Abstract
Acetyl-11-keto-β-boswellic acid (AKBA), a main active constituent from Boswellia serrata resin, is a novel candidate for therapy of cerebral ischemia-reperfusion (I/R) injury. Nevertheless, its poor solubility in aqueous solvent, bioavailability, and rapid clearance limit its curative efficacy. To enhance its potency, in our study, AKBA-loaded o-carboxymethyl chitosan nanoparticle (AKBA-NP) delivery system was synthesized. The transmission electron microscopy and transmission electron microscope images of AKBA-NPs suggested that particle size was 132 ± 18 nm, and particles were spherical in shape with smooth morphology. In pharmacokinetics study, AKBA-NPs apparently increases the area under the curve of plasma concentration-time and prolonged half-life compared with AKBA. The tissue distribution study confirmed that AKBA-NPs had a better brain delivery efficacy in comparison with AKBA. The results from our pharmacodynamic studies showed that AKBA-NPs possess better neuroprotection compared with AKBA in primary neurons with oxygen-glucose deprivation (OGD) model and in animals with middle cerebral artery occlusion (MCAO) model. Additionally, AKBA-NPs modulate antioxidant and anti-inflammatory pathways more effectively than AKBA by increasing nuclear erythroid 2-related factor 2 and heme oxygenase-1 expression, and by decreasing nuclear factor-kappa B and 5-lipoxygenase expression. Collectively, our results suggest that AKBA-NPs serve as a potent delivery vehicle for AKBA in cerebral ischemic therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke, as major cause of death, still leads to severe neurologic disability around the world [1]. Restoration of an adequate blood flow is critical to survival. However, even after the restoration of blood flow, reperfusion causes tissue damage through mechanisms including excitotoxicity, oxidative stress, inflammation, and apoptosis [2]. Although much progress has been made toward treatment of stroke, there is deficiency of strategies to effectively reduce brain damage in clinical [3]. Therefore, the development of novel treatment methods is an urgent need to enhance the prognosis of cerebral ischemia-reperfusion (I/R) injury.
Boswellia serrata (commonly known as “Frankincense” or “Olibanum”) resin extracts have been widely used as an antioxidant and anti-inflammatory agent [4, 5]. They have been studied or used for a variety of diseases including cancer, analgesia, asthma inflammation, arthritis, and colitis [6]. Clinically, B. serrata resin extracts can significantly reduce cerebral edema in patients [7], showing promise as a new agent for cardiovascular diseases. Furthermore, the European Medicines Agency granted extracts of B. serrata gum resin as the orphan drug to treat brain edema [8]. Acetyl 11-keto-β-boswellic acid (AKBA), one of pentacyclic triterpenoids, is the main biologically active principle isolated from resin of B. serrata. A recent study in our laboratory showed that AKBA ameliorates cerebral I/R injury by the nuclear factor erythroid-2-related factor 2 (Nrf2) signal pathway [9]. Among other studies, both of inhibition of 5-lipoxygenase (5-LOX) [10] and the suppression of the nuclear factor kappa-beta (NF-κB) pathway [11] is considered as the main mechanism underlying anti-inflammatory effects of AKBA during different conditions.
However, delivery of AKBA is problematic because of its high lipophilicity [12, 13]. Its poor solubility in water and rapid systemic elimination severely curtails its bioavailability [12, 13]. These characteristics of AKBA lead to issues when making its injection, which could be employed for first aid clinically. Thus, it is necessary to develop a new delivery system for AKBA to treat stroke. Chitosan and its derivatives have attracted numerous interests due to their biocompatibility, biodegradability, and inexpensiveness. Interestingly, chitosan, which is produced mainly from the exoskeleton of crustaceans, has proven neuroprotective effects on membrane sealing [14]. Primary amines in chitosan could also play an essential role protective effect during stroke [15]. Moreover, the chitosan-based nanoparticles have been already exploited as delivery system for brain targeting [16–18]. They can open tight junctions between intestinal epithelial cell and facilitate paracellular transport of drugs transiently [19]. Due to poor solubility of chitosan in an aqueous solution with neutral pH, we applied O-carboxymethyl chitosan (O-CMC), a water-soluble chitosan derivative, which has favorable biocompatibility like chitosan [20, 21].
The purpose of this study was to produce an injectable delivery system for AKBA via a very simple and easy way. In this study, we prepared AKBA-loaded O-CMC nanoparticles (AKBA-NPs) by emulsification and solvent evaporation method. Our study further determined the in vitro and in vivo protective effects of AKBA-NPs on oxygen glucose deprivation (OGD) model and the middle cerebral artery occlusion (MCAO) model, respectively. To elucidate the possible mechanisms of AKBA-NPs in ameliorating cerebral I/R injury, we evaluated the levels of Nrf2, HO-1, NF-κB, and 5-LOX proteins by Western blot analysis.
Materials and Methods
Materials and Animals
All chemicals were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA) unless noted. All other chemicals and reagents used in this study were of analytical grade. Adult male Sprague-Dawley (SD) rats (210 ± 18 g) were supplied by the Experimental Animal Center of Fourth Military Medical University (Xi’an, China). The animals were housed on 12-h dark-light cycle, at 25 ± 2 °C and a humidity of 75 ± 4 % free access to food and water before the experiment. Experimental protocols strictly complied with the National Institutes of Health guidelines. The research was approved by the Ethics Committee for Animal Experimentation of the Fourth Military Medical University.
