Abstract
Diverse practices implementing biopolymer-producing bacteria have been examined in various domains lately. PHAs are among the major biopolymers whose relevance of PHA-producing bacteria in the field of crop improvement is one of the radical unexplored aspects in the field of agriculture. Prolonging shelf life is one serious issue hindering the establishment of biofertilizers. Studies support that PHA can help bacteria survive stressed conditions by providing energy. Therefore, PHA-producing bacteria with Plant Growth-Promoting ability can alter the existing problem of short shelf life in biofertilizers. In the present study, Bacillus subtilis NJ14 was isolated from the soil. It was explored to understand the ability of the strain to produce PHA and augment growth in Solanum lycopersicum and Cicer arietinum. NJ14 strain improved the root and shoot length of both plants significantly. The root and shoot length of S. lycopersicum was increased by 3.49 and 0.41 cm, respectively. Similarly, C. arietinum showed a 9.55 and 8.24 cm increase in root and shoot length, respectively. The strain also exhibited halotolerant activity (up to 10%), metal tolerance to lead (up to 1000 μg/mL) and mercury (up to 100 μg/mL), indicating that the NJ14 strain can be an ideal candidate for a potent biofertilizer.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biofertilizers containing one or more microorganisms can accelerate plant growth by transforming unavailable nutrients into useful forms for plant growth. Scientists currently favor and are attempting to ameliorate biofertilizers due to the negative consequences of chemical fertilizers [1]. The shelf life of biofertilizers is one of the significant challenges hindering their establishment. The bacteria themselves, as well as the carrier used for formulation, determine how long biofertilizers last. Polyhydroxyalkanoates (PHA) synthesizing bacteria have been enhancing their chances of survival under challenging circumstances [2]. Hence, PHA-producing bacteria are promising candidates for refining the quality of biofertilizers. While under stress, different prokaryotes tend to mass other biopolymers as energy sources. One such polymer is the polyesters of hydroxyalkanoates, commonly known as PHAs. This biopolymer is built and accumulated as granules when the prokaryotes are subjected to stress by restricting the intake of any necessary nutrients, such as nitrogen, with excess carbon. These PHA granules are also known to develop the organisms' resistance to various stresses, such as maintaining cell integrity throughout an osmotic shock by acting as an intracellular scaffold in bacterial cells. In contrast, osmotic pressure is present, protecting the cells from severe plasmolysis that could harm the cell membrane [3]. PHA granules also protect against temperature fluctuations; bacterial cells start mobilizing PHA to boost the intracellular concentration of monomers and take advantage of their shielding function to get protection during temperature fluctuations. PHA has also been proven to combat elevated pH, pressure fluctuations, and oxygen pressure [4]. Among the PHAs, the mainly studied class is poly-beta-hydroxybutyric acid or polyhydroxybutyrate (PHB), derived primarily from prokaryotes [5]. The exact role and significance of PHB in enhancing the plant growth ability of rhizobacteria are not studied extensively. Comparing PHA-producing bacteria to mutants defective in PHA production is one way to recognize their role in colonization with plant roots. When Herbaspirillum seropedicae was studied for plant growth promotion of Setaria viridis, the study proved that H. seropedicae SmR1 (PHB producing) appeared to improve total and lateral root area in Setaria viridis compared to mutants (incapable of PHB production) [6].
The biosynthesis of PHA can follow different pathways depending on the substrate types employed. The substrates utilized are sugars, fatty acids, and alkanes/alkenes. Three genes are responsible for converting sugar to PHA: A, B, and C. Acetyl-coenzyme A is generated through pyrolysis, which condenses to yield acetoacetyl-CoA through the catalysis of PHA A gene encoded β-ketoacyl-CoA thiolase. The NADPH-dependent acetoacetyl-CoA dehydrogenase, ciphered by the pha B gene, reduces the acetoacetyl-CoA to (R)-3-hydroxybutyrate monomer. Later, PHA C-encoded PHA synthase enzymes polymerize the monomer units through ester linkage. PHB is a potential candidate for replacing synthetic polyethylene due to its versatile properties [7].
Stress is one of the major reasons that lead to reduced yield in plants. Various types of stresses like temperature, excess of light, pathogens, pests, lack or excess of nutrients, salinity, water, and mechanical stresses can deteriorate normal growth in plants therefore affecting their yield. The harmful effect of chemical fertilizers and moreover the efficiency of biological agents have drawn attention toward Plant Growth-Promoting Bacteria (PGPR) as a sustainable remedy to manage stress tolerance in plants. PGPR is one of the most focused areas of research and is the current trend in agriculture which is capable of replacing chemical fertilizers. Under stress, PGPR produces PHA in the soil, and therefore, application of biopolymer-producing bacteria as a plant growth-promoting agent is the major objective of this study.
Since these PHA granules boost the organisms’ forbearance to a variety of challenges by improving survival, stress tolerance, and cell division, which help extend shelf life, this study focuses on identifying an able PHA-producing applicant that has Plant Growth-Promoting (PGP) activities and on testing its capability to improve growth parameters on a leguminous crop plant Cicer arietinum and a non-leguminous plant Solanum lycopersicum.
Methods
Isolation of Bacillus subtilis NJ14
Soil from the rhizosphere of healthy Coffea arabica plants was sampled from Kodagu district, Karnataka, India. The collected sample was utilized for isolation by adding soil (1 g) to 100 mL of nutrient broth (peptone-3 g, NaCl-5 g, yeast extract-3 g, 1 L distilled water, pH 7), incubated at 37 ± 2 °C, 24 h, for enrichment. Later, serial dilution of the enriched culture was done up to 10−6 and plated onto nutrient agar plates (peptone-3 g, NaCl-5 g, yeast extract-3 g, agar-1.5% (w/v), 1 L distilled water, pH 7). The plates were then placed in a 37 °C incubator for 24 h, and with morphological differences were pure cultured onto fresh nutrient agar plates for maintenance. By evaluating each isolate in terms of their PHA-producing and PGP ability, the potential isolate was determined for further involvement in the study [8].ss
PHA Staining and Extraction
Nile Blue Staining
The Nile blue test was a primary method to find PHA-yielding bacteria among the isolates. 0.003 g of Nile blue dye was added in 10 mL of DMSO. 2 mL of this solution was mixed with 100 mL of nutrient agar media (peptone-3 g, NaCl-5 g, yeast extract-3 g, agar-1.5% (w/v), 1 L distilled water, pH 7). The isolates were inoculated on the plates and were incubated (37 °C) for a day. After incubation, the culture on the plates was viewed under a UV chamber for fluorescence. The fluorescence intensity determined the isolates' PHA-producing potentiality [9].
Sudan Black Staining
The Sudan black test was done to further confirm the presence of PHA granules in the isolates [10]. 0.003% (w/v) of Sudan black solution was prepared using ethanol. The isolates to be tested were smeared on glass slides, and 2 drops of Sudan black solution were added to the slides and placed aside for 10 min for staining. The stain was removed, and the smear was counter-stained using safranin. After 1 min, the smear was gently washed with distilled water to remove excess safranin. The slide was then observed under a Leica microscope (DMi8). PHA granules in bacteria-stained black and vegetative parts stained red indicated positive results.
Extraction of PHA
Overnight culture (1 mL) of the isolate was added to 100 mL of media for extraction of PHA (NaCl-10 g, Na2HPO4-3.7 g, KH2PO4-1 g, (NH4)2HPO4-0.5 g, MgSO40.7H2O-0.2 g, Glycerol-2%(v/v), tryptone-5 g, yeast extract-0.5 g in 1 L of distilled water, MgSO40.7H2O, and glycerol were autoclaved separately and added to media) and incubated at 37 ± 2 °C. After 72 h of incubation, the culture was transferred to pre-weighed centrifuge tubes and centrifuged at 6500 rpm for 10 min to collect the pellets. The tubes were placed at 50 °C for 2 h to air dry the pellets completely. The centrifuge tubes with pellets were weighed to get the cell dry weight (g/100 mL). The pellets were treated with 4% (w/v) sodium hypochlorite at 37 ± 2 °C for 1 h to digest all the cell components except PHA. The mixture was spun at 10,000 rpm for 10 min to collect the pellet, which was washed twice with distilled water followed by a wash with a 1:1:1 ratio of acetone: diethyl ether: methanol. Boiling chloroform was added to the centrifuge tubes after washing to dissolve the pellet and poured into a pre-weighed petri dish, and the chloroform was evaporated. The weight of the petri dish with PHA was noted as a dry weight of PHA (g/100 mL) [11].
