. Mol Neurobiol. 1–17
Kashyap M, Singh A, Siddiqui M, Kumar V, Tripathi V, Khanna V, Yadav S, Jain S, Pant A (2010) Caspase cascade regulated mitochondria mediated apoptosis in monocrotophos exposed PC12 cells. Chem Res Toxicol 23(11):1663–1672
Kashyap MP, Singh AK, Kumar V, Tripathi VK, Srivastava RK, Agrawal M, Khanna VK, Yadav S, Jain SK, Pant AB (2011) Monocrotophos induced apoptosis in PC12 cells: role of xenobiotic metabolizing cytochrome P450s. PLoS One 6(3):e17757
Singh A, Kashyap M, Jahan S, Kumar V, Tripathi V, Siddiqui M, Yadav S, Khanna V, Jain S, Das V (2012) Expression and inducibility of cytochrome P450s (CYP1A1, 2B6, 2E1, 3A4) in human cord blood CD34+ stem cell derived differentiating neuronal cells. Toxicol Sci 129(2):392–410. doi:10.1093/toxsci/kfs213
Singh AK, Kashyap MP, Kumar V, Tripathi VK, Yadav DK, Khan F, Jahan S, Khanna VK, Yadav S, Pant AB (2013) 3-Methylcholanthrene induces neurotoxicity in developing neurons derived from human CD34+ Thy1+ stem cells by activation of aryl hydrocarbon receptor. Neuromolecular Med 15(3):570–592
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676
Alvarado AS, Yamanaka S (2014) Rethinking differentiation: stem cells, regeneration, and plasticity. Cell 157(1):110–119
Okano H, Yamanaka S (2014) iPS cell technologies: significance and applications to CNS regeneration and disease. Mol Brain 7(1):22
Pei Y, Peng J, Behl M, Sipes NS, Shockley KR, Rao MS, Tice RR, Zeng X (2015) Comparative neurotoxicity screening in human iPSC-derived neural stem cells, neurons and astrocytes. Brain Res. doi: 10.1016/j.brainres.2015.07.048
Zhao J, W-j J, Sun C, C-z H, Yang X-m, Gao J-g (2013) Induced pluripotent stem cells: origins, applications, and future perspectives. J Zhejiang Univ Sci B 14(12):1059–1069
Yamanaka S (2009) A fresh look at iPS cells. Cell 137(1):13–17
Takahashi K, Yamanaka S (2013) Induced pluripotent stem cells in medicine and biology. Development 140(12):2457–2461
Khurana V, Tardiff DF, Chung CY, Lindquist S (2015) Toward stem cell-based phenotypic screens for neurodegenerative diseases. Nat Rev Neurol 11(6):339–50. doi:10.1038/nrneurol.2015.79
Sterneckert JL, Reinhardt P, Schöler HR (2014) Investigating human disease using stem cell models. Nat Rev Genet 15(9):625–639
Scott CW, Peters MF, Dragan YP (2013) Human induced pluripotent stem cells and their use in drug discovery for toxicity testing. Toxicol Lett 219(1):49–58
Kumar KK, Aboud AA, Bowman AB (2012) The potential of induced pluripotent stem cells as a translational model for neurotoxicological risk. Neurotoxicology 33(3):518–529
Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M (2010) Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463(7284):1035–1041
Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G (2011) Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 476(7359):224–227
Qiang L, Fujita R, Yamashita T, Angulo S, Rhinn H, Rhee D, Doege C, Chau L, Vanti WB, Moreno H (2011) Directed conversion of Alzheimer’s disease patient skin fibroblasts into functional neurons. Cell 146(3):359
Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, Eggan K (2011) Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell 9(3):205–218
Shtrichman R, Germanguz I, Eldor JI (2013) Induced pluripotent stem cells (iPSCs) derived from different cell sources and their potential for regenerative and personalized medicine. Curr Mol Med 13(5):792–805
Shamir ER, Ewald AJ (2014) Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat Rev Mol Cell Biol 15(10):647–664
Li X, Valadez AV, Zuo P, Nie Z (2012) Microfluidic 3D cell culture: potential application for tissue-based bioassays. Bioanalysis 4(12):1509–1525
Yamada KM, Cukierman E (2007) Modeling tissue morphogenesis and cancer in 3D. Cell 130(4):601–610