Synthesis of AKBA-NPs
O-Carboxymethyl chitosan (12 kDa, degree of deacetylation 61.8 % and degree of substitution 0.54) was purchased from Koyo Chemical Co. Ltd., Japan. The methodology of AKBA-NP synthesis was based on reported methods [22] with slight modification and was carried out to increase the redispersibility of the NPs. For the AKBA-NPs, methanolic AKBA solution was incubated with O-CMC solution in water for overnight followed by TPP cross-linking (1 %) for a volume ratio of 25:1(O-CMC/TPP), followed by incubating with 0.1 ml of 1 % BSA solution for 30 min. The drug-loaded nanoparticles of AKBA-NPs were separated. Pellets were collected and used for further characterization and studies.
Characterization of AKBA-NPs
The AKBA-NPs were dispersed in distilled water at appropriate concentrations. The mean particle size, size distribution, and zeta potential were measured using the DLS Delsa Nano particle analyzer (Beckman Coulter, Inc., CA, USA). The shape and surface morphology of AKBA-NPs were observed using scanning electron microscope (SEM) (Hitachi S-3400 N, Tokyo, Japan) at an accelerating voltage of 5 kV. The measurements were repeated three times.
The entrapment efficiency (EE) and loading efficiency (LE) of AKBA-NP sample was quantified by HPLC method. The protocol involves the complete extraction of AKBA from the nanoparticle pellet through methanol/ethanol, followed by spectrophotometric quantification. The EE and LE were calculated with respect to the amount of entrapped drug and the nanoparticle yield. EE was calculated as the percentage of AKBA recovered from AKBA-NPs in comparison with the initial drug amount. LE was defined as the amount of entrapped AKBA compared to the total AKBA-NPs.
The in vitro release of AKBA from AKBA-NPs was performed using dialysis sacs. The AKBA-NPs were placed in pretreated dialysis sacs which were immersed into 100 ml of phosphate buffer solution (pH 7.4). They were under mechanical agitation at 37 °C. At regular intervals, medium was withdrawn and maintained by the same medium. The supernatant was assessed for AKBA with HPLC method previously described [23]. All evaluations were taken thrice.
Pharmacokinetics and Tissue Distribution
For in vivo pharmacokinetic experiment, animals were divided into two groups (n = 8). The native AKBA (10 mg/kg, the dose was determined based on our previous study [9]) dissolved in CMC-Na solution (0.5 %, w/v) and AKBA-NPs (containing AKBA 10 mg/kg) dispersed in saline solution were administered intravenously. Blood sample (0.5 ml) was collected via caudal vein at predetermined time point. Then, they were centrifuged at 3000 × g for 12 min.
In tissue distribution study, animals were sacrificed at 2 and 6 h after administration of drug. Then, the brain, heart, liver, spleen, and kidney were excised. The organs were quickly washed with cold saline. All of samples were stored at −20 °C until analysis.
All samples were prepared and subjected to HPLC/MS-MS analysis as previously described [23]. AKBA was determined with Agilent 1100 liquid chromatographic system equipped with a reverse-phase column (Dikma, Beijing, China). Pharmacokinetic parameters were calculated with the noncompartment model by WinNonlin 6.2 (Pharsight Corporation, Mountain view, CA, USA). The area under the plasma concentration-time curve (AUC) was calculated using the trapezoidal rule extrapolated to infinity. The terminal elimination half-life (t1/2), the systemic clearance (Cl), mean residence time (MRT), and volume of distribution at steady state (Vss) was obtained.
OGD Model
To test the neuroprotective function of AKBA-NPs against ischemic injury in vitro, the OGD model was utilized in our study as previously described [9]. Cortical neurons were prepared with brains from 1-day-old rats. About 30,000 cells in 50-ml neurobasal medium were seeded into six-well plates. After 2 h, 0.5-ml neurobasal medium was added to each well. After 2 days in vitro, 5 μM cytosine arabinofuranoside was added to inhibit glial proliferation as previously described [24]. Neurons were cultured at 37 °C in a humidified 5 % CO2 atmosphere.
To model ischemia-like conditions in vitro, primary cultured cortical neurons were subjected to transient OGD for 60 min. Then, the neurons were incubated again in the incubator with 95 % air and 5 % CO2 with different treatments for 24 h. We designed several groups: control (no OGD), OGD, OGD + void NPs, OGD + AKBA (50 μM dissolved in DMSO, the dose was determined based on our previous study [9]) treatment and OGD + AKBA-NP (10 mg/ml) treatment. Concentration of DMSO in the medium was kept <0.1 % w/v. Medium-treated cells were used as controls.
Then, for each group the cell viability was determined using the MTT assay as previously described [25]. Lactate dehydrogenase (LDH) release was assessed by a commercially available kit (Jiancheng Biological Engineering Institute, Nanjing, China). Data was expressed as the percentage of control levels.