Characterization of Extracted PHA
Fourier‑Transform Infrared (FTIR) Characterization of PHA
FTIR is a routinely used technique to study the functional groups in the extracted polymer by exposing it to IR rays that create distinctive peaks as a function of the chemical groups present in the polymer. FTIR analysis of the extracted polymer was performed by spreading it (100 mg) on to KBr disc, followed by measuring the spectrum at a wavelength range between 400 and 4000 cm−1 using an FTIR spectrometer (Shimadzu IR Spirit-L) [12].
X‑Ray Diffraction (XRD) Analysis of PHA
The crystalline nature of the extracted polymer was assessed through XRD analysis (Philips’ Xpert PRO instrument) by scanning at a 2θ range between 10 and 80° [13].
Nuclear Magnetic Resonance (NMR) Analysis of PHA
The precise monomeric structure of the extracted PHA was studied using 1H NMR analysis. This relies on the characteristic magnetic spin of the particles present in the polymer to deduce their monomeric composition. The extracted polymer (5 mg) was dissolved in deuterated chloroform (CDCl3), and 1H NMR was recorded (Bruker Avance III spectrometer) at 400 MHz using tetramethyl silane as internal standard [14].
Plant Growth-Promoting Traits
The isolate that confirmed PHA production and was employed for PHA extraction was further subjected to different PGP assays to verify its ability as a significant candidate for bioinoculant.
ACC Deaminase Test
ACC-deaminase activity of the isolate was checked using DF salts minimal media (KH2PO4-4 g, Na2HPO4-6 g, H3BO3-10 μg, MnSO4-10 μg, ZnSO4-70 μg, CuSO4-50 μg, MoO3-10 μg, C6H12O6-2 g, FeSO40.7H2O-1 mg, C6H12O7-2 g, C6H8O7-2 g, MgSO40.7H2O-0.2 g, agar-1.5 g (NH4)2SO4-0.3033 g in 1 L distilled water). The isolate was inoculated on media and incubated at 37 ± 2 °C. After 120 h, the plates were checked for growth which indicated positive results [15, 16].
Phosphate Solubilization Test
The capacity to solubilize phosphate was discerned by inoculating the isolate onto Pikovskaya media (yeast extract-0.5 g, MgSO40.7H2O-0.1 g, dextrose-10 g, Ca3(PO4)2-5 g, FeSO40.7H2O-0.0001 g, (NH4)2SO4-0.5 g, KCl-0.2 g, MnSO4-0.0001 g, agar-15 g, distilled water-1 L). After inoculation, the plates were incubated at 28 ± 2 °C for 7 days, and the clearance zone was checked on the seventh day [17, 18]. The phosphate Solubilization Index (PSI) of the organism was calculated using the following equation [19, 20]:
Zinc Solubilization Test
Pikovskaya media altered with ZnCl2 (yeast extract-0.5 g, MgSO40.7H2O-0.1 g, dextrose-10 g, FeSO40.7H2O-0.0001 g, Ca3(PO4)2-5 g, (NH4)2SO4-0.5 g, KCl-0.2 g, MnSO4-0.0001 g, agar-15 g, ZnCl2-2 g distilled water-1 L) were used to test Zn solubilization capacity of the isolate. The media were inoculated with isolate and left at 28 ± 2 °C for a week. The incubated plates were checked for halo framing of the colonies. The Zinc Solubilization Index (ZSI) was obtained by using the following equation [19, 21]:
Indole Acetic Acid Test
Nutrient broth (5 mL) was altered with 1% (w/v) tryptophan and added with 1 mL of overnight isolate culture. The broth was placed at 28 ± 2 °C incubator for 48 h and was spun at 10,000 rpm at 4 °C (10 min). 99.9% (w/v) orthophosphoric acid (2 drops) followed by Salkowski reagent, 2 mL (0.5 M FeCl3-1.5 mL, distilled water-50 mL, conc. H2SO4-30 mL) was added to 2 mL of supernatant. A blank was prepared by mixing the reagents with 2 mL deionized water instead of the supernatant. IAA was utilized to prepare the standard, with 10 ug/mL to 500 ug/mL concentrations. The mixtures were incubated for half an hour (in the dark), and absorbance was measured at 535 nm. Pink coloration in the sample tube denoted the successful production of IAA [8, 22].
Siderophore Test
The presence of siderophores (small molecular iron chelators) was determined using FeCl3. The overnight culture of the isolate was spun for 10 min (10,000 rpm), and 2 mL of 2% (w/v) FeCl3 was added into 5 mL of the supernatant. The presence of brown color indicated positive results [23, 24].
Ammonia Test
The isolate was tested for ammonia production using Nessler’s reagent. 1 mL of overnight culture was added into 5 mL peptone water (peptone-50 g, NaCl-5 g, distilled water-1 L, pH 7.2) and was placed in a 28 ± 2 °C incubator. After 3 days, the tube was mixed with Nessler’s reagent (2 mL). The change in color to light brown from yellow showed positive results [25, 26].
Nitrogen Fixation Test
The nitrogen-fixation ability of the isolate was tested by inoculating onto Jensen’s agar media (Sucrose-20 g, K2HPO4-1 g, CaCO3-0.1 g, NaCl-0.1 g, MgSO40.7H2O-0.5 g, FeSO40.7H2O-0.1 g, Na2MoO40.2H2O-0.005 g, agar-1.5% (w/v) distilled water-1 L). The inoculated media were incubated in a 28 ± 2 °C incubator for 3 days, and bacterial growth was checked [27, 28].
HCN Test
King’s B media (Peptone-16 g, K2HPO4-1.6 g, MgSO40.7H2O-1.6 g, glycerol-0.1 L, agar 20 g, distilled water-0.99 L) modified with 4.4 g/L glycine were inoculated with the isolate. A sterile Whatman filter paper (no.1) soaked with picric acid solution (1.5% (w/v)-dissolved a picric acid-2.5 g (w/v) and Na2CO-12.5 g (w/v), in 1 L of distilled water) was stuck on the upper plate of the petri dish. The inoculated plates were incubated for 3 days in a 28 ± 2 °C incubator. Positive HCN production was confirmed when yellow filter paper changed its color to brown [8, 29].
Enzymatic Assay
The breakdown of substrates and product generation detect the production of different enzymes. The yield of enzymes by the isolate can increase their potential in industrial applications.
Starch agar (Starch-20 g, peptone-20 g, agar 20 g, distilled water-1 L) was used for checking the amylolytic enzyme production by the isolate. The isolate was inoculated on Sterile starch agar plates, which were incubated at 37 ± 2 °C for a day. After incubation, 20 mL of 1% iodine solution was poured onto the media to observe the hydrolysis zone [30].
Cellulase production of the isolate was tested by inoculating on Carboxymethylcellulose (CMC) agar (yeast extract-2 g, KH2PO4-1 g, MgSO4-5 g, CMC (w/v)-5 g, agar-15 g in 1 L of distilled water). The inoculated plate with isolate was incubated at 37 ± 2 °C for a day, and post-incubation, the plate was flooded with 15 mL 0.1% Congo red, which was washed out with 20 mL 1 M NaCl solution. Afterward, the plates were checked for positive results by observing a hydrolysis zone around the colony [31].
Skimmed milk agar (yeast extract-2.5 g, tryptone-5 g, C6H12O6-1 g, skim milk powder-28 g, agar-15 g, distilled water-1 L) was used for the screening of protease production. The isolate was inoculated and placed in a 37 ± 2 °C incubator for 48 h. A zone of hydrolysis was observed, framing the colony as a positive result [31].
The production of catalase enzymes can be tested using hydrogen peroxide. 6% H2O2 (w/v) was dropped on a clean glass slide. From the overnight nutrient agar plate inoculated with the isolate, a single colony was taken using an inoculation loop and mixed with the drop of H2O2. The positive result was indicated by the formation of air bubbles [32].
Halotolerance Test
The halotolerant test investigates the quality of microorganisms flourishing in varying concentrations of NaCl. Nutrient agar plates with different concentrations of NaCl (2%, 4%, 6%, 8%, 10%, and 15% (w/v)) were inoculated with the isolate. The inoculated plates were incubated at 37 ± 2 °C for 24 h, and the percentage of halotolerant was determined by visually observing the bacterial growth on the plates [33].