Bratt‐Leal AM, Carpenedo RL, McDevitt TC (2009) Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation. Biotechnol Prog 25(1):43–51
Kopanitsa MV, Afinowi NO, Grant SG (2006) Recording long-term potentiation of synaptic transmission by three-dimensional multi-electrode arrays. BMC Neurosci 7(1):61
Musick K, Khatami D, Wheeler BC (2009) Three-dimensional micro-electrode array for recording dissociated neuronal cultures. Lab Chip 9(14):2036–2042
Wallace K, Strickland JD, Valdivia P, Mundy WR, Shafer TJ (2015) A multiplexed assay for determination of neurotoxicant effects on spontaneous network activity and viability from microelectrode arrays. Neurotoxicology 49:79–85. doi:10.1016/j.neuro.2015.05.007
Valdivia P, Martin M, LeFew WR, Ross J, Houck KA, Shafer TJ (2014) Multi-well microelectrode array recordings detect neuroactivity of ToxCast compounds. Neurotoxicology 44:204–217
Robinette BL, Harrill JA, Mundy WR, Shafer TJ (2011) In vitro assessment of developmental neurotoxicity: use of microelectrode arrays to measure functional changes in neuronal network ontogeny. Front Neuroeng 4:1. doi:10.3389/fneng.2011.00001
Frimat J-P, Sisnaiske J, Subbiah S, Menne H, Godoy P, Lampen P, Leist M, Franzke J, Hengstler JG, van Thriel C (2010) The network formation assay: a spatially standardized neurite outgrowth analytical display for neurotoxicity screening. Lab Chip 10(6):701–709
Moors M, Rockel TD, Abel J, Cline JE, Gassmann K, Schreiber T, Schuwald J, Weinmann N, Fritsche E (2009) Human neurospheres as three-dimensional cellular systems for developmental neurotoxicity testing. Environ Health Perspect 117(7):1131–1138
Terrasso AP, Pinto C, Serra M, Filipe A, Almeida S, Ferreira AL, Pedroso P, Brito C, Alves PM (2015) Novel scalable 3D cell based model for in vitro neurotoxicity testing: combining human differentiated neurospheres with gene expression and functional endpoints. J Biotechnol 205:82–92
Baumann J, Gassmann K, Masjosthusmann S, DeBoer D, Bendt F, Giersiefer S, Fritsche E (2015) Comparative human and rat neurospheres reveal species differences in chemical effects on neurodevelopmental key events. Arch Toxicol. 1–13
Sasai Y (2013) Next-generation regenerative medicine: organogenesis from stem cells in 3D culture. Cell Stem Cell 12(5):520–530
Bershteyn M, Kriegstein AR (2013) Cerebral organoids in a dish: progress and prospects. Cell 155(1):19–20
Eiraku M, Sasai Y (2012) Mouse embryonic stem cell culture for generation of three-dimensional retinal and cortical tissues. Nat Protoc 7(1):69–79
Broccoli V, Giannelli SG, Mazzara PG (2014) Modeling physiological and pathological human neurogenesis in the dish. Front Neurosci 8:183. doi:10.3389/fnins.2014.00183
Eiraku M, Sasai Y (2012) Self-formation of layered neural structures in three-dimensional culture of ES cells. Curr Opin Neurobiol 22(5):768–777
Hatherell K, Couraud P-O, Romero IA, Weksler B, Pilkington GJ (2011) Development of a three-dimensional, all-human in vitro model of the blood–brain barrier using mono-, co-, and tri-cultivation Transwell models. J Neurosci Methods 199(2):223–229
Pamies D, Hartung T, Hogberg HT (2014) Biological and medical applications of a brain-on-a-chip. Exp Biol Med 239(9):1096–1107. doi:10.1177/1535370214537738
Lancaster MA, Renner M, Martin C-A, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA (2013) Cerebral organoids model human brain development and microcephaly. Nature 501(7467):373–379
Gjorevski N, Ranga A, Lutolf MP (2014) Bioengineering approaches to guide stem cell-based organogenesis. Development 141(9):1794–1804
Hogberg HT, Bressler J, Christian KM, Harris G, Makri G, O’Driscoll C, Pamies D, Smirnova L, Wen Z, Hartung T (2013) Toward a 3D model of human brain development for studying gene/environment interactions. Studies 13:15