MCAO Model
In chloral hydrate anesthesia, cerebral ischemia was performed on rats by right MCAO with an intraluminal filament as described in our previous study [26]. At 1 h after the induction of ischemia, restoration of MCA blood flow was achieved by withdrawing, and the neck incision was closed. The rats were allowed to survive for 48 h with free access to water and food. The sham control rats received the same surgery procedures, but the MCA was not occluded.
In this study, rats were divided into five groups by random assignment (in a random number table generated by SPSS). (1) sham group; (2) vehicle group, treated with saline solution; (3) NPs group, void NP-treated group (10 mg/kg), treated with received nanoparticles without AKBA (10 mg/kg); (4) AKBA group, treated with native AKBA (10 mg/kg) solution; and (5) AKBA-NPs group, treated with AKBA-NPs (containing AKBA 10 mg/kg) dispersed in saline solution. Vehicle controls, NPs, AKBA, and AKBA-NPs were administered via the intravenous administration at 1 h after reperfusion.
Evaluation of Infarct Volume and Neurological Deficits
At 48 h after reperfusion, the neurologic deficits of animals in each group (n = 8) were determined according to Longa’s five score system [27, 26] as follows: 0, no neurological deficit; 1, failure to extend left forepaw fully; 2, turning to the ipsilateral side when held by the tail; 3, falling to the left; and 4, not being able to walk and no spontaneous locomotor activity.
To calculate infarct volume at 48 h after reperfusion, the animals were euthanized in anesthesia. The brain was quickly removed and cut into 2-mm-thick coronal sections. Slices were subjected to 2,3,5-triphenyltetrazolium chloride (TTC) staining. Unstained areas were defined as infarcts and were measured using image analysis software. The percent of the infarct volume was calculated via the method as the following formula: ([total contralateral hemispheric volume] − [total ipsilateral hemispheric stained volume]) / (total contralateral hemispheric volume) × 100 %.
TUNEL Staining
TUNEL assay was performed on paraffin-embedded sections. Commercially available reagents (Promega, DeadEnd Flurometric Tunel System) were used to conduct TUNEL analysis. An investigator being blinded to the studies counted in five distinct fields for every section the amount of positive cells with light microscopy. The percent was computed as the following formula: (number of apoptotic cells / total number counted) × 100 %.
Estimation of Biochemical Parameters
Rats in each group (n = 8) were sacrificed at 48 h after MCAO. The right cerebral cortex tissue was homogenized in 2 ml of 10 mM phosphate buffer (pH 7.4). Superoxide dismutase (SOD) activity and glutathione peroxidase (GSH-Px) activity are essential indexes for assessing antioxidative effects. Levels of SOD and GSH-Px were quantified with test kit following the manufacturer’s directions (Jiancheng Biological Engineering Institute, Nanjing, China). Meanwhile, the levels of inflammatory cytokines, such as tumor necrosis factor-a (TNF-α) and interleukin-1β (IL-1β) were quantified with ELISA kits (Jiancheng Biological Engineering Institute, Nanjing, China) according to the manufacturer’s directions.
Western Blotting
Western blot analysis was done to examine the molecular mechanism by which the AKBA and AKBA-NPs protect against cerebral I/R injury. Right cortical samples were weighed. The nuclear protein was extracted using a Neuclear-Cytosol Extraction Kit (Applygen Technologies Inc., Beijing), and total protein in extraction was obtained using Total Protein Extraction Kit (Applygen Technologies Inc., Beijing). Equal amounts of total protein extracts or nuclear protein extracts were separated by SDS-PAGE and transferred onto polyvinylidenedifluoride membranes (Millipore, Bedford, MA, USA) by electrophoresis. Membranes were blocked with 5 % nonfat milk in TBST (0.1 % Tween 20 in TBS) for 1 h. Then, the membrane was incubated with antibodies against Nrf2 (catalogue number sc-722), HO-1 (catalogue number sc-7696), NF-κB p65 (catalogue number sc-372), 5-LOX (catalogue number sc-722), histone H3 (catalogue number sc-10809), and β-actin (catalogue number sc-130657) in TTBS overnight at 4 °C. All antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Expressions of protein were detected by Western blotting reagent. The relative band densities were quantified by densitometry.
Statistical Analysis
The statistical analyses were conducted using the SPSS 19.0 software (IBM SPSS, Chicago, IL). Data were expressed as means ± standard deviation (SD), except for the neurological deficit score. One-way ANOVA followed by a post hoc Turkey’s test was used for determining the statistical differences between groups. Neurological deficit scores were expressed as the median with range. This data was analyzed by the Kruskal–Wallis test followed by the Mann–Whitney test and Bonferroni post hoc correction. A P value of <0.05 was considered significant.
Results
Characterization of AKBA-NPs
To confirm the solubility of AKBA-NPs, we found that nanoparticle AKBA dissolved in aqueous solution gave a clear, dispersed formulation, while native AKBA is poorly soluble in aqueous media (Fig. 1a). SEM images showed that the prepared NPs have a smooth surface with spherical morphology (Fig. 1b). The particle data of AKBA-NPs showed a size of 132 ± 18 nm (Fig. 1c), polydispersity indexes of 0.112 ± 0.094, and zeta potentials of 24.5 ± 3.1 mV, which was benefit for crossing the blood–brain barrier. In addition, AKBA-NPs showed the drug entrapment efficiency of 87.5 ± 6.5 % with the drug loading 32.5 ± 5.0 % (Fig. 1d).