Metal Tolerance Test
Metal tolerance can determine a bacterial strain's capacity to mitigate and thrive in an environment with high concentrations of heavy metals. Nutrient agar plates were amended separately with 50, 100, 250, 500, and 1000 µg/mL of Hg, Cd, and Pb heavy metals. The isolate was inoculated on the plates and incubated at 37 ± 2 °C for 24 h. After visual observation of the bacterial growth, the most promising isolate was selected for further research [34]
Antifungal Assay
Four fungal plant pathogens, Talaromyces albobiverticillius, Cladosporium tenuissimum, Aspergillus niger, and Fusarium solani, were employed to check the antifungal activity of the isolate. Fungal discs were prepared by inoculating the fungi onto Sabouraud’s Dextrose Agar (dextrose-4 g, peptone-1 g, agar-1.5 g, in 100 mL of distilled water) with filter paper discs. All four fungi were obtained from soil, and these molecularly identified fungi were preserved in the Department of Life Sciences, CHRIST (Deemed to be University), Bangalore, Karnataka, India. The plates were incubated at room temperature for 5 days for mycelial growth. Meanwhile, SDA plates were prepared by inoculating the bacterial isolate by streaking two parallel lines 6 cm apart. These plates were placed in a 37 ± 2 °C incubator for a day for bacterial growth. Later the prepared fungal discs were placed on the center of SDA plates with the isolate streak and incubated for 7 days at room temperature. SDA plates without the isolate were also prepared and inoculated with the same fungi to serve as a control. Fungal growth was observed for all the fungi, and the positive results were regarded as restricted fungal growth in the presence of the isolate [35].
Biochemical Molecular Characterization [36, 37].
Preliminary characterization, like gram staining and biochemical tests, was performed for the plant growth-promoting bacterial isolate capable of producing PHA before enzyme assays and molecular characterizations. Later 16 s rRNA gene sequencing was done at Barcode Biosciences, Bangalore. After the genomic DNA isolation from the culture, it was resolved using agarose gel electrophoresis (1% agarose gel) followed by amplifying the DNA fragment of 16S rRNA gene through PCR with 16SrRNA-F and 16SrRNA-R primers using BDT v3.1 Cycle sequencing kit on ABI 3730xl genetic analyzer. Aligner sequence software was used to deduce the consensus sequence. BLAST was performed with the ‘nr’ NCBI GenBank database, scrutinizing the first 10 sequences based on the maximum identity score. Multiple sequence alignment was done using Clustal ⍵ software. Distance matrix and phylogenetic tree were constructed using MEGA 10 software [36, 37].
Statistical Optimization of Two-Factor Interaction Model for the Bacterial Biomass
To optimize the measurable output (response) bacterial biomass (g/L) of the isolate, a DOE-based two-level, Reduced Quartic model was set for the multivariate study using Design Expert 13 software. The input parameters were A: Incubation period (h), B: Inoculum level (%), and C: NaCl (g/L). A total of 20 runs with different combinations of input parameters were performed. The run details are shown in Table 2. Finally, the validation of optimized parameters in triplicates was performed within the range of the established design space, as adopted as per ICH Q8 guideline (https://www.ich.org/page/quality-guidelines) and our previous studies [38, 39].
Seed Germination Test of Tomato and Chickpea
Microbial Bioinoculant Preparation
B. subtilis NJ14 were cultured in nutrient broth (100 mL). After 24 h, bacteria were collected from the media by centrifuging the culture at 6000 rpm for 10 min at 4 °C. The cells were procured carefully without cell loss and washed twice with Sterile distilled water (SDW) to remove the media components. The washed cells were resuspended in SDW (100 mL) to acquire ≅ 104 CFU mL−1 rhizobacterial density.
Seed Treatment
The seeds of S. lycopersicum (Arka-Samrat variety from IIHR, Bangalore, Karnataka, India) and C. arietinum (MNK-1 variety from UAS, Raichur, Karnataka, India) were utilized for the study. The seeds were coated with 1% (w/v) CMC adhesive after being surface sterilized using 0.1% (w/v) HgCl2. These seeds were then immersed in bioinoculant prepared and were soaked for 30 min in a shaker incubator at 120 rpm. Seeds soaked in SDW without the bioinoculant were maintained as control. The seeds were then planted in a seedling tray with sterilized soil to monitor seed germination and growth over 27 days [19].
Growth Parameters
The seed germination was calculated based on the observed germination of the total number of seeds sown. To calculate the Total Germination Percentage (TGP), the following formula was utilized:
Other than germination percentage, root length and shoot lengths were observed 4 weeks after sowing. These parameters were used to compare the difference in the growth rate of the sample plant against the control.
Statistical Analysis
The significant difference (p < 0.05) was determined by One-way ANOVA operating DMRT in IBM SPSS Statistics 21. All the values were expressed as Mean ± SE.
Results and Discussion
Isolation and Identification of of B. subtilis NJ14
The rhizospheric soil collected from the rhizosphere of Coffea arabica was used for isolation, and 14 isolates were obtained. The obtained isolates were subjected to preliminary PHA production tests and PGP tests. The most promising isolate NJ14 (Fig. 1a, 1b) which had both traits was biochemically characterized (Table 1) and later molecularly characterized for identification. The isolate was identified as B. subtilis after constructing a phylogenetic tree using Clustal ⍵ and MEGA 6 software. The accession number OM780222 was received for the strain after submitting the sequence to GenBank.
PHA Staining and Extraction
Nile Blue Staining
Nile blue stain is a lipophilic dye used to stain PHA in bacteria. In a work, B. subtilis has been studied to show fluorescence in Nile blue staining, confirming the presence of PHA granules [40]. Another work recommended that B. subtilis NRR-B-941 exhibited PHA production when the Nile blue staining method was applied [41]. When the plates with isolates were placed under a UV chamber, isolate NJ14 with PHA production displayed fluorescence indicating the presence of PHA granules (Fig. 2a).
Sudan Black Staining
Sudan black staining was performed to confirm further the existence of PHA granules in the bacterial cells. A work reported PHA granules in B. subtilis by Sudan black staining method [42]. Another work utilized Sudan black staining and confirmed that B. subtilis could build and use PHA as a carbon source [43]. In this study, the isolate that exhibited fluorescence in Nile blue staining confirmed the presence of PHA by showing black-stained PHA granules (Fig. 2b).
Extraction of PHA
Extraction of PHA is accomplished in 3 steps conventionally: Cell digestion, separation, and recovery. In this study, an alkali salt, 4% sodium hypochlorite, was used for cell digestion, and PHA was separated from the lysed cells by solvent extraction method using acetone: diethyl ether: methanol in a 1:1:1 ratio. And the PHA was dissolved and recovered using boiling chloroform. In a work, B. subtilis yielded 3.09 g/L of PHB at pH 7.0, 37 °C with 10 g/L of glucose [13]. In another work, B. subtilis was reported to produce 2.09 g/100 mL of PHA when 10% sugarcane molasses was employed as carbon substrate [44]. A study reported that B. subtilis produced 22.98 g/L of PHA from 36.98 g/L cell dry mass [42]. In the present study, B. subtilis NJ14 yielded 2.32 g/L of PHA from 9.74 g/L of dry cell mass.
Characterization of PHA
Fourier‑Transform Infrared (FTIR) Characterization of PHA
The FTIR spectrum of the extracted polymer gives a precise understanding of the various functional groups present in the molecule as a function of their wave number. The nature and intensity of the peaks in the FTIR spectrum are used to have a preliminary identification of the nature of biopolymers. The FTIR spectrum of the extracted polymer in this study is depicted in Fig. 3.