Wheeler HE, Wing C, Delaney SM, Komatsu M, Dolan ME (2015) Modeling chemotherapeutic neurotoxicity with human induced pluripotent stem cell-derived neuronal cells. PLoS One 10(2):e0118020
Hubbard K, Beske P, Lyman M, McNutt P (2015) Functional evaluation of biological neurotoxins in networked cultures of stem cell-derived central nervous system neurons. J Vis Exp 96.doi:10.3791/52361
Cao WS, Livesey JC, Halliwell RF (2015) An evaluation of a human stem cell line to identify risk of developmental neurotoxicity with antiepileptic drugs. Toxicol In Vitro 29(3):592–599
Bai X, Bosnjak ZJ (2013) Emerging model in anesthetic developmental neurotoxicity: human stem cells. Int J Clin Anesthesiol 1:1002
Chang S-H, Lee HJ, Kang B, Yu K-N, Minai-Tehrani A, Lee S, Kim SU, Cho M-H (2013) Methylmercury induces caspase-dependent apoptosis and autophagy in human neural stem cells. J Toxicol Sci 38(6):823–831
Meamar R, Dehghani L, Karamali F (2012) Toxicity effects of methamphetamine on embryonic stem cell-derived neuron. J Res Med Sci 17(5):470
Li T, Wang W, Pan Y-W, Xu L, Xia Z (2013) A hydroxylated metabolite of flame-retardant PBDE-47 decreases the survival, proliferation, and neuronal differentiation of primary cultured adult neural stem cells and interferes with signaling of ERK5 MAP kinase and neurotrophin 3. Toxicol Sci 134(1):111–124. doi:10.1093/toxsci/kft083
Mutsaers HA, Tofighi R (2012) Dexamethasone enhances oxidative stress-induced cell death in murine neural stem cells. Neurotox Res 22(2):127–137
Rocha R, Gimeno-Alcaniz J, Martin-Ibanez R, Canals J, Velez D, Devesa V (2011) Arsenic and fluoride induce neural progenitor cell apoptosis. Toxicol Lett 203(3):237–244
Dye BR, Hill DR, Ferguson MA, Tsai Y-H, Nagy MS, Dyal R, Wells JM, Mayhew CN, Nattiv R, Klein OD (2015) In vitro generation of human pluripotent stem cell derived lung organoids. eLife 4:e05098
Xia Y, Sancho-Martinez I, Nivet E, Esteban CR, Campistol JM, Belmonte JCI (2014) The generation of kidney organoids by differentiation of human pluripotent cells to ureteric bud progenitor–like cells. Nat Protoc 9(11):2693–2704
Lancaster MA, Knoblich JA (2014) Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc 9(10):2329–2340
Greggio C, De Franceschi F, Figueiredo-Larsen M, Grapin-Botton A (2014) In vitro pancreas organogenesis from dispersed mouse embryonic progenitors. J Vis Exp 89:e51725–e51725
Koehler KR, Hashino E (2014) 3D mouse embryonic stem cell culture for generating inner ear organoids. Nat Protoc 9(6):1229–1244
Tieng V, Stoppini L, Villy S, Fathi M, Dubois-Dauphin M, Krause K-H (2014) Engineering of midbrain organoids containing long-lived dopaminergic neurons. Stem Cells Dev 23(13):1535–1547
Takebe T, Sekine K, Suzuki Y, Enomura M, Tanaka S, Ueno Y, Zheng Y-W, Taniguchi H (2012) Self-organization of human hepatic organoid by recapitulating organogenesis in vitro. Transplant Proc 4(4):1018–1020, Elsevier
Acknowledgements
The authors are grateful to the director of the CSIR-IITR, Lucknow, India, for his interest. Financial support from the Department of Science and Technology, Ministry of Science & Technology, Government of India, New Delhi, India (Grant No. SR/SO/Z 36/2007/91/10); Department of Biotechnology, Ministry of Science & Technology, Government of India, New Delhi, India (Grant No. 102/IFD/SAN/3533/2014-15); and Council of Scientific & Industrial Research, Government of India, New Delhi, India (Grant No. BSC0111/INDEPTH/ CSIR Network Project) is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Singh, S., Srivastava, A., Kumar, V. et al. Stem Cells in Neurotoxicology/Developmental Neurotoxicology: Current Scenario and Future Prospects. Mol Neurobiol 53, 6938–6949 (2016). https://doi.org/10.1007/s12035-015-9615-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9615-2