The in vitro release profile of AKBA-NPs was shown in Fig. 1d. The AKBA-NPs showed a burst drug release (60 %) within the initial 6 h in solution. Subsequently, the release rate gradually slowed, and the cumulative release of drug was over 70 % within 96 h. The observed initial burst release might be due to the dissociation of surface absorbed drugs present in the polymeric matrix. Subsequently, sustained release activity of the drugs was due to the slow release of drugs entrapped inside the polymer matrix [24].
Pharmacokinetics and Tissue Distribution
Plasma concentration-time profiles after a single dose intravenous administration of AKBA or AKBA-NPs are shown in Fig. 2a. The pharmacokinetic parameters of AKBA-NPs and AKBA are shown in Table 1. Plasma AUC of AKBA-NPs (373.6 ± 65.9 mg min/ml) was 3.7-fold higher than that of the native AKBA (100.2 ± 21.4 mg min/ml); the t1/2 (274.3 ± 68.9), MRTinf (373.6 ± 65.9), and Vss (291.7 ± 58.2) of AKBA in AKBA-NPs were increased by 5.7-, 1.65-, and 3.5-fold, while Cl of AKBA-NPs was decreased from 99.8 ± 11.0 to 26.8 ± 3.2 ml/min/kg compared with the native AKBA. It can be concluded that the slow release of AKBA from nanoparticle system increased the AUC of AKBA and improves circulatory half-life (t1/2). After 24 h, AKBA-NPs could still be detected in the plasma, while AKBA had almost vanished after 12 h from circulation system.
The differences in the tissues distribution between AKBA-NPs and AKBA at 2 and 6 h after injection are presented in Fig. 2b, c. AKBA-NPs were mainly distributed to the blood and liver. Nevertheless, native AKBA was concentrated to the kidney and spleen, suggesting that AKBA may be rapidly phagocytized in reticuloendothelial system and discharged. Furthermore, because of these improvements in pharmacokinetics and tissue distribution, drug concentration in brain of AKBA-NPs group was about three times that of AKBA group (P < 0.05).
Effects of AKBA-NPs on Primary Neurons Exposed to OGD
Figure 3 represents the cell viability. In OGD treatment group, the percentage of cell death was about 64 %. There was no obvious difference found in cell viability between NP and OGD group (P < 0.05). The cell viability in the AKBA-NP (70 ± 5 %) group was higher than that in the OGD group (36 ± 3 %) and the AKBA (54 ± 6 %) group (Fig. 3a) (P < 0.05). To confirm the protective effect of AKBA-NPs, a LDH assay was performed. AKBA-NP treatment decreased OGD-induced LDH release by approximately 47 % (P < 0.05) (Fig. 3b).
Effect of AKBA-NPs on cell viability and LDH release in primary culture of rat cortical neurons exposed to OGD. a The cell viability. b LDH release. The control group (normal cell group) was defined as 100 %. Data represent means ± SD of triplicate independent experiments. +P < 0.05 vs. control group; #P < 0.05 vs. OGD group; *P < 0.05 vs. OGD + AKBA group
Effects of AKBA-NPs on Rats with MCAO Model
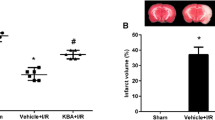
The infarct volume and neurological score in response to I/R injury were analyzed at 48 h after MCAO. There is no infarct or neurological deficit found in the sham group (Fig. 4). By comparison, both of cerebral infarct volume and neurological deficit scores in the vehicle group dramatically elevated compared the sham control treatment (Fig. 4). However, we did not find significant difference between the vehicle group and NPs group (Fig. 4). It suggested that empty NPs did not protect against I/R.
Effect of AKBA-NPs on brain infarct volume and neurological deficits at 48 h after cerebral I/R in rats. a Representative TTC-stained brain sections and quantification of brain infarct volume for each group. b Neurological scores of rats after cerebral I/R for each group. Data are expressed as means ± SD (n = 8). +P < 0.05 vs. sham group; #P < 0.05 vs. vehicle group; *P < 0.05 vs. AKBA group
As shown in Fig. 4, when comparing with AKBA group, AKBA-NPs dramatically reduced cerebral infarct volume by approximately 32 % and lighten the neural function deficit (P < 0.05, Fig. 4), which was similar with the result of TUNEL staining (Fig. 5). At 48 h after exposure to MCAO, the percentage of TUNEL-positive cells of the vehicle group (40 ± 7 %) and empty NPs group (38 ± 5 %) was higher than that of the control group (4 ± 2 %) (P < 0.05). However, the number TUNEL-positive cells of the AKBA-NPs group (15 ± 3 %) was lower than in the vehicle group (40 ± 7 %) or the AKBA group (25 ± 4 %) (P < 0.05).