The central peak at 2927 cm−1 corresponds to aliphatic stretching contributed by the alkane group and the peak at 3428 cm−1 is contributed by the –OH group. A sharp rise at 1720 cm−1 signifies weak c=o stretching contributed by the conjugated carbonyl group, and 1277 cm−1 corresponds to symmetric and asymmetric stretch vibration of C–O–C. The peak at 1052 cm−1 reflects the presence of alkyl halides in the extracted polymer. The peaks obtained in the FTIR spectrum of the extracted polymer confirmed the polymer to be PHA due to the peak similarity with available literature. In a study dealing with PHA production from Bacillus subtilis RS1, major peaks obtained were at 2924 cm−1 (C–H stretching of bonds of methyl group), 1722 cm−1 (C=O stretching of ester groups), and 1278 cm−1(C–O–C stretching) which is similar to the peaks obtained in this study [45]. In another study with Bacillus cereus SH-02, characteristic peaks reflecting PHA were obtained at 2933 cm−1 and 1724 cm−1, which strongly correlates with the result obtained in this study [46]. PHA production study using distillery effluent with Bacillus subtilis NCDC 0671 revealed characteristic peaks at 2920 cm−1 corresponding to C-H vibrations of the methyl group, 1724 cm−1 signifying carbonyl groups C=O from ester and carboxyl groups, and peaks at 1033 cm−1 suggesting C–O–C stretching [12]. This correlates with the peaks obtained in this study.
X‑Ray Diffraction (XRD) Analysis of PHA
The crystalline nature of the PHA can be deduced from the pattern of XRD peaks and is thus a routine method for polymer characterization and identification [47]. In the present study, the XRD spectrum of the extracted biopolymer showed characteristic diffraction (2θ) peaks at 13.54, 17.02, 22.03, 31.76, 45.5, and 56.54 (Fig. 4). These peaks were in correlation with the XRD peaks of PHA reported in other studies and reflected the semi-crystalline nature of PHA. A research study on PHA film preparation using acetic acid-based solvent casting methods obtained characteristic XRD peaks (2θ) at 13.5, 16.85, 21.4, 27.2, and 44. These peaks were similar to those reported in this study [48]. In another study reporting the production of PHB using fish solid waste hydrolysate as a cost-effective medium, characteristic XRD peaks (2θ) were obtained at 13.34, 16.96, 21.95, 25.92, 27.29, 31.77, 45.70, and 56.39 which reflects the crystalline lattice of the biopolymer [13].
Nuclear Magnetic Resonance (NMR) Analysis of PHA
1H NMR analysis is a susceptible technique to understand the precise monomeric units and functional groups present in the biopolymers. The 1H NMR spectrum of the biopolymer extracted in this study revealed characteristic peaks suggesting the polymer to be PHB (the most widely studied class of PHA). In the 1H NMR spectrum, a doublet peak at 1.2 ppm indicates the presence of a methyl group (–CH3) of the hydroxybutyrate (HB). Similarly, the cliffs at 2.4 ppm, 2.5 ppm, and 2.6 ppm denote the methylene group (–CH2) in HB, and the multiple peaks at 5.2 ppm indicate the methine group (–CH) of HB. The sharp peak at 7.2 ppm is contributed by the deuterochloroform (CDCl3) used for sample preparation (Fig. 5).
1H NMR spectrum of PHB synthesized from Bacillus sp. NII2 using damaged wheat grains (DWG) revealed characteristic peaks at 1.268–1.281 ppm, 2.453– 2.630 ppm, and 5.239–5.302 ppm [49]. These results were in agreement with the 1H NMR spectrum reported in this study. The 1H NMR spectrum of PHB extracted from Bacillus cereus strain 4N revealed characteristic peaks at 1.24 ppm, 2.48 ppm, 2.5 ppm, and 5.45 ppm, similar to that reported in this study [50].
Plant Growth-Promoting Tests
ACC Deaminase Test
Production of ACC-deaminase is a mechanism of Plant Growth-Promoting Rhizobacteria (PGPR) that elevates plant growth in stress conditions. According to a study, tomatoes' capability to withstand drought stress has been boosted by B. subtilis Rhizo SF, which induces ACC-deaminase [51]. Another study indicated that B. subtilis (NBRI 28B), B. subtilis (NBRI 33 N), and B. safensis (NBRI 12 M) yielded ACC-deaminase and helped mitigate salt stress in Zea mays [52]. A study reported that ACC-deaminase producing B. subtilis BERA 71 appeared to stimulate salt stress in Chickpeas [53]. In this study, B. subtilis NJ14 has shown ACC-deaminase-production indicating its potential to boost plant growth in stress conditions (Fig. 6a).
Phosphate Solubilization Test
Plants utilize P only in 2 forms, HPO4 (monobasic) or as H2PO4 (dibasic) ions; [54]. A study by [55] B. megaterium mj1212 has proved to have phosphate solubilization and enhanced carbohydrates and amino acids in mustard plants. Another work by [56] dealing with thermotolerant B. subtilis strain displayed phosphate solubilization. Another work by [57] revealed that B. velezensis strain Ag75 appeared to have phosphate solubilization and promoted growth in maize and soybean crops. In this study, B. subtilis NJ14 has shown phosphate solubilization and had 1.431 ± 0.12 cm of PSI (Fig. 6b).
Zinc Solubilization Test
Zn insufficiency is considered a significant global risk factor for human and plant health [58]. The test for Zinc solubilizing bacteria was performed with Pikovskaya media containing insoluble ZnCl2. A work reported a zone of 4.3 ± 0.3 cm in CDK25 species of B. megaterium [59]. Another study has suggested that Bacillus spp. IA16 exhibited Zn solubilization to enhance cotton growth [60]. Another work involving B. subtilis RH5 showed that the strain could solubilize Zn and protect rice from Sheath blight disease from Rhizoctonia solani [61]. In the current study, B. subtilis NJ14 showed Zn solubilization and expressed ZSI of 1.223 ± 0.31 cm (Fig. 6c).
Indole Acetic Acid Test
IAA is also appreciated for its involvement in inducing flowering and fruiting in plants. In a study B. subtilis was inferred to produce 4 ± 0.2 ug/mL IAA [62]. In another study it was observed that, after 24 h of incubation, B. subtilis (Mt3b) expressed 322.6 μg/mL, and B. cereus (So3II) displayed 241.6 μg/mL auxin concentrations [63]. In the present study, B. subtilis NJ14 yielded 30.55 μg/mL of IAA, thus can help in root elongation (Fig. 6d).
Siderophore Test
Ferric ions play an essential role in monitoring oxygen during ATP synthesis, heme formation, and deteriorating ribotide precursors of DNA [64]. Siderophores yielding bacteria residing near the roots of plants help in this process [65]. Research has studied bacillibactin siderophores from B. subtilis and applied them in plant growth and oil production from sesame [66]. In another study, B. subtilis MF497446 having siderophores production has shown to control Cephalosporium maydis in Maize plants [67]. Another work has shown that B. subtilis CAS15-inducing siderophores helped control Fusarium wilt and elevated plant growth in pepper [68]. In this study, B. subtilis NJ14 produced siderophores and thus can help in the chelation of Fe ions (Fig. 6e).
Ammonia Test
The influential repression caused due to the increased pH in soil by the production of ammonia (pH 9–9.5) keeps away certain harmful fungi and other Nitrobacter and undermines spore formation in many fungi. A study has indicated ammonia production in 95% of Bacillus spp [69]. Another study explored ammonia production of ammonia in B. subtilis as a volatile compound with antifungal activity against Rhizoctonia solani and Pythium ultimum [70]. Another research showed B. cereus CUAMS116 has shown ammonia yielding and supporting the growth of Phaseolus vulgaris L [71]. In this study, B. subtilis NJ14 produced ammonia as another promising PGP trait (Fig. 6f).
Nitrogen Fixation Test
The Diazotrophy pathway is mediated by diazotrophs which are prokaryotic. A work describing Bacillus spp. that was isolated from Egyptian soil has reported nitrogen-fixing activity [72]. Bacillus spp., which can fix nitrogen, has been isolated from a tropical estuary and studied by [73]. Another work has concluded that B. subtilis is a nitrogen-fixing agent [74]. In the current study, B. subtilis NJ14 has grown on Jensen’s nitrogen-free media confirming its nitrogen-fixing ability (Fig. 6g).
HCN Test
HCN production in bacteria is majorly correlated to its antagonistic trait, thereby can hinder the growth of other phytopathogens as the producers are resistant [75]. Trace amounts of HCN near the plant roots are considerably less harmful to the plants. In research they investigated B. subtilis for HCN production and used it to stimulate sesame plant growth and oil production [66]. In another work, Bacillus spp. is studied and reported to induce HCN as a trait of plant growth promotion [76]. In another work B. subtilis PF1 has shown HCN production [77]. In the current study, B. subtilis NJ14 exhibited HCN production, contributing to its antagonistic trait (Fig. 6h).
Enzymatic Assay
Protease and cellulase production can help in plant growth promotion by cell wall degradation of pathogenic fungi [78]. Amylase helps plant growth by degrading organic compounds like starch in the soil [79]. Bacteria produce catalase to protect themselves from hydrogen peroxide, which is poisonous to bacteria and plant roots [80]. Cellulase production from B. licheniformis has been reported [81]. The effect of the C:N ratio on alpha-amylase production has been studied [82]. Other studies have shown the production of alpha-amylase from B. licheniformis using pearl millet as a medium 5. Catalase activity has been reported in Bacillus spp. in a work [83]. In the current study, B. subtilis NJ14 showed positive results in all four lytic enzyme assays (Fig. 7).
Halotolerance Test
Halotolerant PGPR ameliorates plant growth by activating enzymes such as peroxidase, catalase, and superoxide dismutase which are part of the antioxidant defense in plants. The cumulation of hazardous Na+ ions is reduced by boosting the selective ingestion of K+ ions that maintains the K+/Na+ ratio level. Exopolysaccharide (EPS) evacuated by bacteria can bind Na+ ions and increase soil macropores, which meliorates water retention near the roots. Many PGPR genera, including Bacillus, have been inferred to produce EPS and accelerate biofilm formation. A work proved that B. subtilis HG-15 displayed halotolerant (NaCl) up to 30% [84]. Another work dealing with B. subtilis BBK-1 showed NaCl tolerance of up to 16% and production of biosurfactants up to 8% in NaCl [85]. Another report concluded that halotolerant B. subtilis helped mitigate salt stress in hydroponically grown soybeans [86]. In the current study, B. subtilis NJ14 could tolerate NaCl up to 10% and be utilized in salt-stressed soil (Fig. 8).
Metal Tolerance Test
Metal tolerance in bacteria helps them thrive in metal-contaminated soil and makes them a promising applicant for heavy metal removal. A work has reported a novel Bacillus spp. with Pb resistance [87]. Another work concluded that B. anthracis and B. subtilis showed tolerance to Cr, Cu, and Hg [88]. Biosorption of Pb has been studied using B. subtilis MTCC 2423 [89]. In the present study, B. subtilis NJ14 could tolerate Pb up to 1000 μg/mL, Hg up to 100 μg/mL, and did not display any tolerance to Cd (Fig. 9).
Antifungal Assay
Antagonism against pathogenic fungi is one of the vital traits of a PGPR. Fusarium spp. is a threatening pathogen to many plants, including tomatoes, that causes Fusarium wilt disease [90]. Some strains of Cladosporium are also known as saprophytic plant pathogens and affect many plant hosts [91]. An antifungal compost has been developed from the B. licheniformis KJ-9 strain in research [92]. Aspergillus flavus is an opportunistic pathogen affecting maize plants weakened by drought or heat stress [93]. Talaromyces are generally non-pathogenic to plants and are studied as an applicant for biofertilizers. A work has inferred that B. subtilis GM5 expressed antagonism against Fusarium oxysporum [94]. Another work revealed that B. subtilis and B. amyloliquefaciens antagonize against Aspergillus parasiticus [95]. Talaromyces trachyspermus has shown antibacterial activity against B. subtilis, confirming antifungal activity [96]. In the current study, B. subtilis NJ14 exhibited antagonism against all four fungi recommending its ability to control phytopathogens (Fig. 10).
Statistical Optimization of Two-Factor Interaction Model for the Bacterial Biomass
The data in Table 2 were utilized to construct a two-factor interaction model representing the response variable (bacterial biomass g/L) of B. subtilis NJ14. An empirical equation was obtained from the data; the Y response (bacterial biomass (g/L) defined as a function of A: Incubation period (h), B: Inoculum level (%), and C: NaCl (g/L). The proposed equation is,
Y = 0.968333 + 0.0297302A + 0.347843B + 0.00891905C + 0.0224858AB + 0.0624022AC − 0.0523812BC − 0.0842636A2 + 0.108423B2 − 0.103709C2 − 0.300395A2B − 0.116255 A2C − 0.0671419AB2 − 0.130936A2B2.
The input variables, namely Incubation period (A), Inoculum level (B), AB, AC, and BC, were observed to be statistically significant (p ≤ 0.05) for the bacterial biomass (g/L) (Table 3). F value for the model was noted as 231.31. The F value 231.31 showed that there was only a 0.01% chance that the result could occur due to noise. The regression coefficient of the selected factorial model, 99.8%, indicated adequate precision in the response prediction.
Establishment of the Design Space
Following the guideline outlined by ICH Q8 (https://www.ich.org/page/quality-guidelines) and according to our previous works [38, 39], an overlaid plot also known as design space that decides a standard range of interacting input variables for specific responses for the optimized process in terms of the response bacterial biomass was established.
In the present study, interacting variables Incubation period (h) and Inoculum level (%) (AB) were selected as the x (× 1) and y (× 2) axis of the plot, respectively (Fig. 11).
Another variable NaCl (g/L) was maintained as a constant parameter. The yellow space of the plot represented by the incubation period within the range of 33.73–62.27 h, the inoculum level within the range of 1.81–4.19 g/L, and NaCl 4.91 g/L could provide the best possible optimized solution as ≥ 0.84 g/L bacterial biomass. The interaction plot revealed that an increase in the Incubation period (h) and Inoculum level (%) could jointly enhance the bacterial biomass. This result was further apprised by a 3D contour plot (Fig. 12).
Design Space Validation
The design space was validated by selecting random values from the optimized variable ranges. Selected values were 58.8 h during the Incubation period, 3.73% Inoculum level, and 4.9 g/L NaCl. The validation experiments showed the bacterial biomass as 1.05 ± 0.02 g/L. The value fitted well within the prediction interval range with 95% confidence as provided by the software (1.01 to 1.09 g/L).
Plant Growth Study on S. lycopersicum and C. arietinum
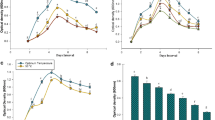
After confirming PHA production and PGP traits in B. subtilis NJ14, a Plant Growth study was carried out using the dip method [33] on a leguminous plant C. arietinum (MNK-1 variety from UAS, Raichur) and a non-leguminous plant S. lycopersicum (Arka-Samrat variety collected from IIHR, Bangalore), and the study was carried out for 27 days. The results are presented in Fig. 13. The plants were healthy and free from fungal attack throughout the study, possibly due to the antagonistic trait of B. subtilis NJ14 against fungi. The total Germination Percentage (TGP) was the same for both the control and sample in S. lycopersicum, which was 80.56%. But in C. arietinum, the sample expressed higher TGP than the control, 72, and 68%, respectively. There was a significant difference in root length of the control and sample of S. lycopersicum which were 8.42 ± 0.53 and 11.91 ± 0.7 cm, respectively, and 15.42 ± 0.65 and 23.66 ± 0.81 cm for C. arietinum, respectively (Figs. 14, 15). Shoot length did not display a notable significant difference in S. lycopersicum which were 9.08 ± 0.21 and 9.49 ± 0.33 cm in control and sample, respectively. However, the sample showed improved shoot growth compared to the control in C. arietinum which were 38.94 ± 0.51 and 48.49 ± 0.93 cm, respectively.
Conclusion
Bacillus subtilis NJ14 is a significant applicant for fabricating a reliable biofertilizer that can enhance the growth of S. lycopersicum and C. arietinum. The ability of B.subtilis NJ14 to produce PHA assures augmenting the shelf life of biofertilizer. Plant Growth-Promoting traits like the production of ACC-deaminase, Indole Acetic Acid, HCN, Siderophores, Ammonia, lytic enzymes (Protease, Catalase, Cellulase, and Amylase); Nitrogen fixation; Phosphate and Zinc solubilization; and antagonism against fungi make B.subtilis NJ14 a definitive PGPR that can ameliorate plant growth. This work can be extended by implementing B.subtilis NJ14 in formulating a considerable biofertilizer with remarkable shelf life by utilizing a suitable carrier. A product of B. subtilis NJ14 can also be used in salt-stressed land (up to 10%) and lead-stressed land due to its halotolerant and metal tolerance properties, respectively. The PHA-producing ability of B. subtilis NJ14 can also be employed in various other applications in the field of agriculture, like the preparation of mulch. Therefore, PHA-producing PGPR B. subtilis NJ14 is an ideal candidate for biofertilizers that can replace chemical fertilizers in the market.