Effect of AKBA-NPs on neuron apoptosis. Representative fluorescent micrographs of TUNEL staining. The TUNEL-positive neurons were shown in green, and the nuclei were stained blue with DAPI (×400 magnification). Scale bars = 20 mm. Representative photomicrographs of sham group, vehicle group, void NPs group, AKBA group, and AKBA-NPs group. Statistical analysis of the numbers of TUNEL-positive neurons in each group. Data are expressed as means ± SD (n = 8). +P < 0.05 vs. sham group; #P < 0.05 vs. vehicle group; *P < 0.05 vs. AKBA group
Estimation of Biochemical Parameters
The results of estimation of biochemical parameters are shown in Table 2. Void NP treatment did not show any significant effect on any of these oxidative stress or inflammation parameters.
SOD and GSH-Px activity in the cortex of vehicle group was decreased by 38 and 43 % in average compared with the sham group, but (SOD and GSH-Px), while AKBA and AKBA-NPs markedly restored them (P < 0.05). There were also notable increases in both of SOD and GSH-Px of AKBA-NP-treated group in comparison with the AKBA group by 21 and 19 % in average (P < 0.05).
TNF-α and interleukins, which are the mediators of inflammation, also serve as biological markers for inflammation. In this study, there was an elevation in TNF-α and IL-β levels in cortex of vehicle group as compared to sham group (P < 0.05). A significant reduction in both these parameters was observed with AKBA and AKBA-NPs groups. Especially, AKBA-NP formulation significantly reduced TNF-α level and IL-β levels more than AKBA by 34 and 33 % in average (P < 0.05).
Neuroprotective Mechanism of AKBA-NPs
To explore the mechanism of action, we assessed the effects of AKBA-NPs on proteins of important antioxidant and anti-inflammatory pathways involved in cerebral I/R. In Fig. 6, the data of Western blotting showed that I/R injury elevated the levels of nuclear Nrf2, total HO-1, NF-κB, and 5-LOX compared to sham group. After intravenously AKBA, and especially AKBA-NP treatment, protein expressions were noticeably regulated. Compared with AKBA, after administered with AKBA-NPs, the expressions of NF-κB in nuclear and 5-LOX were markedly suppressed, while the expressions of Nrf2 in nuclear and HO-1 were significantly increased. The data indicated that AKBA-NPs may exert its improved protection against cerebral I/R than AKBA through mechanisms related to enhancement of antioxidant capacity and prevention of inflammatory cascades.
Neuronal protective mechanism of AKBA-NPs. Antioxidant and anti-inflammatory protein expressions of cortex measured 48 h after MCAO in each group: nuclear Nrf2 (a), HO-1 (b), NF-κB (c), and 5-LOX (d). Data are expressed as means ± SD (n = 8). +P < 0.05 vs. sham group; #P < 0.05 vs. vehicle group; *P < 0.05 vs. AKBA group
Discussion
As far as we know, this is the first report on the O-CMC nanoparticle as an injectable delivery system for AKBA to treat cerebral I/R. AKBA-NPs was developed to overcome major obstacles associated with AKBA, such as poor solubility, rapid degradation, and poor bioavailability. The SEM image indicated that the particles of AKBA-NPs were uniform, segregated, spherical, and subspherical in shape (Fig. 1b). The blood-brain barrier (BBB) is characterized by tight junctions between cerebral endothelial cells that guard the selective diffusion of blood borne compounds from entering the brain [28]. For AKBA-NPs, in our study, the particle size was below 200 nm (Fig. 1c), that was able to enhance the endocytosis by the brain capillary cells as well as prevent spleen filtering [29]. Besides, the desired diameter of intravascular long-circulating NPs has been suggested within a relatively narrow range (70–200 nm) [30]. The zeta potential value of AKBA-NPs was about 24.5 mV, which agrees the adsorptive endocytosis of the cationic nanoparticles and allows absorptive-mediated transcytosis to cross the BBB. Additionally, after the initial burst drug release in 6 h (60 %), the controlled release pattern of AKBA-NPs was characterized by in vitro release experiments (Fig. 1d), which was suitable for stroke treatment.
Because of poor solubility of boswellic acids, oral administration is the main route for them; however, this formulation showed low bioavailability [12, 13]. As shown in our study, even if AKBA was intravenously administered, the short t1/2 made AKBA could not concentrate in brain to exert more pharmacodynamic action. Compared with AKBA, AKBA-NPs significantly prolong the t1/2 and MRTinf of AKBA (Table 1) and increase the AUC of AKBA and the drug levels of brain (Fig. 2). A longer retention time in the brain may also be achieved through the enhanced permeability and retention (EPR) effect of this nanosized delivery system. By the enhancement in pharmacokinetics and tissue distribution, AKBA-NPs were able to improve the therapeutic effects of AKBA.
Both of in vitro and in vivo pharmacodynamic studies found that the loading of AKBA into NPs significantly improved the protective properties of this natural compound against the cerebral I/R injury. At first, the therapeutic benefits of AKBA and AKBA-NPs against I/R injury were evaluated in an OGD model in vitro. Our results showed that AKBA-NPs more efficient in increasing primary neuronal cell viability and reducing LDH release after OGD injury, suggesting that AKBA-NPs exerted a better neuroprotective effect on neurons. In addition, MCAO model has been well characterized and often used to assess drug efficacy on cerebral I/R [31]. Thus, this model was applied in our study. In rats with MCAO, AKBA-NPs were more effective in alleviating the neurological deficiencies, decreasing the infarct volume than AKBA (P < 0.05, Fig. 4). Numerous studies have shown that OGD injury can induce neuronal cell apoptosis [25]; hence, the neuronal apoptosis were assessed with TUNEL staining during this study. There was an obvious decrease in the percentage of TUNEL-positive cells in AKBA-NPs group compared to AKBA group (P < 0.05, Fig. 5). These more beneficial effects compared to AKBA-NPs were in accordance with the greater accumulation of AKBA-NPs in the brain. Above all, the results revealed that AKBA-NPs protected against ischemic injury in the brain better than AKBA.