Data Availability
The original data related to this research work will be available on special request to the authors.
References
Kaushik, B. D., Kumar, D., & Shamim, M. (2019). Biofertilizers and Biopesticides in Sustainable Agriculture. Role of Rhizospheric Microbes in Soil (pp. 377–398). CRC Press.
Correa, J. P., Molina, V., Sanchez, M., Kainz, C., Eisenberg, P., & Massani, M. B. (2017). Improving ham shelf life with a polyhydroxybutyrate/polycaprolactone biodegradable film activated with nisin. Food Packaging and Shelf Life, 11, 31–39.
Sedlacek, P., Slaninova, E., Koller, M., Nebesarova, J., Marova, I., Krzyzanek, V., & Obruca, S. (2019). PHA granules help bacterial cells to preserve cell integrity when exposed to sudden osmotic imbalances. New Biotechnology, 49, 129–136.
Obruca, S., Sedlacek, P., & Koller, M. (2021). The underexplored role of diverse stress factors in microbial biopolymer synthesis. Bioresource Technology, 326, 124767.
Sehgal, R., & Gupta, R. (2020). Polyhydroxyalkanoate and its efficient production: An eco-friendly approach towards development. 3 Biotech, 10(12), 1–14.
Silveira Alves, L. P., Plucani do Amaral, F., Kim, D., Todo Bom, M., Piñero Gavídia, M., Silvano Teixeira, C., & Stacey, G. (2019). Importance of poly-3-hydroxybutyrate metabolism to the ability of Herbaspirillum seropedicae to promote plant growth. Applied and Environmental Microbiology, 85(6), 1–14.
Umesh, M., Adhithya Sankar, S., & Thazeem, B. (2021). Fruit waste as Sustainable Resources for Polyhydroxyalkanoate (PHA) production. Bioplastics for Sustainable Development, 307, 205–229.
Gohil, R. B., Raval, V. H., Panchal, R. R., & Rajput, K. N. (2022). Plant growth-promoting activity of Bacillus sp. PG-8 isolated from fermented panchagavya and its effect on the growth of arachis hypogea. Frontiers in Agronomy, 4, 1–13.
Jaber, N. N. (2019). Isolation and identification of polyhydroxyalkanoates from two strains of Clostridium bifermentans isolated from the soil near the gas station in Basrah city. Biomedical Journal of Scientific & Technical Research, 13(2), 9888–9892.
Balakrishna Pillai, A., Jaya Kumar, A., Thulasi, K., & Kumarapillai, H. (2017). Evaluation of short-chain-length polyhydroxyalkanoate accumulation in Bacillus aryabhattai. Brazilian Journal of Microbiology, 48(3), 451–460.
Priyanka, K., Umesh, M., Thazeem, B., & Preethi, K. (2020). Polyhydroxyalkanoate biosynthesis and characterization from optimized medium utilizing distillery effluent using Bacillus endophyticus MTCC 9021: A statistical approach. Biocatalysis and Biotransformation, 39(1), 16–28.
Priyanka, K., Umesh, M., & Preethi, K. (2022). Distillery effluent valorization through cost effective production of polyhydroxyalkanoate: Optimization and characterization. Biomass Conversion and Biorefinery. https://doi.org/10.1007/s13399-022-03333-z
Mohapatra, S., Sarkar, B., Samantaray, D. P., Daware, A., Maity, S., Pattnaik, S., & Bhattacharjee, S. (2017). Bioconversion of fish solid waste into PHB using Bacillus subtilis based submerged fermentation process. Environmental Technology, 38(24), 3201–3208.
Ray, S., Prajapati, V., Patel, K., & Trivedi, U. (2016). Optimization and characterization of PHA from isolate Pannonibacter phragmitetus ERC8 using glycerol waste. International Journal of Biological Macromolecules, 86, 741–749.
Dworkin, M., & Foster, J. W. (1958). Experiments with some microorganisms which utilize ethane and hydrogen. Journal of Bacteriology, 75(5), 592–603.
Husen, E., Wahyudi, A. T., Suwanto, A., & Saraswati, R. (2016). Soybean seedling root growth promotion by 1-aminocyclopropane-1-carboxyate deaminase-producing Pseudomonads. Indonesian Journal of Agricultural Science, 10(1), 19.
Nautiyal, C. S. (1999). An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiology Letters, 170(1), 265–270.
Sanchez-Gonzalez, M. E., Mora-Herrera, M. E., Wong-Villarreal, A., De La Portilla-López, N., Sanchez-Paz, L., Lugo, J., & Yañez-Ocampo, G. (2022). Effect of pH and Carbon source on phosphate solubilization by bacterial strains in pikovskaya medium. Microorganisms, 11(1), 49.
Singh, T. B., Sahai, V., Goyal, D., Prasad, M., Yadav, A., Shrivastav, P., & Dantu, P. K. (2020). Identification, Characterization and evaluation of multifaceted traits of plant growth promoting rhizobacteria from soil for sustainable approach to agriculture. Current Microbiology, 77(11), 3633–3642.
Pande, A., Kaushik, S., Pandey, P., & Negi, A. (2020). Isolation, characterization, and identification of phosphate-solubilizing Burkholderia cepacia from the sweet corn cv. Golden Bantam rhizosphere soil and effect on growth-promoting activities. International Journal of Vegetable Science, 26(6), 591–607.
Eshaghi, E., Nosrati, R., Owlia, P., Malboobi, M. A., Ghaseminejad, P., & Ganjali, M. R. (2019). Zinc solubilization characteristics of efficient siderophore-producing soil bacteria. Iranian Journal of Microbiology, 11(5), 419–430.
Meudt, W. J., & Gaines, T. P. (1967). Studies on the oxidation of indole-3-acetic acid by peroxidase enzymes. I. Colorimetric determination of indole-3-acetic acid oxidation products. Plant Physiology, 42(10), 1395–1399.
Lugtenberg, B., & Kamilova, F. (2009). Plant-growth-promoting rhizobacteria. Annual Review of Microbiology, 63, 541–556.
Akinrinlola, R. J., Yuen, G. Y., Drijber, R. A., & Adesemoye, A. O. (2018). Evaluation of strains for plant growth promotion and predictability of efficacy by physiological traits. International Journal of Microbiology, 53, 1–14.
Ahemad, M., & Kibret, M. (2014). Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. Journal of King Saud University - Science, 26(1), 1–20.
James, N., Umesh, M., Sarojini, S., Shanmugam, S., Nasif, O., Alharbi, S. A., & Brindhadevi, K. (2023). Unravelling the potential plant growth activity of halotolerant Bacillus licheniformis NJ04 isolated from soil and its possible use as a green bioinoculant on Solanum lycopersicum L. Environmental Research, 216(2), 114620.
Jensen, H. L. (1940). Contributions to the Nitrogen Economy of Australian Wheat Soils, with Particular Reference to New South Wales.
Ahmad, F., Ahmad, I., & Khan, M. S. (2008). Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiological Research, 163(2), 173–181.
Bakker, A. W., & Schippers, B. (1987). Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas SPP-mediated plant growth-stimulation. Soil Biology & Biochemistry, 19(4), 451–457.
Simair, A. A., Qureshi, A. S., Khushk, I., Ali, C. H., Lashari, S., Bhutto, M. A., & Lu, C. (2017). Production and partial characterization of α-amylase enzyme from bacillus sp. BCC 01–50 and potential applications. BioMed Research International, 2017, 1–9.
Ayyachamy, M., & Vatsala, T. M. (2007). Production and partial characterization of cellulase free xylanase by Bacillus subtilis C 01 using agriresidues and its application in biobleaching of nonwoody plant pulps. Letters in Applied Microbiology, 45(5), 467–472.
Gogoleva, O. A., Nemtseva, N. V., & Bukharin, O. V. (2012). Catalase activity of hydrocarbon-oxidizing bacteria. Applied Biochemistry and Microbiology, 48(6), 552–556.
Sharma, A., Dev, K., Sourirajan, A., & Choudhary, M. (2021). Isolation and characterization of salt-tolerant bacteria with plant growth-promoting activities from saline agricultural fields of Haryana, India. Journal, Genetic Engineering & Biotechnology, 19(1), 99.