Accumulating evidences have suggested that the superabundant reactive oxygen species (ROS) are associated with cerebral I/R injury and oxidative stress is a important pathological mechanism in stroke [32]. Scavenging of the free radicals by SOD and GSH-Px protects the brain against I/R injury [33]. Consistent with our previous studies [9, 34], significant reductions during the activities of SOD and GSH-Px in the brain tissues were shown after I/R in this study (Table 2). Nevertheless, AKBA-NPs noticeably raised SOD and GSH-Px compared AKBA, indicating its neuroprotective effects could come from improving action of endogenous antioxidants. It has been definitely confirmed that Nrf2 and its gene targets phase II enzymes, including HO-1, play a critical role among endogenous antioxidative systems [35, 36]. Previously, we have proved that increasing the activity of Nrf2/HO-1 pathway exerts highly neuroprotective effects against oxidative, metabolic, and excitotoxic insults relevant to I/R injury both in cell and animal models [9, 34]. In the present study, we found that AKBA induced the protein expressions of Nrf2 and HO-1 in brain tissues, and these effects were significantly enhanced by NP formulation (Fig. 6).
Inflammation is another major factor involved in ischemic injury [2]. Cerebral ischemia caused a significant induction of cytokines and peaks during hours of reperfusion. TNF-α and IL-1β represent important mediators of the inflammatory response and apoptosis. Both of them are key mediators in neurodegeneration caused by cerebral I/R [37, 38]. The elevated levels of IL-1β and TNF-α in the vehicle group was remarkably improved by AKBA and AKBA-NPs in the treatment groups (Table 2). Therefore, the neuroprotection of AKBA during cerebral I/R may be partly mediated through modulation of the injury caused by proinflammatory cascades. NF-κB has been widely demonstrated as crucial regulator of inflammation. The inhibition on NF-κB reduced the levels of inflammatory mediators including proinflammatory cytokines (TNF-α and IL-1β) [39]. We found that the neuroprotection of AKBA-NPs alleviated the increased contents of proinflammatory cytokines more effectively than AKBA (Table 2). In addition, many reports demonstrated that AKBA activity mainly arises from suppression of leukotriene biosynthesis and 5-LOX [40]. In our comparative analysis of the protective effects of AKBA and AKBA-NPs, AKBA-NPs displayed significantly greater inhibition in NF-κB and 5-LOX expression (Fig. 6).
Both oxidative stress and inflammation are important elements in the pathogenesis of cerebral I/R [2]. The evidence has implicated that AKBA ameliorates oxidative stress and inflammatory mediators in a wide variety of human diseases. The results from our studies on mechanism indicated that improved pharmacodynamic activity of AKBA-NPs in I/R models could attribute to its enhanced antioxidant potential and inhibitory effects of AKBA, achieved by packaging AKBA into a suitable carrier system and improved uptake of AKBA by brain via this formulation. In this study, we demonstrated a marked therapeutic effect of AKBA nanoparticles by intravenous route at 1 h postrefusion, indicating the promising potential of AKBA-NPs for treating stroke in clinical settings.
In this study, AKBA-NPs were successfully prepared by a very simple and easy method. They were nanosized and monodispersed with good encapsulation efficiency and sustained release patterns. The pharmacokinetic and biodistribution studies illustrated that AKBA-NPs could substantially raise AUC and concentrate into the brain. We showed that nanoformulation of AKBA leads to enhanced protection of AKBA against cerebral ischemic insult by improving antioxidant defense (SOD and GSH-Px) and decreasing inflammation (TNF-α and IL-1β). It was also supported by enhanced activation in Nrf2/HO-1 pathway and inhibition of inflammatory protein expressions (NF-κB and 5-LOX). It can be concluded that O-CMC nanoparticles represent an interesting delivery system for AKBA and, hence, offer an effective and promising candidate for the treatment of stroke.