Lima de Silva, A. A., de Carvalho, M. A. R., de Souza, S. A. L., Dias, P. M. T., da Silva Filho, R. G., de Meirelles Saramago, C. S., & Hofer, E. (2012). Heavy metal tolerance (Cr, Ag AND Hg) in bacteria isolated from sewage. Brazilian Journal of Microbiology, 43(4), 1620–1631.
Anith, K. N., Nysanth, N. S., & Natarajan, C. (2021). Novel and rapid agar plate methods for in vitro assessment of bacterial biocontrol isolates’ antagonism against multiple fungal phytopathogens. Letters in Applied Microbiology, 73(2), 229–236.
Kimura, M. (1980). A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution, 16(2), 111–120.
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35(6), 1547–1549.
Choudhuri, I., Khanra, K., Maity, P., Patra, A., Maity, G. N., Pati, B. R., & Bhattacharyya, N. (2021). Structure and biological properties of exopolysaccharide isolated from Citrobacter freundii. International Journal of Biological Macromolecules, 168, 537–549.
Liya, S. M., Umesh, M., Nag, A., Chinnathambi, A., Alharbi, S. A., Jhanani, G. K., & Brindhadevi, K. (2023). Optimized production of keratinolytic proteases from Bacillus tropicus LS27 and its application as a sustainable alternative for dehairing, destaining and metal recovery. Environmental research, 221, 115283.
Edkie, G. N., & Prasad, D. T. (2014). Bacillus subtilis isolated from sugarcane rhizosphere produces PHA to defend NaCl induced stress. Research Journal of Microbiology, 9(3), 115.
Mascarenhas, J., & Aruna, K. (2017). Screening of polyhydroxyalkonates (pha) accumulating bacteria from diverse habitats. Journal of Global Biosciences, 6(3), 4835–4848.
Gomaa, E. Z. (2014). Production of polyhydroxyalkanoates (PHAs) by Bacillus subtilis and Escherichia coli grown on cane molasses fortified with ethanol. Brazilian Archives of Biology and Technology, 57, 145–154.
Anjali, M., Sukumar, C., Kanakalakshmi, A., & Shanthi, K. (2014). Enhancement of growth and production of polyhydroxyalkanoates by Bacillus subtilis from agro-industrial waste as carbon substrates. Composite Interfaces, 21(2), 111–119.
Nair, A. M., Annamalai, K., Kannan, S. K., & Kuppusamy, S. (2014). Utilization of sugarcane molasses for the production of polyhydroxyalkanoates using Bacillus subtilis. Malaya Journal of Biosciences, 1(1), 24–30.
Rathika, R., Janaki, V., Shanthi, K., & Kamala-Kannan, S. (2019). Bioconversion of agro-industrial effluents for polyhydroxyalkanoates production using Bacillus subtilis RS1. International Journal of Environmental Science and Technology, 16(10), 5725–5734.
Hamdy, S. M., Danial, A. W., Gad El-Rab, S. M. F., Shoreit, A. A. M., & Hesham, A.E.-L. (2022). Production and optimization of bioplastic (Polyhydroxybutyrate) from Bacillus cereus strain SH-02 using response surface methodology. BMC Microbiology, 22(1), 183.
Samrot, A. V., Samanvitha, S. K., Shobana, N., Renitta, E. R., Senthilkumar, P., Kumar, S. S., & Thirumurugan, R. (2021). The synthesis, characterization and applications of Polyhydroxyalkanoates (PHAs) and PHA-based nanoparticles. Polymers, 13(19), 3302.
Anbukarasu, P., Sauvageau, D., & Elias, A. (2015). Tuning the properties of polyhydroxybutyrate films using acetic acid via solvent casting. Scientific Reports, 5, 17884.
Sirohi, R. (2021). Sustainable utilization of food waste: Production and characterization of polyhydroxybutyrate (PHB) from damaged wheat grains. Environmental Technology & Innovation, 23, 101715.
Martínez-Herrera, R. E., Alemán-Huerta, M. E., Almaguer-Cantú, V., Rosas-Flores, W., Martínez-Gómez, V. J., Quintero-Zapata, I., & Rutiaga-Quiñones, O. M. (2020). Efficient recovery of thermostable polyhydroxybutyrate (PHB) by a rapid and solvent-free extraction protocol assisted by ultrasound. International Journal of Biological Macromolecules, 164, 771–782.
Gowtham, H. G., Singh, B., Murali, M., Shilpa, N., Prasad, M., Aiyaz, M., Amruthesh, K. N., & Niranjana, S. R. (2020). Induction of drought tolerance in tomato upon the application of ACC deaminase producing plant growth promoting rhizobacterium Bacillus subtilis Rhizo SF 48. Microbiological Research, 234, 126422.
Misra, S., & Chauhan, P. S. (2020). ACC deaminase-producing rhizosphere competent spp. mitigate salt stress and promote growth by modulating ethylene metabolism. 3 Biotech, 10(3), 119.
Singh, R. P., Ma, Y., & Shadan, A. (2022). Perspective of ACC-deaminase producing bacteria in stress agriculture. Journal of Biotechnology, 352, 36–46.
Khan, M. S., Zaidi, A., & Musarrat, J. (2014). Phosphate Solubilizing Microorganisms: Principles and Application of Microphos Technology. Springer.
Kang, S.-M., Radhakrishnan, R., You, Y.-H., Joo, G.-J., Lee, I.-J., Lee, K.-E., & Kim, J.-H. (2014). Phosphate solubilizing Bacillus megaterium mj1212 regulates endogenous plant carbohydrates and amino acids contents to promote mustard plant growth. Indian Journal of Microbiology, 54(4), 427–433.
Swain, M. R., Laxminarayana, K., & Ray, R. C. (2012). Phosphorus solubilization by thermotolerant bacillus subtilis isolated from cow dung microflora. Agricultural Research, 1(3), 273–279.
Mosela, M., Andrade, G., Massucato, L. R., de Araújo Almeida, S. R., Nogueira, A. F., de Lima Filho, R. B., & Gonçalves, L. S. A. (2022). Bacillus velezensis strain Ag75 as a new multifunctional agent for biocontrol, phosphate solubilization and growth promotion in maize and soybean crops. Scientific Reports, 12(1), 15284.
Joshi, D., Negi, G., Vaid, S., & Sharma, A. (2013). Enhancement of wheat growth and Zn content in grains by zinc solubilizing bacteria. International Journal of Agriculture Environment and Biotechnology, 6(3), 363.
Bhatt, K., & Maheshwari, D. K. (2020). Zinc solubilizing bacteria (Bacillus megaterium) with multifarious plant growth promoting activities alleviates growth in Capsicum annuum L. 3 Biotech, 10(2), 36.
Ahmad, I., Ahmad, M., Hussain, A., & Jamil, M. (2021). Integrated use of phosphate-solubilizing Bacillus subtilis strain IA6 and zinc-solubilizing Bacillus sp. strain IA16: a promising approach for improving cotton growth. Folia Microbiologica, 66(1), 115–125.
Jamali, H., Sharma, A., & Roohi, & Srivastava, A. K. (2020). Biocontrol potential of Bacillus subtilis RH5 against sheath blight of rice caused by Rhizoctonia solani. Journal of Basic Microbiology, 60(3), 268–280.
Patel, R. R., Patel, D. D., Thakor, P., Patel, B., & Thakkar, V. R. (2015). Alleviation of salt stress in germination of Vigna radiata L. by two halotolerant Bacilli sp. isolated from saline habitats of Gujarat. Plant Growth Regulation, 76(1), 51–60.
Wagi, S., & Ahmed, A. (2019). Bacillus spp.: potent microfactories of bacterial IAA. PeerJ, 7, 7258.
Neilands, J. B. (1995). Siderophores: structure and function of microbial iron transport compounds. Journal of Biological Chemistry, 270(45), 26723–26726.
Kobayashi, T., & Nishizawa, N. K. (2012). Iron uptake, translocation, and regulation in higher plants. Annual Review of Plant Biology, 63(1), 131–152.
Nithyapriya, S., Lalitha, S., Sayyed, R. Z., Reddy, M. S., Dailin, D. J., El Enshasy, H. A., & Herlambang, S. (2021). Production, purification, and characterization of bacillibactin siderophore of Bacillus subtilis and Its application for improvement in plant growth and oil content in sesame. Sustainability: Science Practice and Policy, 13(10), 5394.