Abbreviations
- AKBA:
-
Acetyl-11-keto-β-boswellic acid
- AKBA-NPs:
-
AKBA-loaded o-carboxymethyl chitosan nanoparticles
- EPR:
-
Enhanced permeability and retention
- GPx:
-
Glutathione peroxidase
- IL-1β:
-
Interleukin-1β
- I/R:
-
Ischemia-reperfusion
- HO-1:
-
Heme oxygenase-1
- 5-LOX:
-
5-Lipoxygenase
- MCAO:
-
Middle cerebral artery occlusion
- MDA:
-
Malondialdehyde
- MTT:
-
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- Nrf2:
-
Nuclear factor erythroid-2-related factor 2
- NF-κB:
-
Nuclear factor kappa-beta
- O-CMC:
-
O-Carboxymethyl chitosan
- OGD:
-
Oxygen and glucose deprivation
- SD:
-
Standard deviation
- SEM:
-
Scanning electron microscope
- SOD:
-
Superoxide dismutase
- TNF-α:
-
Tumor necrosis factor-α
- TTC:
-
2,3,5-Triphenyltetrazolium chloride
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick-end labeling
References
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S et al (2013) Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 127(1):e6–e245. doi:10.1161/CIR.0b013e31828124ad
Iadecola C, Anrather J (2011) Stroke research at a crossroad: asking the brain for directions. Nat Neurosci 14(11):1363–1368
Marder VJ, Jahan R, Gruber T, Goyal A, Arora V (2010) Thrombolysis with plasmin: implications for stroke treatment. Stroke J Cereb Circ 41(10 Suppl):S45–S49. doi:10.1161/STROKEAHA.110.595157
Ernst E (2008) Frankincense: systematic review. BMJ 337:a2813. doi:10.1136/bmj.a2813
Ammon HP (2006) Boswellic acids in chronic inflammatory diseases. Planta Med 72(12):1100–1116. doi:10.1055/s-2006-947227
Moussaieff A, Mechoulam R (2009) Boswellia resin: from religious ceremonies to medical uses; a review of in-vitro, in-vivo and clinical trials. J Pharm Pharmacol 61(10):1281–1293. doi:10.1211/jpp/61.10.0003
Kirste S, Treier M, Wehrle SJ, Becker G, Abdel-Tawab M, Gerbeth K, Hug MJ, Lubrich B et al (2011) Boswellia serrata acts on cerebral edema in patients irradiated for brain tumors: a prospective, randomized, placebo-controlled, double-blind pilot trial. Cancer 117(16):3788–3795. doi:10.1002/cncr.25945
Skarke C, Kuczka K, Tausch L, Werz O, Rossmanith T, Barrett JS, Harder S, Holtmeier W et al (2012) Increased bioavailability of 11-keto-beta-boswellic acid following single oral dose frankincense extract administration after a standardized meal in healthy male volunteers: modeling and simulation considerations for evaluating drug exposures. J Clin Pharmacol 52(10):1592–1600. doi:10.1177/0091270011422811
Ding Y, Chen M, Wang M, Wang M, Zhang T, Park J, Zhu Y, Guo C et al (2014) Neuroprotection by acetyl-11-keto-beta-boswellic acid, in ischemic brain injury involves the Nrf2/HO-1 defense pathway. Sci Rep 4:7002. doi:10.1038/srep07002
Sailer ER, Subramanian LR, Rall B, Hoernlein RF, Ammon H, Safayhi H (1996) Acetyl‐11‐keto‐β‐boswellic acid (AKBA): structure requirements for binding and 5-lipoxygenase inhibitory activity. Br J Pharmacol 117(4):615–618
Cuaz-Pérolin C, Billiet L, Baugé E, Copin C, Scott-Algara D, Genze F, Büchele B, Syrovets T et al (2008) Antiinflammatory and antiatherogenic effects of the NF-κB inhibitor acetyl-11-keto-β-boswellic acid in LPS-challenged ApoE−/− mice. Arterioscler Thromb Vasc Biol 28(2):272–277
Karlina M, Pozharitskaya O, Kosman V, Ivanova S (2007) Bioavailability of boswellic acids: in vitro/in vivo correlation. Pharm Chem J 41(11):569–572
Kruger P, Daneshfar R, Eckert GP, Klein J, Volmer DA, Bahr U, Muller WE, Karas M et al (2008) Metabolism of boswellic acids in vitro and in vivo. Drug Metab Dispos Biol Fate Chem 36(6):1135–1142. doi:10.1124/dmd.107.018424
Cho Y, Shi R, Borgens RB (2010) Chitosan produces potent neuroprotection and physiological recovery following traumatic spinal cord injury. J Exp Biol 213(9):1513–1520
Lee HJ, Park J, Yoon OJ, Kim HW, Kim DH, Lee WB, Lee N-E, Bonventre JV et al (2011) Amine-modified single-walled carbon nanotubes protect neurons from injury in a rat stroke model. Nat Nanotechnol 6(2):121–125
De Giglio E, Trapani A, Cafagna D, Sabbatini L, Cometa S (2011) Dopamine-loaded chitosan nanoparticles: formulation and analytical characterization. Anal Bioanal Chem 400(7):1997–2002. doi:10.1007/s00216-011-4962-y
Xie YT, Du YZ, Yuan H, Hu FQ (2012) Brain-targeting study of stearic acid-grafted chitosan micelle drug-delivery system. Int J Nanomedicine 7:3235–3244. doi:10.2147/Ijn.S32701
Malatesta M, Galimberti V, Cisterna B, Costanzo M, Biggiogera M, Zancanaro C (2014) Chitosan nanoparticles are efficient carriers for delivering biodegradable drugs to neuronal cells. Histochem Cell Biol 141(5):551–558. doi:10.1007/s00418-013-1175-9
Mistry A, Stolnik S, Illum L (2009) Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharmaceut 379(1):146–157. doi:10.1016/j.ijpharm.2009.06.019
Zhao L, Zhu B, Jia Y, Hou W, Su C (2013) Preparation of biocompatible carboxymethyl chitosan nanoparticles for delivery of antibiotic drug. BioMed Res Int 2013:236469. doi:10.1155/2013/236469
Smitha KT, Sreelakshmi M, Nisha N, Jayakumar R, Biswas R (2014) Amidase encapsulated O-carboxymethyl chitosan nanoparticles for vaccine delivery. Int J Biol Macromol 63:154–157. doi:10.1016/j.ijbiomac.2013.10.045
Nagpal K, Singh SK, Mishra DN (2013) Formulation, optimization, in vivo pharmacokinetic, behavioral and biochemical estimations of minocycline loaded chitosan nanoparticles for enhanced brain uptake. Chem Pharm Bull 61(3):258–272
Reising K, Meins J, Bastian B, Eckert G, Mueller WE, Schubert-Zsilavecz M, Abdel-Tawab M (2005) Determination of boswellic acids in brain and plasma by high-performance liquid chromatography/tandem mass spectrometry. Anal Chem 77(20):6640–6645. doi:10.1021/ac0506478
Wu J, Li Q, Wang X, Yu S, Li L, Wu X, Chen Y, Zhao J et al (2013) Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PLoS One 8(3):e59843. doi:10.1371/journal.pone.0059843
Deng B, Gou X, Chen H, Li L, Zhong H, Xu H, Jiang F, Zhao Z et al (2013) Targeted delivery of neurogenin-2 protein in the treatment for cerebral ischemia-reperfusion injury. Biomaterials 34(34):8786–8797. doi:10.1016/j.biomaterials.2013.07.076
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke J Cereb Circ 20(1):84–91
Lu Y-M, Huang J-Y, Wang H, Lou X-F, Liao M-H, Hong L-J, Tao R-R, Ahmed MM et al (2014) Targeted therapy of brain ischaemia using Fas ligand antibody conjugated PEG-lipid nanoparticles. Biomaterials 35(1):530–537
Rubin L, Staddon J (1999) The cell biology of the blood-brain barrier. Annu Rev Neurosci 22(1):11–28
Huwyler J, Wu D, Pardridge WM (1996) Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci 93(24):14164–14169
Stolnik S, Illum L, Davis S (2012) Long circulating microparticulate drug carriers. Adv Drug Deliv Rev 64:290–301
Durukan A, Tatlisumak T (2007) Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav 87(1):179–197. doi:10.1016/j.pbb.2007.04.015
Chan PH (1994) Oxygen radicals in focal cerebral ischemia. Brain Pathol 4(1):59–65
Siesjo BK, Agardh CD, Bengtsson F (1989) Free radicals and brain damage. Cerebrovasc Brain Metab Rev 1(3):165–211
Ding Y, Chen M, Wang M, Li Y, Wen A (2014) Posttreatment with 11-keto-β-boswellic acid ameliorates cerebral ischemia–reperfusion injury: Nrf2/HO-1 pathway as a potential mechanism. Mol Neurobiol 1–10. doi:10.1007/s12035-014-8929-9
Ding Y, Zhang B, Zhou K, Chen M, Wang M, Jia Y, Song Y, Li Y et al (2014) Dietary ellagic acid improves oxidant-induced endothelial dysfunction and atherosclerosis: role of Nrf2 activation. Int J Cardiol 175(3):508–514. doi:10.1016/j.ijcard.2014.06.045
Maines MD (1988) Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J Off Publ Fed Am Soc Exp Biol 2(10):2557–2568
Loetscher H, Gentz R, Zulauf M, Lustig A, Tabuchi H, Schlaeger EJ, Brockhaus M, Gallati H et al (1991) Recombinant 55-kDa tumor necrosis factor (TNF) receptor. Stoichiometry of binding to TNF alpha and TNF beta and inhibition of TNF activity. J Biol Chem 266(27):18324–18329
Touzani O, Boutin H, Chuquet J, Rothwell N (1999) Potential mechanisms of interleukin-1 involvement in cerebral ischaemia. J Neuroimmunol 100(1):203–215
Killeen MJ, Linder M, Pontoniere P, Crea R (2014) NF-kappabeta signaling and chronic inflammatory diseases: exploring the potential of natural products to drive new therapeutic opportunities. Drug Discov Today 19(4):373–378. doi:10.1016/j.drudis.2013.11.002
Siemoneit U, Hofmann B, Kather N, Lamkemeyer T, Madlung J, Franke L, Schneider G, Jauch J et al (2008) Identification and functional analysis of cyclooxygenase-1 as a molecular target of boswellic acids. Biochem Pharmacol 75(2):503–513
Acknowledgments
This research was financially supported by the Key Technologies for New Drug Innovation and Development of China (Nos. 2011ZXJ09202-13 and 2012BAK25B00) and the National Natural Science Foundation of China (Nos. 81373947 and 81201985).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yi Ding and Youbei Qiao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ding, Y., Qiao, Y., Wang, M. et al. Enhanced Neuroprotection of Acetyl-11-Keto-β-Boswellic Acid (AKBA)-Loaded O-Carboxymethyl Chitosan Nanoparticles Through Antioxidant and Anti-Inflammatory Pathways. Mol Neurobiol 53, 3842–3853 (2016). https://doi.org/10.1007/s12035-015-9333-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9333-9