Ghazy, N., & El-Nahrawy, S. (2020). Siderophore production by Bacillus subtilis MF497446 and Pseudomonas koreensis MG209738 and their efficacy in controlling Cephalosporium maydis in maize plant. Archives of Microbiology, 203(3), 1195–1209.
Yu, X., Ai, C., Xin, L., & Zhou, G. (2011). The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. European Journal of Soil Biology, 47(2), 138–145.
Joseph, B., Ranjan Patra, R., & Lawrence, R. (2012). Characterization of plant growth promoting rhizobacteria associated with chickpea (Cicer arietinum L.). International Journal of Plant Production, 1(2), 141–152.
Fiddaman, P. J., & Rossall, S. (1993). The production of antifungal volatiles by Bacillus subtilis. The Journal of Applied Bacteriology, 74(2), 119–126.
Sebastian, A. M., Umesh, M., Priyanka, K., & Preethi, K. (2020). Isolation of plant growth-promoting Bacillus cereus from soil and its use as a microbial inoculant. Arabian Journal for Science and Engineering, 46(1), 151–161.
Wahab, A. M. A., Abdel Wahab, A. M., & El-Sharouny, H. M. (1979). Nitrogen-fixing Bacillus species from Egyptian soils: Acetylene reduction and cultural conditions. Plant and Soil, 51(2), 187–196.
Yousuf, J., Thajudeen, J., Rahiman, M., Krishnankutty, S., Alikunj, P., & A., & A Abdulla, M. H. (2017). Nitrogen fixing potential of various heterotrophic Bacillus strains from a tropical estuary and adjacent coastal regions. Journal of Basic Microbiology, 57(11), 922–932.
Hashem, A., Tabassum, B., Allah, F. A., & E. (2019). A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi Journal of Biological Sciences, 26(6), 1291–1297.
Muthezhilan, R., Sindhuja, B. S., Hussain, A. J., & Jayaprakashvel, M. (2012). Efficiency of plant growth promoting rhizobacteria isolated from sand dunes of Chennai coastal area. Pakistan Journal of Biological Sciences: PJBS, 15(16), 795–799.
Pathak, E., Sanjyal, A., Regmi, C. R., Paudel, S., & Shrestha, A. (2021). Screening of potential plant growth promoting properties of Bacillus species isolated from different regions of Nepal. Nepal Journal of Biotechnology, 9(1), 79–84.
Bhange, K., Chaturvedi, V., & Bhatt, R. (2016). Ameliorating effects of chicken feathers in plant growth promotion activity by a keratinolytic strain of Bacillus subtilis PF1. Bioresources and Bioprocessing, 3(13), 1–10.
Bhattacharyya, C., Banerjee, S., Acharya, U., Mitra, A., Mallick, I., Haldar, A., & Ghosh, A. (2020). Evaluation of plant growth promotion properties and induction of antioxidative defense mechanism by tea rhizobacteria of Darjeeling India. Scientific Reports, 10(1), 15536.
Choubane, S., Cheba, B. A., & Benourrad, A. (2016). Screening and phenotypic diversity of amylase producing rhizospheric bacteria from some North African plants. Procedia Technology, 22, 1197–1204.
Bumunang, E. W., & Babalola, O. O. (2014). Characterization of rhizobacteria from field grown genetically modified (GM) and non-GM maizes. Brazilian Archives of Biology and Technology, 57(1), 1–8.
Shah, F., Ranawat, B., Dubey, S., & Mishra, S. (2021). Optimization of fermentation conditions for higher cellulase production using marine Bacillus licheniformis KY962963: An epiphyte of Chlorococcum sp. Biocatalysis and Agricultural Biotechnology, 35, 102047.
P., V. D. A., & Dharani Aiyer P., V. (2004). Effect of C: N ratio on alpha amylase production by Bacillus licheniformis SPT 27. African Journal of Biotechnology, 3(10), 519–522.
Ghazy, N., & El-Nahrawy, S. (2021). Siderophore production by Bacillus subtilis MF497446 and Pseudomonas koreensis MG209738 and their efficacy in controlling Cephalosporium maydis in maize plant. Archives of Microbiology, 203(3), 1195–1209.
Ji, C., Tian, H., Wang, X., Song, X., Ju, R., Li, H., & Liu, X. (2022). Bacillus subtilis HG-15, a halotolerant rhizoplane bacterium, promotes growth and salinity tolerance in wheat (Triticum aestivum). BioMed Research International, 2022, 9506227.
Roongsawang, N., Thaniyavarn, J., Thaniyavarn, S., Kameyama, T., Haruki, M., Imanaka, T., & Kanaya, S. (2002). Isolation and characterization of a halotolerant Bacillus subtilis BBK-1 which produces three kinds of lipopeptides: bacillomycin L, plipastatin, and surfactin. Extremophiles: Life Under Extreme conditions, 6(6), 499–506.
Yasmin, H., Naeem, S., Bakhtawar, M., Jabeen, Z., Nosheen, A., Naz, R., & Hassan, M. N. (2020). Halotolerant rhizobacteria Pseudomonas pseudoalcaligenes and Bacillus subtilis mediate systemic tolerance in hydroponically grown soybean (Glycine max L) against salinity stress. PLoS ONE, 15(4), e0231348.
Cai, Y., Li, X., Liu, D., Xu, C., Ai, Y., Sun, X., & Yu, H. (2018). A novel Pb-resistant Bacillus subtilis bacterium isolate for Co-biosorption of hazardous Sb(III) and Pb(II): Thermodynamics and application strategy. International Journal of Environmental Research and Public Health, 15(4), 702.
Sekhar, C. H., & S. M. (2013). Heavy metal tolerance of Bacillus spp. Progressive Research, 8(10), 315–318.
Gupta, S., Surendran, A., & JosephThatheyus, A. (2020). Biosorption of Lead Using the Bacterial Strain, Bacillus subtilis (MTCC 2423). Journal of Biotechnology and Biomedical Science, 2(3), 1–14.
Scheffer, R. P. (1952). The Wilting Mechanism in Fusarium Wilt of Tomato.
Răut, I., Călin, M., Capră, L., Gurban, A.-M., Doni, M., Radu, N., & Jecu, L. (2021). Cladosporium sp. isolate as fungal plant growth promoting agent. Agronomy, 11(2), 392.
Seo, D.-C., Ko, J.-A., & Lee, S.-W. (2010). Production of antifungal compost by using Bacillus licheniformis KJ-9. Journal of Life Science, 20(9), 1339–1344.
Dolezal, A. L., Shu, X., OBrian, G. R., Nielsen, D. M., Woloshuk, C. P., Boston, R. S., & Payne, G. A. (2014). Aspergillus flavus infection induces transcriptional and physical changes in developing maize kernels. Frontiers in Microbiology, 5, 384.
Mardanova, A. M., Fanisovna Hadieva, G., Tafkilevich Lutfullin, M., Valer’evna Khilyas, I., Farvazovna Minnullina, L., Gadelevna Gilyazeva, A., & Rashidovna Sharipova, M. (2017). Bacillus subtilis strains with antifungal activity against the phytopathogenic fungi. Agricultural Sciences in China/Sponsored by the Chinese Academy of Agricultural Sciences, 08(01), 1–20.
Siahmoshteh, F., Hamidi-Esfahani, Z., Spadaro, D., Shams-Ghahfarokhi, M., & Razzaghi-Abyaneh, M. (2018). Unraveling the mode of antifungal action of Bacillus subtilis and Bacillus amyloliquefaciens as potential biocontrol agents against aflatoxigenic Aspergillus parasiticus. Food Control, 89, 300–307.
Farhat, H., Urooj, F., Sohail, N., Hameedi, S. F., Ali, M. S., & Ehteshamul-Haque, S. (2022). Evaluation of antibacterial potential of endophytic fungi and GC-MS profiling of metabolites from Talaromyces trachyspermus. South African Journal of Botany, 150, 240–247.
Funding
Not Applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical Approval
This research work does not involve the use of any human and/ or animal studies and thus ethical approval is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
James, N., Umesh, M. Multifarious Potential of Biopolymer-Producing Bacillus subtilis NJ14 for Plant Growth Promotion and Stress Tolerance in Solanum lycopercicum L. and Cicer arietinum L: A Way Toward Sustainable Agriculture. Mol Biotechnol 66, 1031–1050 (2024). https://doi.org/10.1007/s12033-023-01001-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-01001-9